
Abstract
Aims: The aim of this study was to investigate short-term and five-year follow-up results from patients randomised to coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) with paclitaxel-eluting stents in the SYNTAX trial, focusing on patients with chronic kidney disease (CKD).
Methods and results: Baseline glomerular filtration rate estimates (eGFR) were available in 1,638 patients (PCI=852 and CABG=786). The Kidney Disease: Improving Global Outcomes (KDIGO) threshold was used to define staging of CKD. At five years, death was significantly higher in patients with CKD compared to patients with normal kidney function after PCI (26.7% vs. 10.8%, p<0.001) and CABG (21.2% vs. 10.6%, p=0.005). Comparing PCI with CABG, there was a significant interaction according to kidney function for death (pint=0.017) but not the composite endpoint of death/stroke/MI (pint=0.070) or MACCE (pint=0.15). In patients with CKD, the rate of MACCE was significantly higher after PCI compared with CABG (42.1% vs. 31.5%, p=0.019), driven by repeat revascularisation (21.9% vs. 8.9%, p=0.004) and all-cause death (26.7% vs. 21.2%, p=0.14). In patients with CKD who also had diabetes, PCI versus CABG was significantly worse in terms of death/stroke/MI (47.9% vs. 24.4%, p=0.005) and all-cause death (40.9% vs. 17.7%, p=0.004).
Conclusions: During a five-year follow-up, adverse event rates were comparable between PCI and CABG patients with moderate CKD but significantly higher compared to the patients with impaired or normal kidney function. The negative impact of CKD on long-term outcome following PCI appears to be stronger when compared to CABG, especially in the CKD patients with diabetes and extensive coronary disease. ClinicalTrials.gov Identifier: NCT00114972
Abbreviations
CABG: coronary artery bypass grafting
CAD: coronary artery disease
CKD: chronic kidney disease
MACCE: major adverse cardiac and cerebrovascular events
PCI: percutaneous coronary intervention
Introduction
Chronic kidney disease (CKD) is a worldwide healthcare problem with an increasing incidence1. Despite the magnitude of resources committed to its treatment, CKD is one of the leading causes of death in Western countries2. End-stage renal disease, but also the early stages of CKD are found to be strong independent predictors of developing coronary artery disease (CAD) with subsequently markedly increased rates of cardiovascular events and high mortality3. The high prevalence of diabetes among patients with CKD contributes to the progression of renal disease, thereby promoting accelerated atherosclerosis that results in diffuse coronary artery calcifications and represents a group at high risk of cardiac mortality4.
It remains unclear whether percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) should be preferred in patients with CKD. According to the 2014 ESC/EACTS guidelines on myocardial revascularisation, CABG is preferable over PCI in patients with CKD if the life expectancy is more than one year, while PCI is recommended in patients with a life expectancy of less than one year. However, the current guidelines are based on observational studies with inherent limitations5,6. A subgroup analysis of the ARTS trial provides data from a randomised comparison of CABG with PCI using bare metal stents (BMS) in patients with CKD, but these results are difficult to interpret in the current DES era7. Moreover, data on the optimal revascularisation strategy in patients with a combined disease burden of CKD and diabetes are only available from the FREEDOM trial in which the authors were unable to report any differences among diabetic/non-diabetic patients because the population consisted only of diabetic patients with CKD, as mentioned in their limitations8.
In the SYNTAX trial, PCI with first-generation paclitaxel-eluting stents was compared with CABG for patients with de novo three-vessel and/or left main (LM) disease. Unlike previous trials9, patients with CKD were not routinely excluded10. Therefore, this study presents unique data of patients with CKD by comparing five-year outcomes between CABG and PCI along the spectrum of kidney function.
Methods
STUDY DESIGN
The SYNTAX trial design and methods have been described previously10. Briefly, SYNTAX was a prospective, multinational, randomised clinical trial in which 1,800 patients were randomly assigned to undergo PCI with first-generation paclitaxel-eluting stents (TAXUS™ Express™; Boston Scientific, Marlborough, MA, USA) or CABG. Patients were randomly assigned in a 1:1 fashion to undergo PCI (n=903) or CABG (n=897).
This study was carried out according to the principles of the Declaration of Helsinki. The trial is registered with number NCT00114972 at the ClinicalTrials.gov website.
DEFINITIONS AND ENDPOINTS
The definitions used for the classification of adverse events have been reported previously10,11. The primary endpoint of the SYNTAX trial was the composite rate of major adverse cardiac and cerebrovascular events (MACCE), defined as all-cause death, stroke, myocardial infarction (MI), and repeat revascularisation. Secondary endpoints in this study included the composite safety endpoint of death/stroke/MI and rates of the individual MACCE components.
Estimation of the glomerular filtration rate (eGFR) was used to assess the degree of kidney failure. The eGFR was calculated from the baseline serum creatinine level, which was available in 1,638 patients (PCI=852, 94.3% and CABG=786, 87.6%). Of the remaining 162 (9.0%) patients, the baseline creatinine could not be determined (Supplementary Table 1). For each patient, the eGFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation12. The Kidney Disease: Improving Global Outcomes (KDIGO) threshold was used to define the CKD population13. According to KDIGO classifications, patients were classified according to the eGFR: stage 1, patients with a normal kidney function had an eGFR of ≥90 mL/min per 1.73 m2; stage 2, impaired renal function, defined by an eGFR between 60 and 89 mL/min per 1.73 m2; and stages 3-5, CKD, defined by an eGFR <60 mL/min per 1.73 m2. Patients with CKD were further subdivided into stage 3 (eGFR 30-59 mL/min per 1.73 m2), stage 4 (eGFR 15-29 mL/min per 1.73 m2) and stage 5 (chronic dialysis treatment)13. Using these classifications, the following subgroups were defined and analysed in the current study: i) normal kidney function (stage 1); ii) impaired kidney function (stage 2); and iii) CKD (stages 3, 4 and 5), the latter being pooled together into a single group due to the low number of patients in stages 4 and 5 subgroups.
STATISTICAL ANALYSIS
Data are presented using descriptive statistics, as a percentage, count of sample size, or mean±standard deviation (SD). Either the Student’s t-test or the Kruskal-Wallis test was used to compare continuous variables. Differences in discrete variables were compared with a χ2 or Fisher’s exact test, where appropriate.
All analyses were based on the intention-to-treat principle. Short-term outcomes were defined within 30 days after the procedure. Five-year rates of adverse events were estimated using the Kaplan-Meier method, and comparisons between groups were made using log-rank tests. P-values for interaction were acquired using a logistic regression chi-square test. Cox proportional hazard models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CI) with PCI as the reference group. The proportionality for Cox models was tested with Schoenfeld residuals and confirmed no significant departures from the proportionality assumption. A two-sided p-value of <0.05 was considered to be statistically significant. Analyses were performed using SPSS Statistics, Version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Of the 1,638 patients, only 219 (25.7%) patients randomised to PCI and 209 (26.6%) patients randomised to CABG had a normal kidney function (Figure 1). The majority of patients had impaired kidney function (PCI=475 [55.8%] and CABG=426 [54.2%]), whereas CKD was present in 158 (18.5%) patients randomised to PCI and 151 (19.2%) patients randomised to CABG. Among patients with CKD, 24 patients (PCI=13 [8.2%] and CABG=11 [7.3%]) had severe CKD (stage 4) and six patients (PCI=3 [1.9%] and CABG=3 [2.0%]) were on chronic dialysis (stage 5). Patients without information on GFR had similar baseline characteristics and five-year outcomes to patients with information on GFR (Supplementary Table 1).
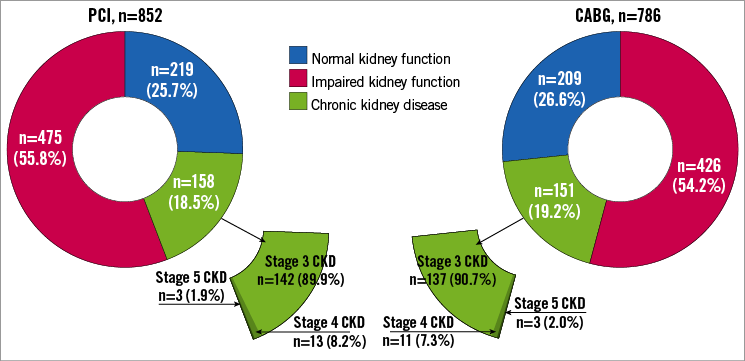
Figure 1. Distribution of patients enrolled in the SYNTAX randomised cohort according to kidney function. Groups consist of patients with normal kidney function as defined by an eGFR ≥90 mL/min per 1.73 m2, impaired kidney function as defined by an eGFR=60-89 mL/min per 1.73 m2, and chronic kidney disease as defined by an eGFR <60 mL/min per 1.73 m2. The group of patients with CKD includes patients in stage 3 CKD as defined by an eGFR=30-59 mL/min per 1.73 m2, stage 4 CKD as defined by an eGFR=15-29 mL/min per 1.73 m2, and stage 5 CKD as defined by an eGFR <15 mL/min per 1.73 m2. CABG: coronary artery bypass grafting; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; PCI: percutaneous coronary intervention
PATIENT CHARACTERISTICS
The risk profile of patients was comparable between PCI and CABG in all categories of patients (Supplementary Table 2). Patients with CKD (mean eGFR 47.6±10.8 mL/min/1.73 m2) had a markedly higher risk profile at baseline than patients with normal or impaired kidney function, reflecting an overall higher logistic EuroSCORE. There were no differences regarding baseline coronary disease complexity as determined by the SYNTAX score (Table 1, Supplementary Table 3). However, a subgroup of CKD patients with diabetes had a significantly higher SYNTAX score compared to non-diabetic CKD patients (32.2±12.1 vs. 28.2±11.7, p=0.008).
PROCEDURAL CHARACTERISTICS AND DISCHARGE MEDICATION
Off-pump CABG was performed more often in patients with CKD compared to patients with impaired or normal kidney function (22.1% vs. 13.1% vs. 15.8%, respectively; p=0.035) (Supplementary Table 4). The use of multiple arterial grafts was lower in CKD patients; in particular, bilateral internal mammary arteries (BIMA) were used less frequently (18.8% vs. 28.5% vs. 34.2%, respectively; p=0.007). The number of distal anastomoses and the completeness of revascularisation did not differ between groups.
In the PCI group, there were no differences among the groups in the procedural aspect regarding stent use (Supplementary Table 4). However, the rate of complete revascularisation was substantially lower in patients with CKD versus those with impaired kidney function or normal kidney function (46.2% vs. 60.5% vs. 56.5%, respectively; p=0.007), while a comparable number of patients underwent staged procedures (14.6% vs. 12.0% vs. 17.4%, respectively; p=0.16).
Acetylsalicylic acid was prescribed less often in patients with CKD compared to patients with impaired kidney function or normal kidney function after PCI (93.0% vs. 96.4% vs. 99.1%, respectively; p=0.006) and CABG (83.0% vs. 89.2% vs. 91.7%, respectively; p=0.043) (Supplementary Table 4). No differences in the administration of statins and beta-blockers were found in either treatment group. In general, secondary preventive medication was prescribed more often after PCI than after CABG (Supplementary Table 2).
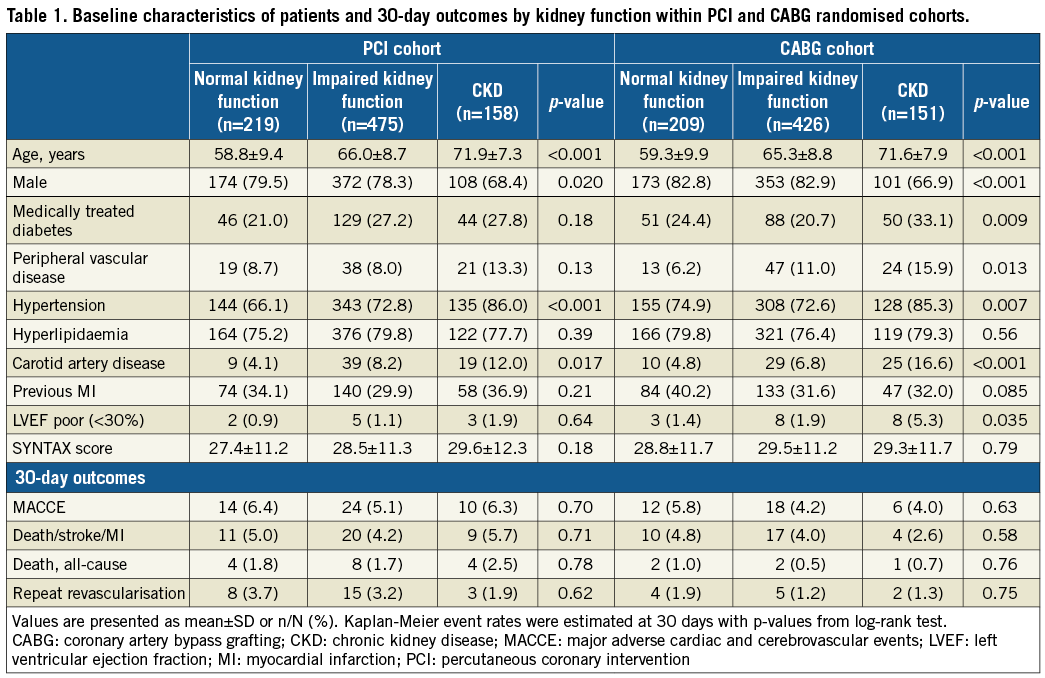
SHORT-TERM OUTCOMES
Within the PCI and CABG groups, incidences of 30-day adverse events were comparable in patients with CKD, impaired kidney function, and normal kidney function (Table 1). Comparing outcomes between PCI and CABG in patients with CKD, there were no significant differences in rates of MACCE (6.3% vs. 4.0%, respectively, p=0.34), the composite of death/stroke/MI (5.7% vs. 2.6%, respectively, p=0.18), all-cause death (2.5% vs. 0.7%, respectively, p=0.19), or repeat revascularisation (1.9% vs. 1.3%, respectively, p=0.68) (Supplementary Table 2).
FIVE-YEAR OUTCOMES
In separate groups of both PCI and CABG, rates of adverse events were not significantly different between patients with normal vs. impaired kidney function, while patients with CKD vs. normal kidney function had significantly higher rates of MACCE, the composite safety endpoint of death/stroke/MI, and all-cause death (Supplementary Table 5). Rates of repeat revascularisation were lower in patients with impaired kidney function and CKD.
Overall, differences between PCI and CABG in rates of adverse events were larger in patients with CKD than in other groups (Table 2, Supplementary Table 6, Figure 2). There was a significant treatment-by-kidney function interaction for all-cause death (pint=0.017), while interactions for MACCE (pint=0.15) or the composite endpoint of death/stroke/MI (pint=0.070) did not reach statistical significance.
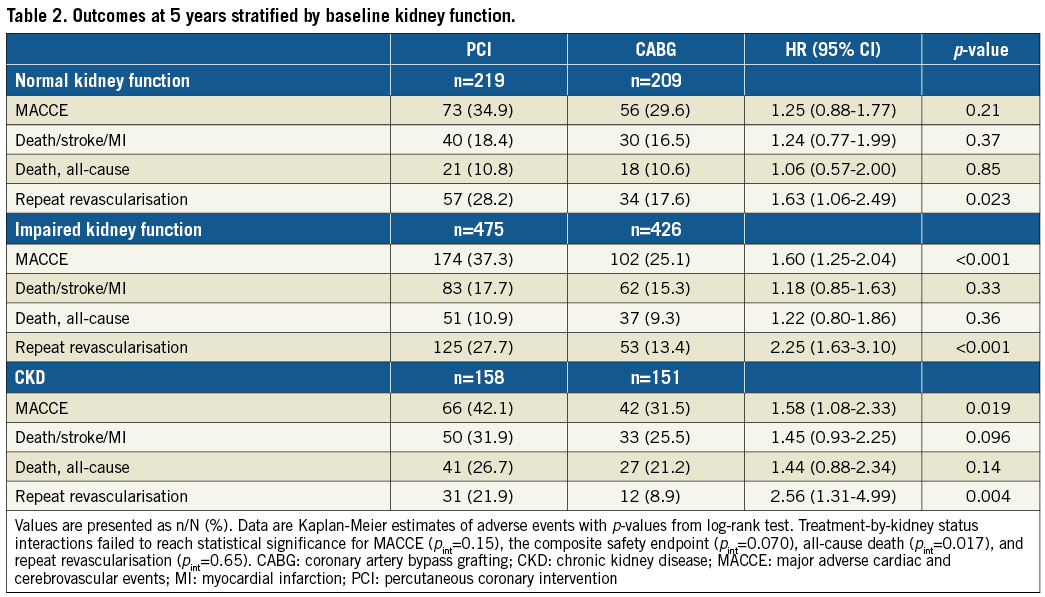
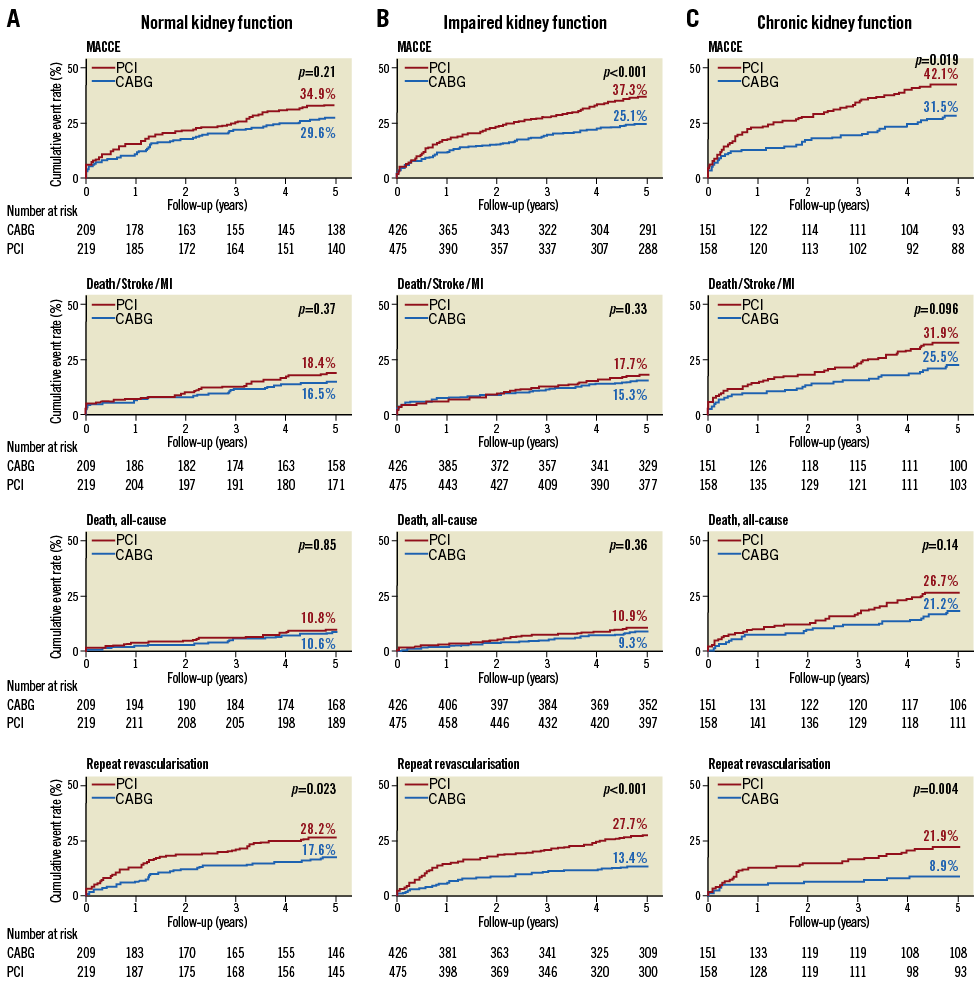
Figure 2. Kaplan-Meier cumulative event curves by the status of kidney function in the SYNTAX randomised cohort. p-values are from log-rank test. CABG: coronary artery bypass grafting; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; MI: myocardial infarction; PCI: percutaneous coronary intervention
In patients with CKD, the rate of MACCE was significantly higher after PCI than after CABG (42.1% vs. 31.5%, respectively; p=0.019) (Table 2, Figure 2). Rates of the composite endpoint of death/stroke/MI were 31.9% vs. 25.5%, respectively (p=0.096), and for all-cause death 26.7% vs. 21.2%, respectively (p=0.14).
SUBGROUP ANALYSIS
The rates of the composite of death/stroke/MI were significantly lower in patients with normal or impaired kidney function compared to CKD patients irrespective of the patient’s baseline characteristics (Supplementary Figure 1).
Overall, subgroup analyses among patients with CKD demonstrated a largely consistent benefit of CABG over PCI (Figure 3). However, significant interactions were found for gender, SYNTAX score and diabetes.
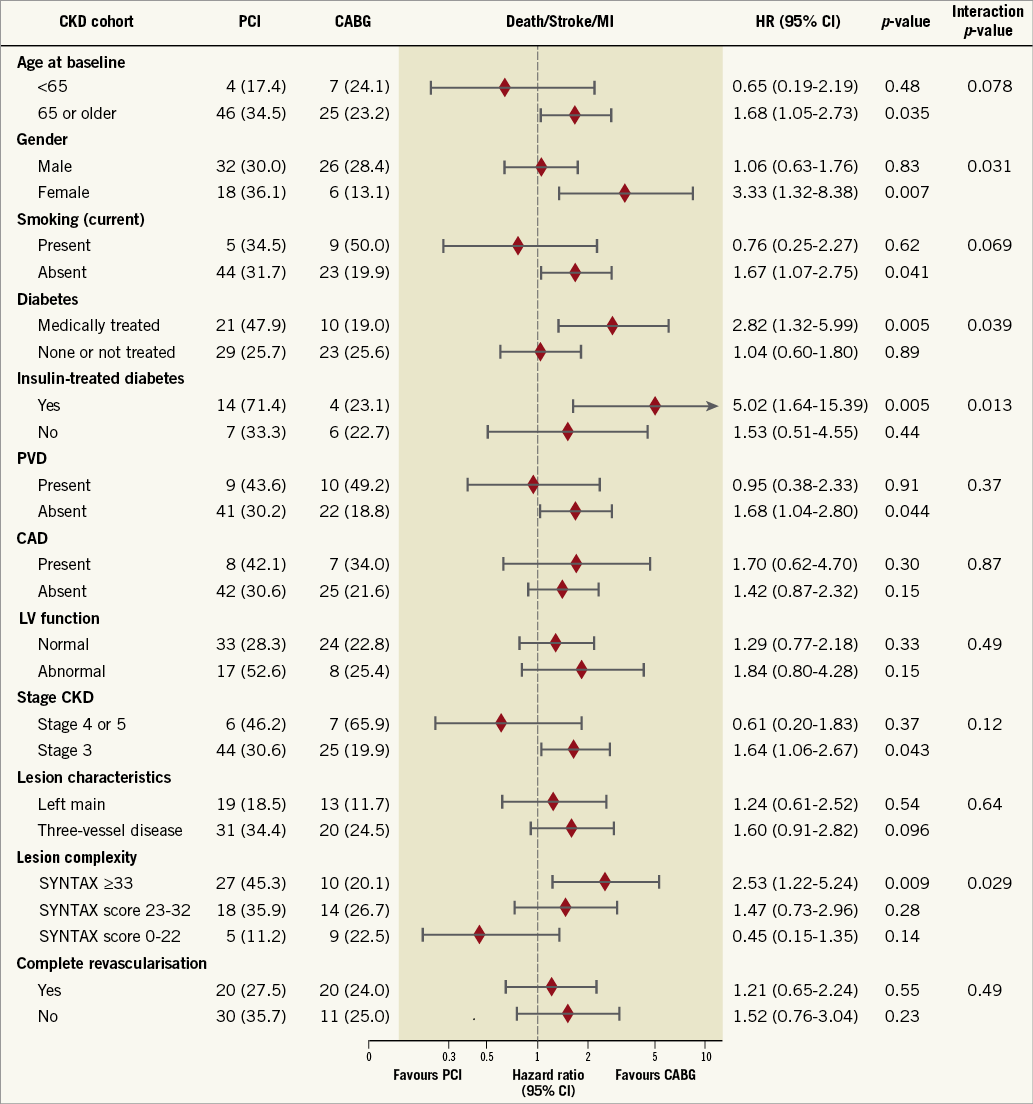
Figure 3. Hazard ratios of PCI versus CABG according to subgroups based on patient characteristics by the status of kidney function. Values are Kaplan-Meier event rates at five years with p-values from log-rank test. CABG: coronary artery bypass grafting; CAD: carotid artery disease; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; HR: hazard ratio; LV: left ventricular; MI: myocardial infarction; PCI: percutaneous coronary intervention; PVD: peripheral vascular disease
Subgroup analyses according to diabetic status show a persistent increase in the difference in adverse events between CABG and PCI with increasing kidney failure (from normal kidney function to CKD) (Supplementary Table 7). Among CKD groups, non-diabetic patients had comparable rates between PCI and CABG regarding the composite of death/stroke/MI (HR 1.04, 95% CI: 0.60-1.80; p=0.89) and all-cause death (HR 0.94, 95% CI: 0.51-1.71; p=0.83) (Figure 4). In contrast, diabetic patients assigned to PCI compared to CABG had significantly higher rates of the composite of death/stroke/MI (HR 2.82, 95% CI: 1.32-5.99; p=0.005 with pint=0.039) and all-cause death (HR 3.39, 95% CI: 1.41-8.13; p=0.004 with pint=0.018) (Figure 4).
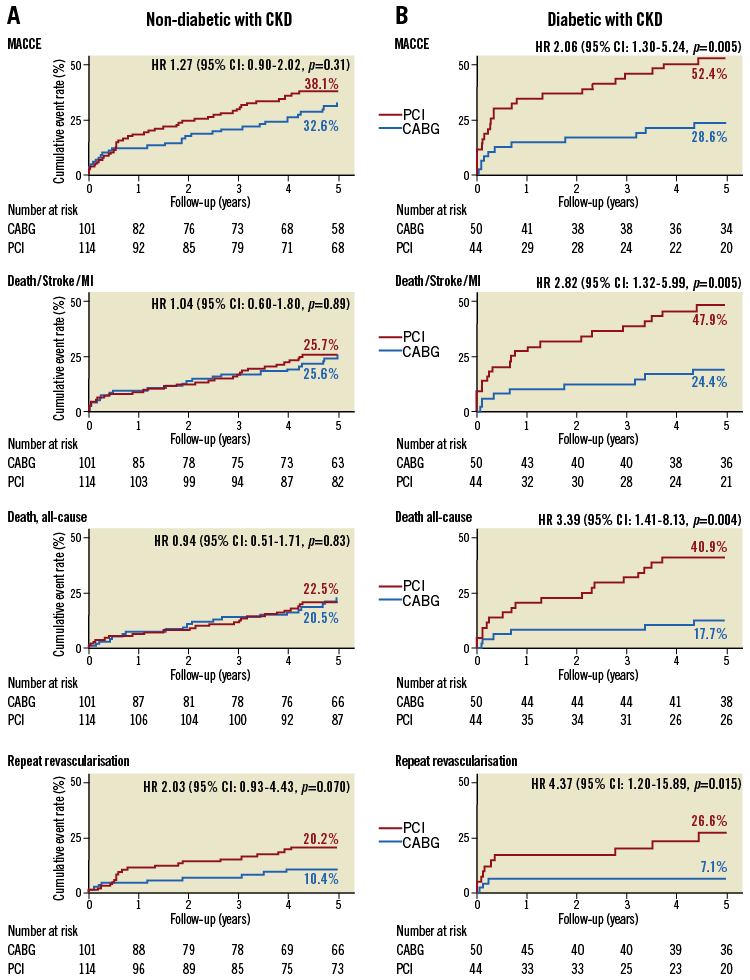
Figure 4. Kaplan-Meier cumulative event curves in patients with chronic kidney disease stratified by medically treated diabetes in the SYNTAX randomised cohort. Curves are separated for (A) non-diabetic patients and (B) medically treated diabetic patients. Treatment-by-diabetes interactions: MACCE (pint=0.10), the composite safety endpoint (pint=0.039), all-cause death (pint=0.018) and repeat revascularisation (pint=0.39). p-values are from log-rank test. CABG: coronary artery bypass grafting; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; HR: hazard ratio; MI: myocardial infarction; PCI: percutaneous coronary intervention
Discussion
This five-year analysis of the SYNTAX trial provides unique data on patients with and without CKD undergoing revascularisation. Important findings are that: i) the treatment plan for patients with versus without CKD differed significantly with CABG (e.g., less BIMA and more off-pump) and PCI (e.g., less complete revascularisation); ii) patients with CKD suffer from receiving less guideline-directed secondary prevention; iii) patients with CKD have significantly poorer outcomes after both PCI and CABG than patients with normal or impaired kidney function; and iv) differences in the five-year adverse event rates between PCI and CABG were minimal in patients with a normal kidney function but in favour of CABG in patients with CKD, particularly if diabetes was also present.
There is an increasing focus on patients with CKD because its presence in patients who require myocardial revascularisation is growing14. Data on the comparison between PCI with DES and CABG in this patient group are limited to observational studies15,16. In a propensity-matched analysis of 893 pairs of patients with a GFR <60 ml/min/1.73 m2, Chan and co-authors reported that three-year MACCE and survival were significantly lower after CABG than PCI with DES15. In contrast, in a larger propensity-matched analysis of 2,960 pairs, Bangalore and co-authors found CABG to be associated with short-term death, stroke and repeat revascularisation with only a benefit over PCI with DES regarding four-year rates of MI and repeat revascularisation but not death16. However, in these retrospective analyses, adjustment for selection bias is not always possible, and many confounding factors may influence the outcomes. Therefore, the results of the current randomised comparison add crucial information to the available body of evidence.
We found that the impact of kidney function on the long-term outcomes of PCI and CABG was significant. Patients with a decreased eGFR between 60 and 90 mL/min/1.73 m2 had similar rates of hard clinical endpoints of death/stroke/MI when compared to patients with normal kidney function after both CABG and PCI, irrespective of SYNTAX score group, but with a significantly increased risk of repeat revascularisation after PCI. However, patients with CKD had substantially higher rates of the composite of death/stroke/MI and particularly all-cause death than patients with normal or impaired kidney function. This calls for more dedicated attempts to improve outcomes in this select group. In this regard, CABG was performed more often off-pump to prevent cardiopulmonary bypass circuit-induced adverse effects on renal function. Despite strong evidence supporting its efficacy after CABG or PCI, the prescription of guideline-directed secondary prevention in patients with CKD was less than in other patients. A lack of evidence, the possibility of dosing errors, a higher incidence of major bleeding, and no clear guideline recommendations for patients with CKD lead to uncertainty among clinicians about the optimal post-treatment medication strategy. Nevertheless, recent publications show that statins significantly reduce cardiovascular events and might be associated with a slower progression of kidney damage17. Also, benefits of low-dose aspirin on long-term survival without an excess of major bleeding were also noted in patients with CKD18.
Kidney function was also found to have a significant impact on differences between PCI and CABG outcomes. Patients with a normal kidney function had similar adverse event rates with PCI vs. CABG except for repeat revascularisation that was higher with PCI. In patients with an impaired kidney function, CABG also failed to show a benefit regarding the composite of death/stroke/MI or all-cause death. However, in patients with CKD, there was a clear benefit of CABG over PCI. The difference in MACCE was driven by higher rates of repeat revascularisation, but also due to markedly higher rates of the composite of death/stroke/MI (absolute difference 6.4%) driven by all-cause death (absolute difference 5.5%). This improved survival might be related to the fact that patients with CKD have a higher risk of thrombotic events with PCI as a result of different complex haemostatic properties, severe atherosclerosis, and a lack of antiplatelet treatments.
Interestingly, several subgroups of patients with CKD were at particularly increased risk of adverse events after PCI. While the significant interaction for SYNTAX score and gender is consistent with the overall results of the SYNTAX trial, we found a positive interaction between diabetes and mortality risk of PCI relative to CABG that was not found in the overall SYNTAX trial result11. The survival advantage of CABG in this context might be due to the high baseline complexity of coronary disease and aggressive nature of the atherosclerotic disease in diabetic patients. In a subgroup analysis of the FREEDOM trial8, patients with an eGFR of 30-59 mL/min/1.73 m2 versus those with an eGFR ≥60 mL/min/1.73 m2 also had significantly higher rates of MACCE and particularly death, similar to the current analysis. However, the relative difference between PCI and CABG was consistent in patients with and without CKD, suggesting that the combination of CKD and diabetes does not increase the benefit of CABG over PCI, in contrast to the current analysis. Unfortunately, the FREEDOM trial could not distinguish between diabetic and non-diabetic patients with CKD as the study included only diabetic patients.
Limitations
Some limitations of the current analysis need to be acknowledged. Analyses according to CKD were not predefined in the trial protocol, and the use of TAXUS stents in clinical practice was superseded by second-generation DES, which have been shown to improve long-term outcomes significantly. Therefore, results should be interpreted as observational and hypothesis-generating. Second, of the CKD patients who were analysed, only 10% had severe and end-stage CKD. Thus, our findings should be restricted to the patients with stage 3 CKD (eGFR 30-60 mL/min/1.73 m²) before index revascularisation. Third, despite the primarily used CKD-EPI and KDIGO guidelines to define CKD populations that require “the presence of kidney damage at least over three months”, estimations of eGFR were only based on the creatinine level at hospital admission. Due to a lack of laboratory data during the periprocedural time, we were unable to determine contrast-induced nephropathy (CIN) and its impact on early and long-term outcomes.
Conclusions
Patients with CKD have an increased risk of adverse events, particularly mortality, after both PCI and CABG. Differences in five-year event rates between PCI and CABG are shown to have a significant interaction according to kidney function. The negative impact of CKD on long-term outcome following PCI appears to be stronger when compared to CABG, especially in the CKD patients with diabetes and extensive coronary disease. Patients with a normal or impaired kidney function may be candidates for PCI based on a similar risk for adverse events between PCI and CABG except for more repeat revascularisation. These results should provide a more substantiated evidence basis for clinical guideline recommendations for patients with CKD. Nevertheless, an adequately powered, dedicated, randomised trial is needed to provide the evidence for an optimal treatment strategy for patients with CKD who require myocardial revascularisation.
| Impact on daily practice The present study demonstrates a profound negative impact of baseline moderate kidney failure on five-year survival following both PCI and CABG. Importantly, patients are suboptimally treated, although the benefit of a more intense antithrombotic and lipid-lowering postoperative therapy on the outcome is established after surgical and interventional procedures. For treatment decision making, the Heart Team should take into consideration kidney failure, particularly in the presence of diabetes together with higher anatomic complexity as measured by the SYNTAX score. |
Guest Editors
This paper was guest edited by Philippe Kohl, MD, PhD; Department of Biomedical and Preclinical Sciences, University of Liège, Liège, Belgium and Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard and University Paris VII, Paris, France
Funding
This study was supported by Boston Scientific Corporation.
Conflict of interest statement
K.D. Dawkins owns stock in and is an employee of Boston Scientific. A.P. Kappetein is an employee of Medtronic. The other authors have no conflicts of interest to declare. The Guest Editor Philippe Kohl has no conflicts of interest to declare and the Guest Editor Alec Vahanian is a consultant for Edwards Lifesciences.
Supplementary data
Supplementary Table 1. Baseline characteristics and 5-year outcomes between the patients with and without baseline glomerular filtration rate estimates.
Supplementary Table 2. Baseline characteristics, 30-day outcomes, and discharge therapy comparison between PCI and CABG groups of patients defined by kidney function.
Supplementary Table 3. Baseline characteristics of patients by kidney function within PCI and CABG randomised cohorts.
Supplementary Table 4. Procedural characteristics, 30-day outcomes and discharge therapies of patients by kidney function within PCI and CABG randomised cohorts.
Supplementary Table 5. Outcomes at 5 years stratified by baseline kidney function in the PCI and CABG cohorts.
Supplementary Table 6. Outcomes at 5 years stratified by baseline kidney function.
Supplementary Table 7. Five-year clinical outcomes according to kidney function and diabetes status.
Supplementary Figure 1. Hazard ratios of PCI versus CABG according to subgroups based on patient characteristics by the status of kidney function.
To read the full content of this article, please download the PDF.

