
Abstract
Aims: Outcomes according to the status of renal insufficiency have not been fully evaluated in left main coronary artery disease (LMCAD). In the present study therefore, we sought to evaluate clinical outcomes in patients with significant LMCAD stratified by the degree of renal insufficiency and the relative clinical outcomes after PCI and CABG stratified by the differential levels of renal function using data from the large multinational “all-comers” Interventional Research Incorporation Society-Left MAIN Revascularization (IRIS-MAIN) registry.
Methods and results: Among 4,894 patients with LMCAD, renal insufficiency was graded according to the estimated glomerular filtration rate (eGFR). The primary outcome was major adverse cardiac and cerebrovascular events (MACCE), defined as death, myocardial infarction, stroke, or any revascularisation. The patients were stratified into three groups according to eGFR: 3,824 (78%) in group 1 (eGFR ≥60 ml·min–1·1.73 m2), 838 (17%) in group 2 (eGFR ≥30 and <60), and 232 (5%) in group 3 (eGFR <30). At two years, after adjustment, compared with group 1, the risk of MACCE was significantly higher in group 2 (hazard ratio [HR] 1.46, 95% confidence interval [CI]: 1.18-1.79) and in group 3 (HR 3.39, 95% CI: 2.61-4.40). The p interaction for MACCE across groups was 0.20. The adjusted risk of MACCE was similar between percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) in groups 1 and 2. However, PCI was associated with a significantly higher risk of MACCE compared to CABG (HR 1.88, 95% CI: 1.08-3.25) in group 3.
Conclusions: The degree of renal insufficiency was proportionately associated with unfavourable outcomes in patients with LMCAD. In group 3, PCI was associated with a higher risk of MACCE compared with CABG. Also, the effect of PCI versus CABG on MACCE was consistent, with PCI being associated with less bleeding and CABG being associated with less repeat revascularisation.
Introduction
Among several anatomical types of obstructive coronary artery disease (CAD), left main coronary artery disease (LMCAD) is associated with the worst clinical outcomes1. Coronary artery bypass graft (CABG) surgery has traditionally been the standard of care for revascularisation treatment of unprotected LMCAD. However, over the last two decades, percutaneous coronary intervention (PCI) has become an alternative strategy for selected patients with LMCAD2,3. Owing to a higher rate of major cardiovascular events and mortality in patients with significant LMCAD, identification of clinical factors associated with worse clinical outcomes and risk stratification is clinically important in the real world.
The relationship between chronic kidney disease (CKD) and an increased risk of cardiovascular events has been shown by many epidemiologic studies4,5. Furthermore, several studies have suggested that patients with CKD have poor outcomes after coronary revascularisation6,7. Previous studies have identified clinical risk factors associated with poorer outcomes in patients with LMCAD8,9,10. However, little is known about the effect of renal insufficiency on clinical outcomes in patients with LMCAD. In the present study therefore, we evaluated clinical outcomes in patients with significant LMCAD stratified by the degree of renal insufficiency and the relative clinical outcomes after PCI and CABG stratified by the differential levels of renal function using data from the large multinational “all-comers” Interventional Research Incorporation Society-Left MAIN Revascularization (IRIS-MAIN) registry.
Methods
STUDY POPULATION
The study population was part of the IRIS-MAIN registry (ClinicalTrials.gov Identifier: NCT01341327). The IRIS-MAIN registry is a non-randomised, multinational, observational registry which consists of a cohort of consecutive patients with significant unprotected LMCAD who were treated with PCI, CABG, or medication alone. Data were collected on patients who were diagnosed as having significant LMCAD (>50% by visual estimation) at approximately 65 centres in the Asia-Pacific region. From the registry, 5,566 consecutive patients from January 2003 to September 2017 were evaluated. Among them, 118 patients who had incomplete data, 145 patients who did not have the creatinine level, and 164 patients who did not have angiographic data were excluded. After further excluding patients who had cardiogenic shock, prior CABG, or valvular heart disease, 4,894 patients were included in the current analysis (Figure 1). The institutional review board at each hospital approved the use of clinical information in those patients for this study.
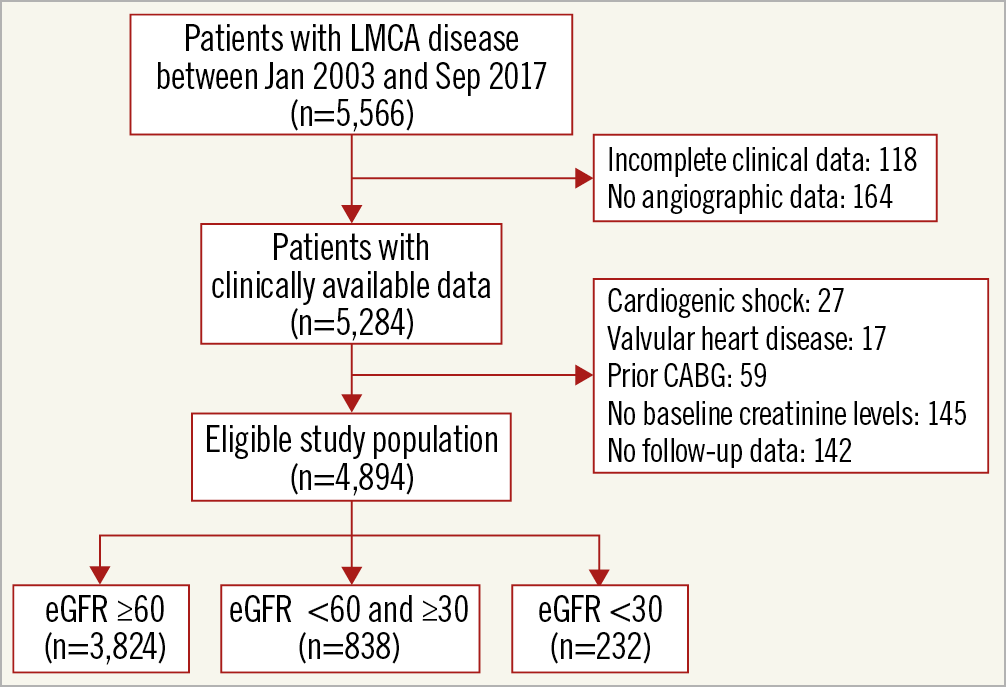
Figure 1. Study population.
Variables and outcome data were collected by specialised personnel using an electronic case report form at each centre. Monitoring and verification of registry data were periodically performed in participating hospitals by the staff of the coordinating centre (Clinical Research Center, Asan Medical Center, Seoul, South Korea). Follow-up was conducted during hospitalisation and at 1, 6, and 12 months after the index treatment and annually thereafter via an office visit or telephone contact.
OUTCOMES AND DEFINITIONS
The primary outcome was a major adverse cardiac and cerebrovascular event (MACCE), which was defined as a composite of death from any cause, myocardial infarction (MI), stroke, or any revascularisation. Death was considered as cardiac unless an unequivocal non-cardiac cause could be established. MI was defined as follows: if occurring within 48 hours following the index treatment, a combination of at least a fivefold increase in the CK-MB with either new pathological Q-waves or new bundle branch block, with either new graft or native coronary occlusion documented on angiography, new regional wall motion abnormality or loss of viable myocardium on imaging studies11,12. Stroke was defined as a loss of neurological function caused by an ischaemic or haemorrhagic event with residual symptoms at least 24 hours after the onset or leading to death and was confirmed by a neurologist on the basis of imaging modalities. Any revascularisation included any type of percutaneous or surgical revascularisation procedure, regardless of whether the procedure was performed on a target or non-target lesion. Thrombolysis In Myocardial Infarction (TIMI) major bleeding was defined as overt clinical bleeding associated with a drop in haemoglobin of greater than 5 g/dL or in haematocrit of greater than 15% (absolute). All events were based on the clinical diagnoses assigned by the patient’s physician and were centrally adjudicated by an independent group of clinicians.
STATISTICAL ANALYSIS
Continuous variables were expressed as median (interquartile range), and categorical variables were presented as numbers and percentages. Differences between the groups, categorised according to the estimated glomerular filtration rate (eGFR), were compared using analysis of variance (ANOVA) or the Kruskal-Wallis test for continuous variables, and the chi-square test or Fisher’s exact test for categorical variables as appropriate. Post hoc tests were performed using ANOVA with the Tukey method or the Kruskal-Wallis test with Bonferroni correction. Cumulative rates of clinical events were calculated using Kaplan-Meier survival analysis, and the log-rank test was used for comparisons across the groups.
A univariate Cox proportional hazards regression model was used to evaluate potential predictors of clinical outcomes. The proportional hazards assumption was checked for all screened covariates; no relevant violations were found. To assess the independent association of eGFR category to clinical outcome, multivariate Cox proportional hazards regression was performed using variables with a p-value of <0.10 in univariate analysis. Using the group of eGFR ≥60 ml/min/1.73 m² as the reference category, we estimated the hazard ratios and 95% confidence intervals for the groups of eGFR <60 and ≥30 and eGFR <30 ml/min/1.73 m². Finally, we compared the rates of primary outcome after PCI and CABG according to the eGFR at baseline. To adjust for the differences in baseline characteristics, multivariate Cox proportional hazards regression was performed using clinically relevant variables and statistically significant variables with a p-value <0.10 by univariate analysis. All reported p-values were two-sided and were not adjusted for multiple testing. All statistical analyses were performed using SPSS Statistics, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
BASELINE CHARACTERISTICS
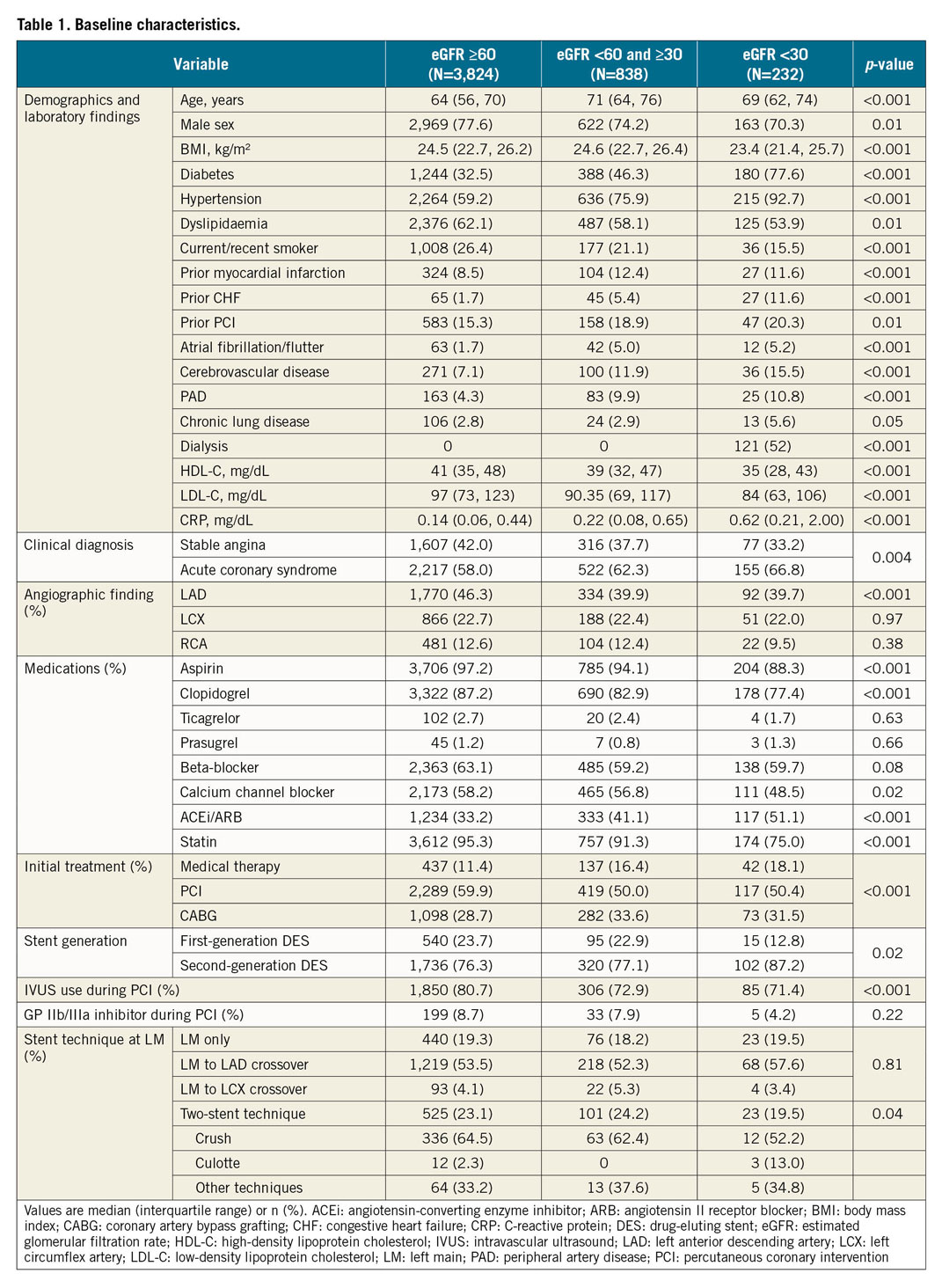
Patients were divided into three groups according to the eGFR at baseline: group 1 including patients with an eGFR ≥60 ml·min–1·1.73 m² (n=3,824, 78.1%), group 2 with an eGFR <60 and ≥30 (n=838, 17.1%), and group 3 with an eGFR <30 (n=232, 4.7%). One hundred and twenty-one (121) patients (52%) in group 3 were on dialysis. Baseline clinical characteristics were substantially different across the three groups (Table 1). Group 3 had higher risk factor profiles. With regard to treatment strategy, PCI was most frequently used in the three groups, whereas medical therapy alone was most frequently selected in group 3. Regarding the information related to PCI, group 3 tended to have a higher proportion of second-generation drug-eluting stents (DES). The use of intravascular ultrasound (IVUS) during PCI was less frequent in group 3. There was no significant difference in the stent technique at the left main lesion among the three groups on the whole, while bifurcation stenting was more prevalent in groups 1 and 2 compared to group 3. In terms of drug therapy, antiplatelet agents and statins were less frequently used in group 3 at baseline as well as during follow-up (Supplementary Table 1).
CLINICAL OUTCOMES
During the median follow-up duration of 1,289 (interquartile range, 729-1,913) days, there were 314 deaths, 39 MIs, 70 cerebrovascular events, and 205 any revascularisation. Overall, the cumulative incidence of MACCE at two years was lowest in group 1 (9.1%) and highest in group 3 (36.2%). This trend was consistent regardless of whether the patient received CABG, PCI or medical therapy (Figure 2). The incidences of the individual outcome of death, MI, or stroke were significantly higher in patients with a higher degree of renal insufficiency, whereas the rate of any revascularisation was comparable among the three groups (4.2% in group 1 vs 3.8% in group 2 vs 4.7% in group 3, p=0.79). The incidence of major bleeding events (8.5% in group 1 vs 10.3% in group 2 vs 12.5% in group 3, p=0.043) was also associated in proportion to the severity of renal insufficiency (Supplementary Table 2).
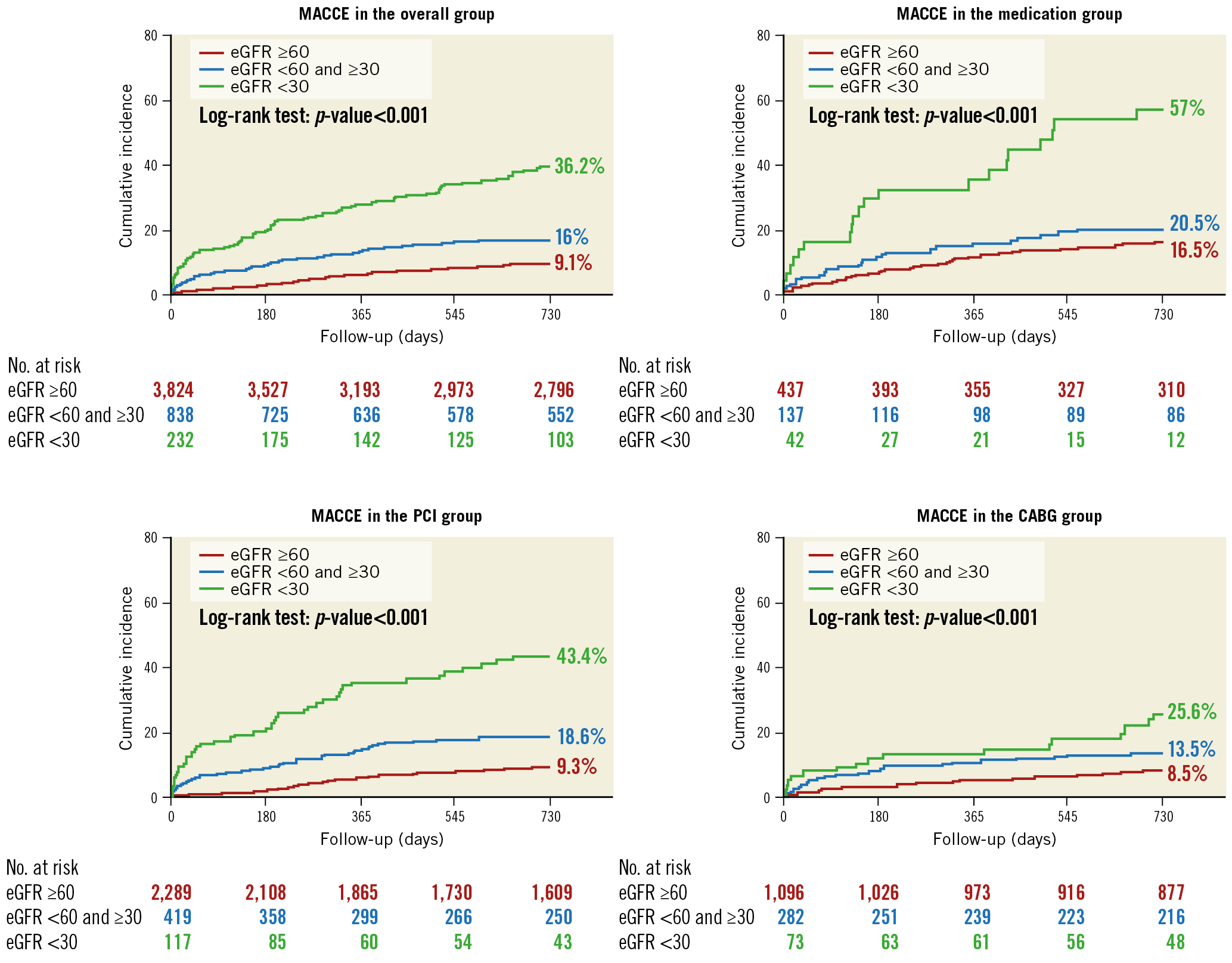
Figure 2. Kaplan-Meier curves of the primary composite outcome according to the levels of baseline renal function.
The landmark analysis revealed that the difference of MACCE according to the eGFR occurred mostly within one year. According to the 30-day landmark analysis, there was no significant difference in the rate of MACCE between groups 2 and 3. However, after one year, patients in group 3 consistently had the highest risk of MACCE, whereas there was no significant difference between groups 1 and 2 (Figure 3). After multivariate adjustment for the baseline differences among the three groups, the adjusted risk of MACCE was significantly higher in group 3 compared with group 1 or group 2 and was driven mainly by the higher risks of death and MI (Table 2).
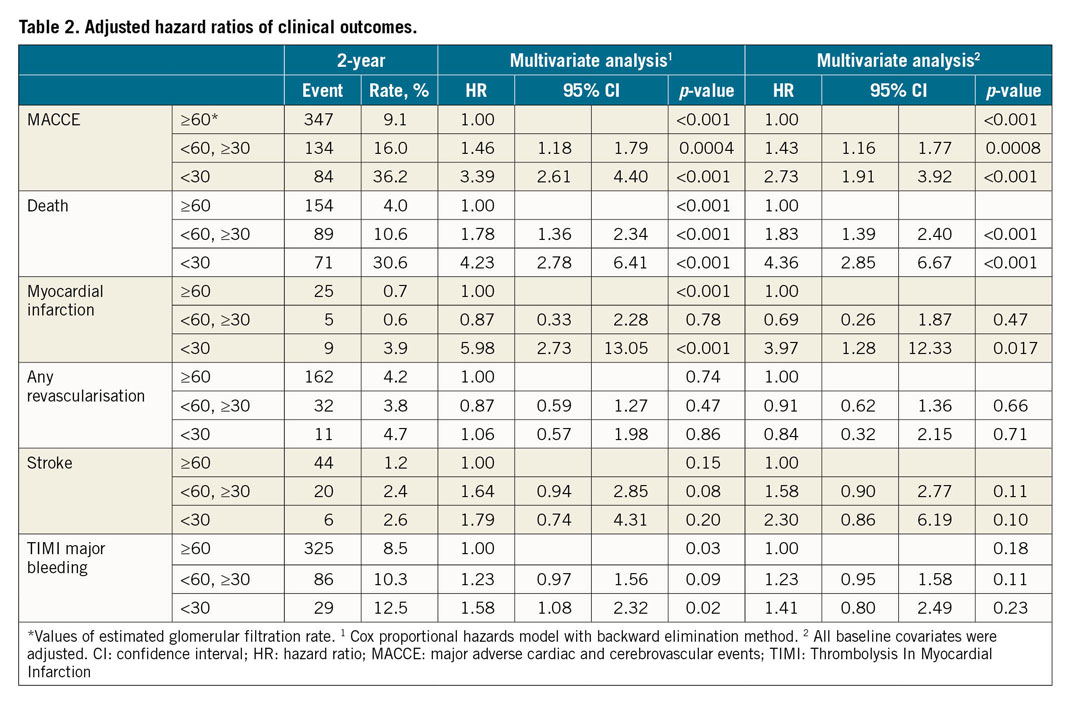
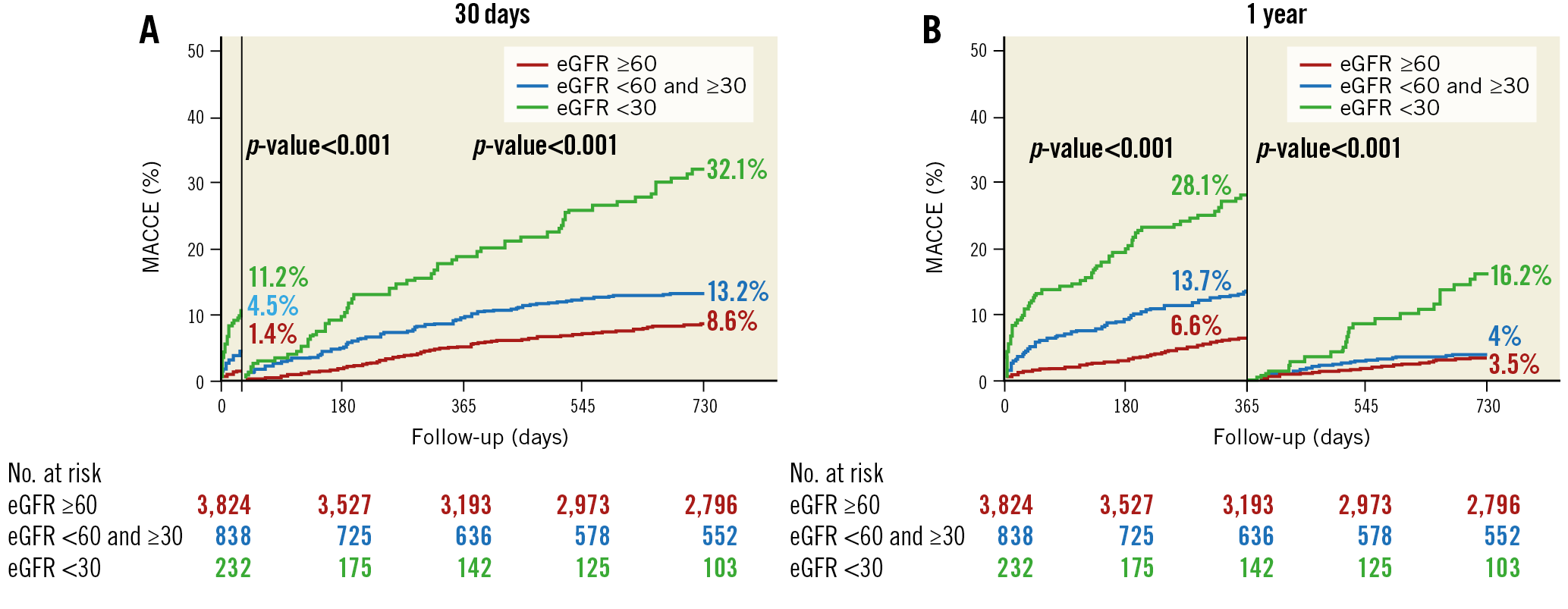
Figure 3. Kaplan-Meier curves with landmark analyses of the primary composite outcome according to the levels of baseline renal function. A) At 30 days. B) At 1 year.
PCI VERSUS CABG ACCORDING TO THE STATUS OF RENAL FUNCTION
The Kaplan-Meier two-year survival estimates for MACCE after PCI and CABG stratified by the status of baseline renal function are shown in Figure 4. The cumulative rates of MACCE did not differ between PCI and CABG among patients in group 1 or group 2. In contrast, there was a significantly higher rate of MACCE with PCI than with CABG in group 3 (38.5% vs 24.7% at two years, p=0.01, p for interaction=0.08). Clinical outcomes after adjusting for possible confounders using the Cox regression model are summarised in Table 3. The risk of MACCE was significantly higher with PCI than with CABG in group 3 (adjusted hazard ratio 1.88, 95% confidence interval [CI]: 1.08-3.25, p=0.02), whereas it was similar between PCI and CABG in patients in group 1 or group 2. Statistical interaction was not found between the status of renal function and revascularisation modality with regard to MACCE (p for interaction=0.20). The risk of any revascularisation tended to be higher with PCI, whereas the risk of TIMI major bleeding was higher with CABG regardless of eGFR level. The results of the sensitivity analysis excluding patients who received first-generation DES were largely consistent (Supplementary Table 3).
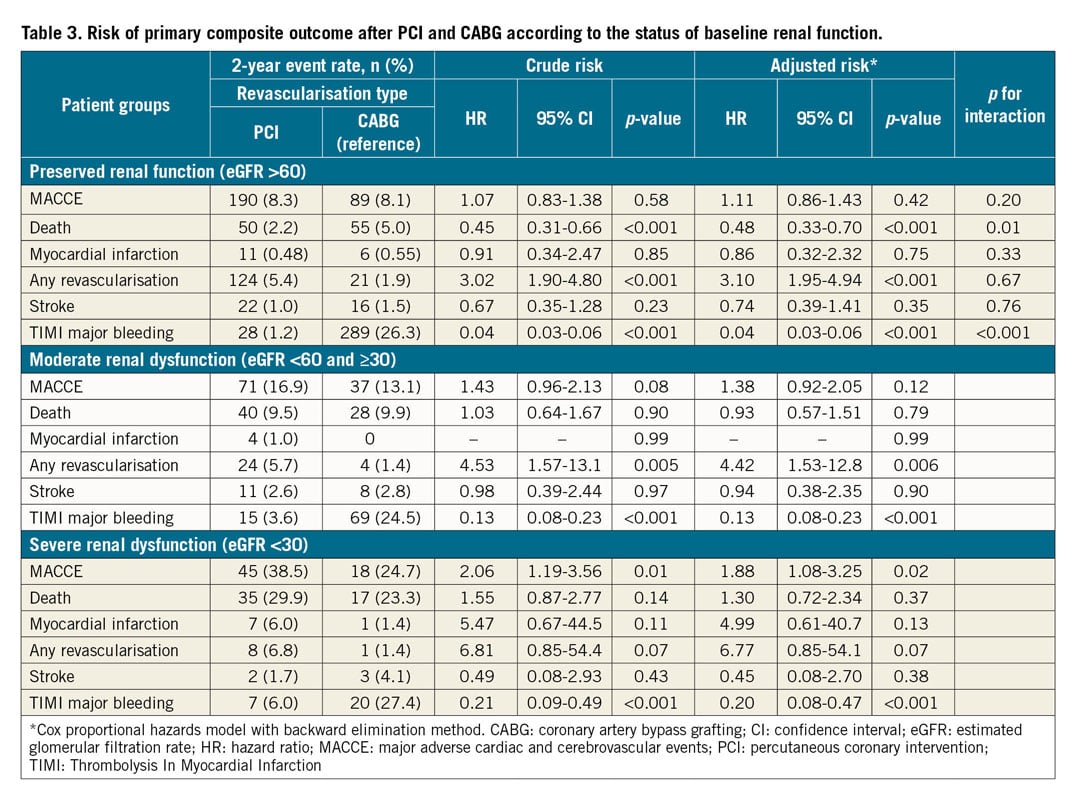
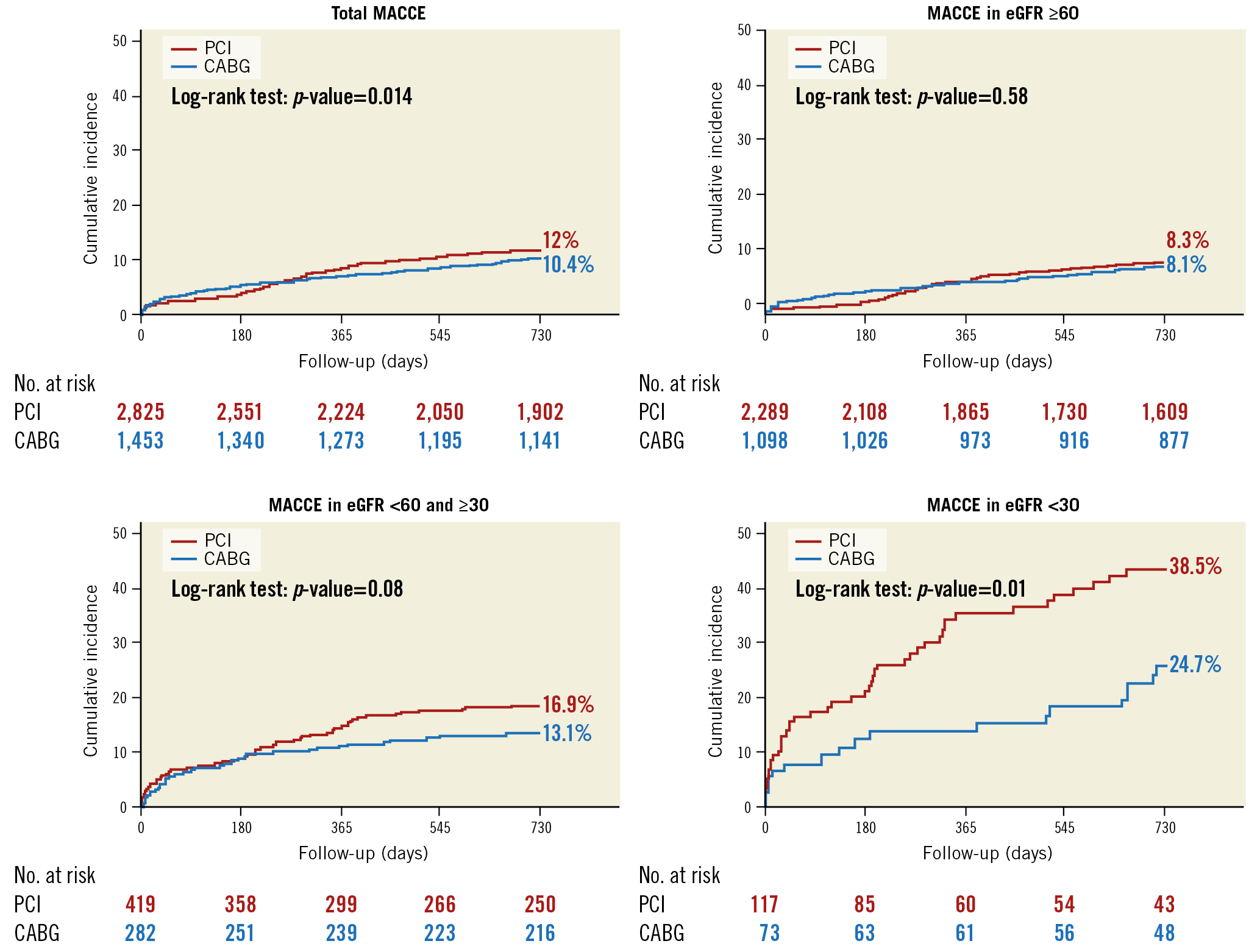
Figure 4. Kaplan-Meier curves of the primary composite outcome between PCI and CABG according to the levels of baseline renal function.
Discussion
From this large, all-comers registry involving patients with LMCAD, we found that the severity of renal insufficiency was proportionately associated with an increased risk of serious adverse events, regardless of the initial treatment strategy. Among patients with preserved or moderate renal dysfunction, the risk of MACCE after PCI and CABG was comparable, whereas the MACCE risk was significantly higher with PCI than with CABG in patients with severe renal dysfunction. Although a statistically significant interaction was not observed, further studies are required to confirm this observation and to help guide decision making between CABG and PCI in LMCAD patients with CKD.
Although some studies have suggested a lesser association between renal function and clinical outcomes after PCI in patients with obstructive CAD13,14, the majority of studies have shown that patients with renal insufficiency were significantly associated with unfavourable outcomes7,15,16. However, patients with LMCAD were mostly excluded in prior studies, thus data on the clinical relevance of renal impairment in patients with such complex lesions were still lacking. In our study involving this high-risk group of patients, we found that renal insufficiency had a detrimental effect on outcomes including death and MACCE which was proportional to the levels of eGFR. Of note, patients with severe renal insufficiency showed higher cumulative event rates sustained beyond one year in the landmark analysis. The association between the severity of renal dysfunction and ischaemic cardiovascular events shown in our study is not surprising given the well-known biopathological features of renal dysfunction such as negative plaque characteristics, heightened states of arterial inflammation, or sympathetic nervous system activation17,18,19,20. However, our study adds more of a real-world explanation of this observation. Patients with a lower eGFR received suboptimal medical therapies of antiplatelet agents and statins, possibly because of concerns about pharmacokinetic issues of the drugs related to renal excretion and increased bleeding tendency. This treatment pattern seems to be in line with the preferential selection of medical therapy alone rather than PCI or CABG in LMCAD patients with severe renal insufficiency. Furthermore, less frequent use of IVUS-supported PCI in patients with a lower eGFR may imply a more complicated or suboptimal procedure which may be related to a worse prognosis.
A comparison between PCI and CABG in patients with LMCAD and CKD has recently been reported in the subgroup analysis of the randomised EXCEL trial21. There were no significant differences between PCI and CABG in terms of death, stroke, or MI at three years after the procedures in patients with and without CKD. However, the results should be interpreted with caution as the number of CKD patients was relatively small (n=361) and the majority of the CKD patients had a moderate degree of renal impairment. Additional in our study was the inclusion of a larger number of real-world patients and the demonstration of the comparative effectiveness between PCI and CABG in LMCAD patients with severe renal dysfunction, who have usually been excluded from randomised trials. This higher-risk subgroup seemed to benefit more after CABG than after PCI in terms of serious ischaemic adverse events. A plausible explanation would be that patients with advanced renal impairment may have severe coronary artery characteristics including a higher degree of calcification and atherosclerotic plaque burden, and consequently may distinctly benefit from bypass grafts which provide a more durable and protective role against future ischaemic events. Because the presence of poor renal function is frequently encountered in daily clinical practice during Heart Team discussions concerning whether to opt for PCI or CABG, subsequent studies will be critical for the development of optimal treatment strategies according to the degree of CKD for high-risk patients with LMCAD.
Limitations
This study has several limitations. First, there were different risk profiles, comorbidities, and anatomical disease extent or complexity in each CKD group as well as the PCI versus CABG groups (Supplementary Table 4-Supplementary Table 7). Although confounding covariates were adjusted in the multivariable models, the results are vulnerable to unmeasured confounders. Second, variables that are known in clinical practice to have a profound influence on the choice of revascularisation (e.g., SYNTAX score or patient frailty) were not available for this analysis. A lack of such information could have penalised the CABG group relative to the PCI group. Third, the number of patients included in group 3 was relatively small. Although the different outcome after PCI and CABG in these patients was one major finding of our study, interpretation of the results should be cautious, and the findings should be considered hypothesis-generating only. Fourth, the impact of incomplete revascularisation on outcome between PCI and CABG could not be assessed as the registry does not capture this variable for CABG. Finally, relevant information regarding the renal outcomes such as acute renal failure or new requirement of dialysis was not available in our study.
Conclusions
The presence and severity of renal dysfunction were associated with an increased risk of serious adverse events in real-world patients with LMCAD. Among LMCAD patients with severe renal dysfunction, CABG was associated with a lower risk of MACCE as compared with PCI. Also, the effect of PCI versus CABG on MACCE was consistent, with PCI being associated with less bleeding and CABG being associated with less repeat revascularisation. Further studies are required to confirm the differential effect of PCI and CABG by degree of renal function, which may help to guide decision making in patients with LMCAD.
|
Impact on daily practice This analysis of the IRIS-MAIN registry showed the clinical implications of renal insufficiency in LMCAD patients. Patients with decreasing levels of renal function had higher risk profiles of baseline clinical, anatomical, and procedural characteristics and also had unfavourable clinical outcomes. According to the eGFR levels, CABG showed favourable results in patients with advanced renal insufficiency compared with PCI in LMCAD patients, while PCI and CABG showed no significant difference in patients with less severe renal insufficiency. |
Funding
This work was supported by a grant from the CardioVascular Research Foundation, Seoul, South Korea (2015-09).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

