Abstract
Background: The EXCEL trial reported similar five-year rates of the primary composite outcome of death, myocardial infarction (MI), or stroke after percutaneous coronary intervention (PCI) compared with coronary artery bypass grafting (CABG) for treatment of obstructive left main coronary artery disease (LMCAD).
Aims: We sought to determine whether these outcomes remained consistent regardless of geography of enrolment.
Methods: We performed a prespecified subgroup analysis based on regional enrolment.
Results: Among 1,905 patients randomised to PCI (n=948) or CABG (n=957), 1,075 (56.4%) were recruited at 52 European Union (EU) centres, and 752 (39.5%) were recruited at 67 North American (NA) centres. EU versus NA patients varied according to numerous baseline demographics, anatomy, pharmacotherapy and procedural characteristics. Nonetheless, the relative rates of the primary endpoint after PCI versus CABG were consistent across EU versus NA centres at 30 days and 5 years. However, NA participants had substantially higher late rates of ischaemia-driven revascularisation (IDR) after PCI, driven predominantly by the need for greater target vessel and lesion revascularisation. This culminated in a significant difference in the relative risk of the secondary composite outcome of death, MI, stroke, or IDR at 5 years (pinteraction=0.02).
Conclusions: In the EXCEL trial, the relative risks for the 30-day and five-year primary composite outcome of death, MI or stroke after PCI versus CABG were consistent irrespective of geography. However, five-year rates of IDR after PCI were significantly higher in NA centres, a finding the Heart Team and patients should consider when making treatment decisions. ClinicalTrials.gov identifier: NCT01205776
Introduction
Percutaneous coronary intervention (PCI) is now established as the most commonly used technique for coronary revascularisation, while coronary artery bypass grafting (CABG) is still considered the gold standard for complex coronary artery disease (CAD). Coronary intervention with drug-eluting stents (DES), however, is increasingly utilised in higher-risk patients, including those with obstructive left main (LM) CAD. Furthermore, in the LMCAD subgroup of patients in the SYNTAX trial with low and moderate anatomic complexity (SYNTAX score ≤32), PCI with first-generation DES was comparable to CABG at five-year follow-up12. This hypothesis-generating subgroup analysis from SYNTAX was the catalyst for the Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial, in which select patients with LMCAD had fewer adverse events within 30 days and similar three-year and five-year rates of the primary composite outcome of all-cause mortality, myocardial infarction (MI), or stroke at three years after PCI with everolimus-eluting stents (EES) and CABG34.
The relative outcomes after PCI or CABG, however, may vary according to patient demographics, operator expertise and technique, and adjunctive pharmacotherapies used with respect to the different locations enrolling in EXCEL, a large, international trial that recruited individuals from 17 countries. We thus performed a prespecified substudy of the EXCEL trial by analysing the relative differences in the short- and long-term outcomes from the EXCEL trial at European Union (EU) and North American (NA) recruitment centres. Our aim was to determine whether outcomes remained consistent despite known geographic variations in patient characteristics and clinical practice.
Methods
The present report describes a prespecified subgroup analysis according to geographic region of enrolment in the EXCEL trial. Outcomes were assessed among patients enrolled in EU versus NA centres. As per the original trial protocol, the current subgroup analysis would be considered exploratory and hypothesis-generating as the trial was underpowered for comparative assessment of PCI versus CABG in any subgroup. There was no predefined time point for the current investigation, so all data to five-year follow-up have been analysed.
STUDY DESIGN
The EXCEL trial design and rationale along with the principal three- and five-year results have been reported previously345. In brief, EXCEL was a prospective, international, open-label, multicentre randomised trial that compared the safety and effectiveness of PCI using the XIENCE cobalt-chromium EES (CoCr-EES; Abbott Vascular) platform versus CABG for obstructive LMCAD. Patients were enrolled from 126 centres in the EU, USA, Canada, South America, Asia, and Australia.
Inclusion criteria were unprotected LMCAD with a visually estimated angiographic diameter stenosis ≥70%, or ≥50% but <70% if deemed haemodynamically significant by invasive or non-invasive testing; site-assessed SYNTAX score ≤32; and clinical equipoise for revascularisation by PCI or CABG as per a local Heart Team consensus. Complete details of the inclusion and exclusion criteria have been reported35. The trial was conducted according to the Declaration of Helsinki and Good Clinical Practice and was approved by the investigational review board or research ethics committee at each participating centre. All participants gave their informed consent.
Randomisation was stratified according to recruiting site, the presence or absence of medically treated diabetes, and site-assessed SYNTAX score. Procedural recommendations were per standard of care35. Intravascular ultrasound (IVUS) to guide PCI was strongly recommended. For CABG, arterial bypass conduits and epi-aortic and transoesophageal echocardiography were recommended. Daily aspirin was mandatory in all patients. An adenosine diphosphate (ADP) platelet receptor antagonist was initiated prior to PCI and was continued for at least one year. Its use was optional after CABG according to local practice. Clinical follow-up was performed at 1 month, 6 months, 1 year, and annually up to five years.
ENDPOINTS
The primary endpoint was the composite of all-cause mortality, MI, or stroke at three years. Major powered secondary endpoints included the composite endpoint at 30 days, and the composite of death, MI, stroke, or ischaemia-driven revascularisation (IDR) at 3 years. All patients were followed up to 5 years. The definitions of all endpoints have been reported previously35. An independent committee adjudicated primary and secondary events after review of original source documents. An independent angiographic core laboratory assessed the extent of CAD at baseline and residual SYNTAX scores5.
STATISTICAL ANALYSIS
Discrete variables are expressed as percentages with frequencies and were compared by the χ2 or Fisher’s exact tests. Continuous variables are reported as mean±standard deviation and were compared by t-test if normally distributed or the Wilcoxon rank-sum test for a non-parametric distribution. Event rates were based on Kaplan-Meier estimates in time-to-first-event analyses. Hazard ratios (HR) with 95% confidence intervals (CI) were determined by Cox regression analysis, and event rates were compared with the log-rank test. Interaction testing was performed to determine whether the relative risk of the major outcome measures at 30 days, 3 years and 5 years after PCI versus CABG varied by geographic location. All analyses were performed in the intention-to-treat population. A two-sided p-value <0.05 was considered significant. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Between September 2010 and March 2014, 1,905 patients with LMCAD were randomised to PCI (n=948) versus CABG (n=957). Of those, 1,075 (56.4%) patients were recruited at 52 EU centres and 752 (39.5%) were recruited at 67 NA centres (including 549 from the USA and 203 from Canada). The remaining 78 (4.1%) participants were recruited from seven sites in Argentina, Brazil, South Korea, and Australia.
BASELINE PATIENT CHARACTERISTICS, PROCEDURES, AND MEDICATIONS
As shown in Table 1, there were significantly more women recruited in the NA cohort. The NA cohort also had a higher body mass index and more cases of hypertension, diabetes, history of anaemia, and prior PCI compared to those in the EU; however, the EU cohort recruited individuals with more complex coronary anatomy (higher SYNTAX scores and more distal LM bifurcation involvement). The baseline demographic and angiographic characteristics in patients randomised to PCI versus CABG within each region were generally well balanced (Supplementary Table 1).
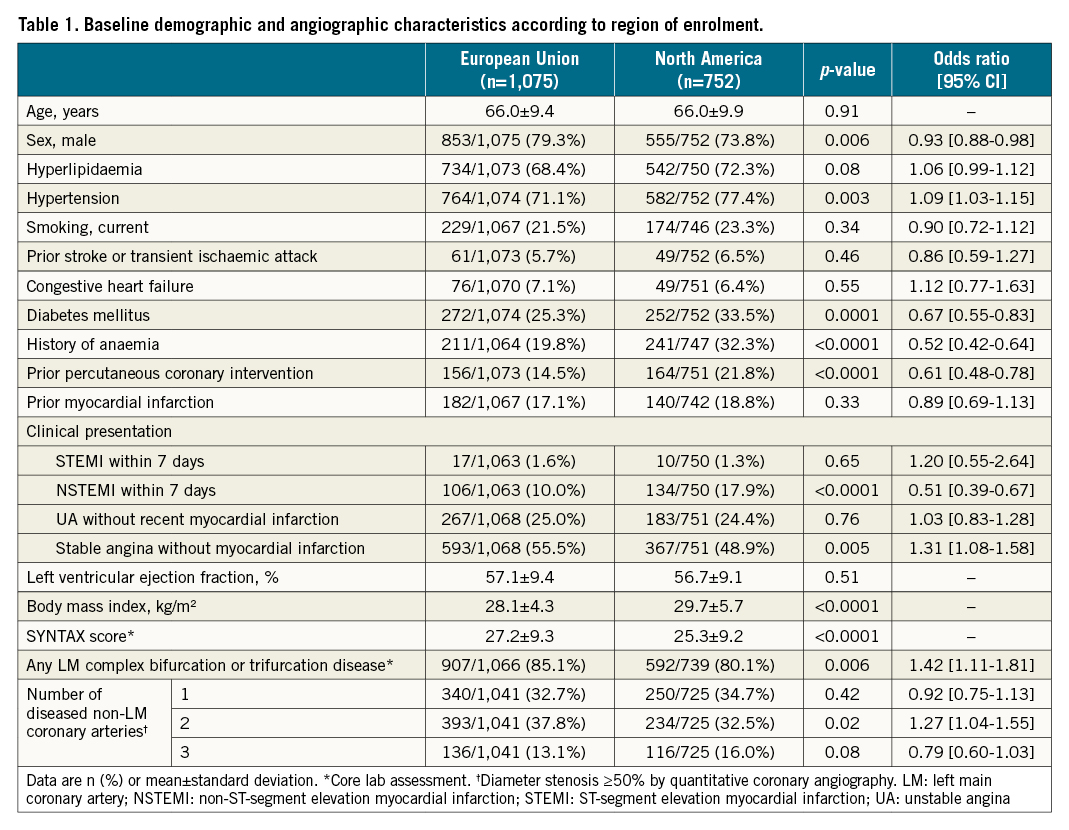
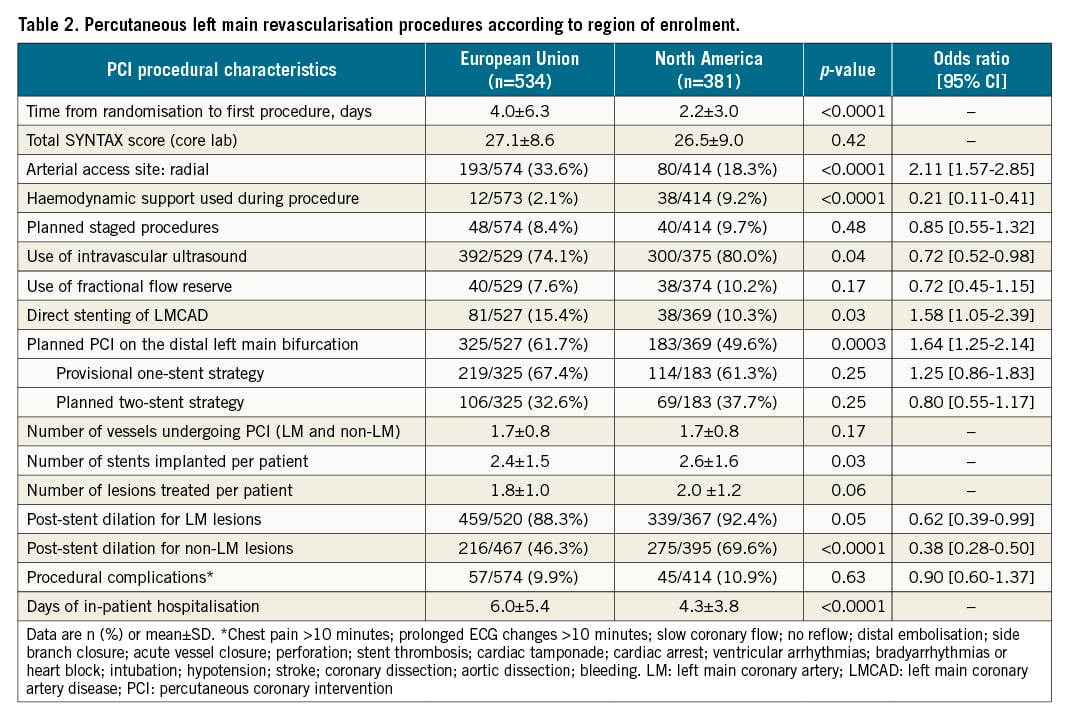
PCI procedures in the EU compared with NA had a longer delay from randomisation to intervention, were more likely to use radial access but less likely to employ haemodynamic support, had fewer treated lesions with fewer stents used despite a similar SYNTAX score, were less likely to have post-dilatation performed, and had a longer total hospital stay (Table 2). CABG procedures in the EU compared with NA also had a greater time interval between randomisation and surgery, were more likely to be off-pump and use all arterial grafts (although fewer vessels were bypassed despite a higher SYNTAX score), were less likely to employ ultrasound screening, and were shorter in duration but had a longer total hospital stay (Table 3).
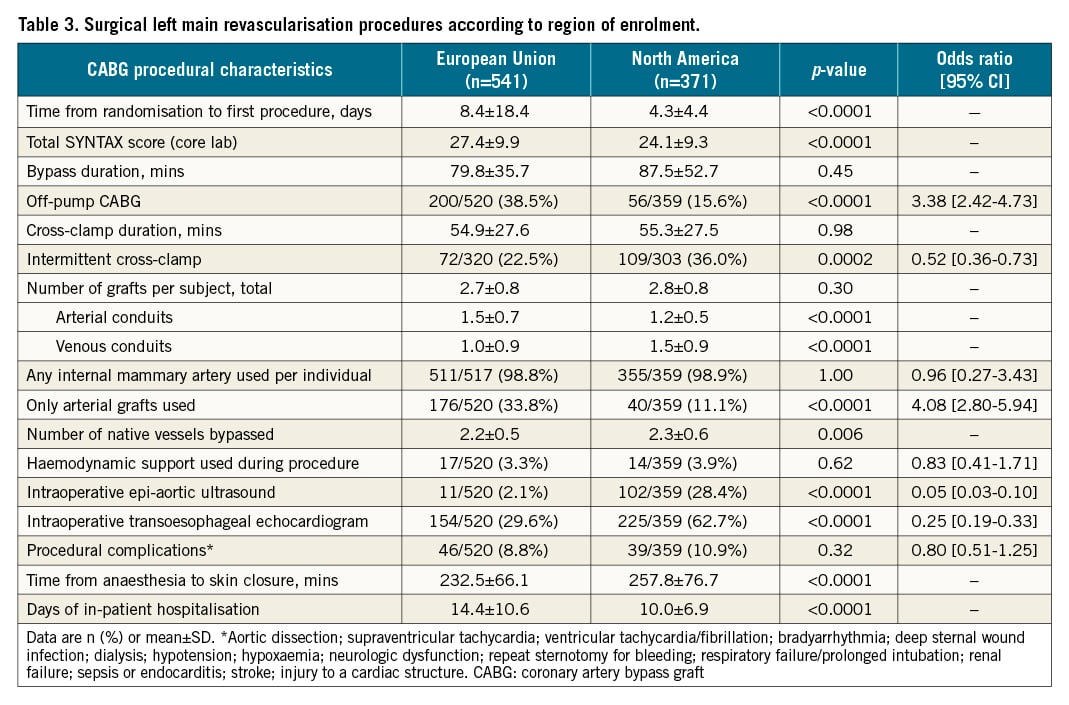
There were numerous regional differences in medication use (Supplementary Table 2, Supplementary Table 3). In general, EU sites were less likely to use more potent P2Y12 platelet inhibitors and bivalirudin in PCI patients, were less likely to treat post-CABG patients with dual antiplatelet therapy at hospital discharge, used lower doses of aspirin, and were more likely to use antagonists of the renin-angiotensin system.
OUTCOMES
The 30-day composite secondary endpoint of death, protocol-defined MI, or stroke and its individual components did not vary substantially overall with respect to region of enrolment (Supplementary Table 4), although all-cause mortality was greater in the EU. At 3 and 5 years there were similar death rates by region, but IDR was more common among patients enrolled in NA compared with EU sites, which culminated in a significant difference in the combined endpoint of death, MI, stroke, or IDR at 5 years (Supplementary Table 4).
Thirty-day, 3-year, and 5-year outcomes according to PCI versus CABG by region are shown in Table 4. The 30-day composite of death, MI, or stroke occurred less frequently after PCI compared with CABG, driven by fewer MI events. This reduction was consistent in EU and NA centres (pinteraction=0.09).
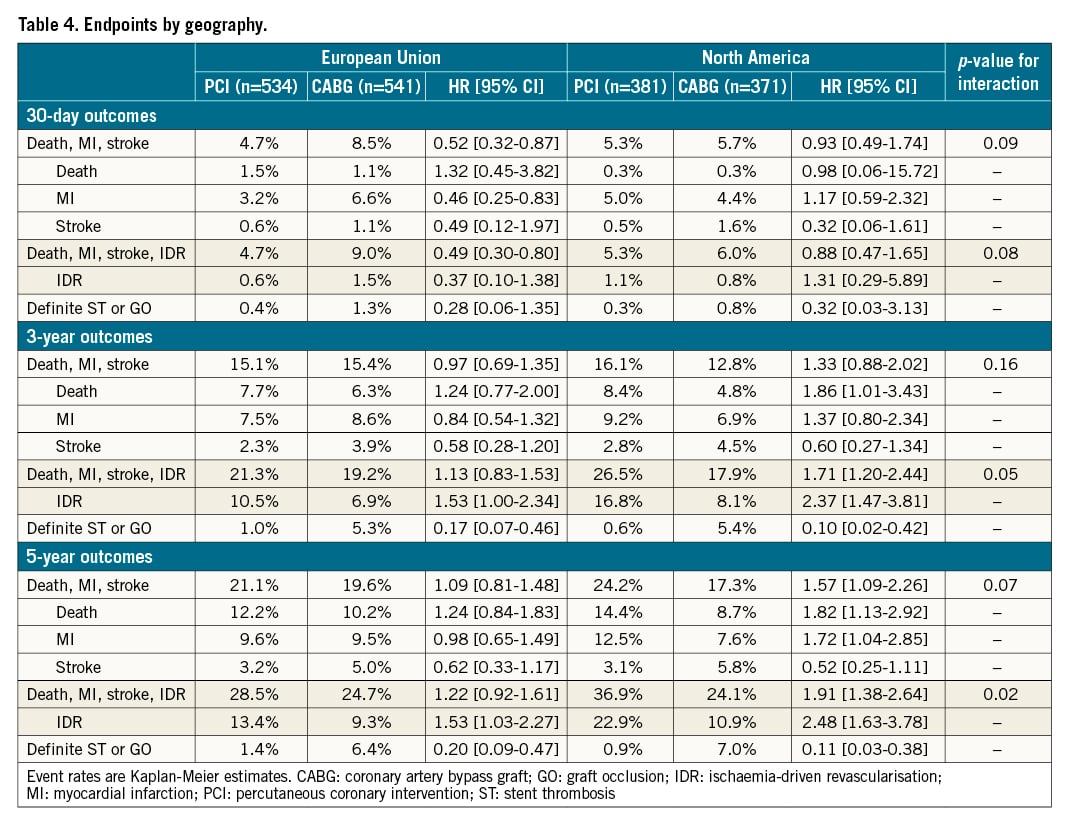
At 3 years, there were no significant differences in the primary composite endpoint of death, MI, or stroke between PCI and CABG according to geographic recruitment region. The relative 3-year rates of death, MI, stroke, or IDR, however, continued to diverge between the treatment groups in the NA cohort, driven by significantly higher IDR rates after PCI. A test for interaction demonstrated a borderline significant difference for the effect of PCI versus CABG on the 3-year composite of death, MI, stroke, or IDR between the EU and NA (pinteraction=0.05) (Table 4).
At 5-year follow-up the relative rates of the composite of death, MI, or stroke after PCI and CABG were not different between enrolment regions. However, the greater rate of IDR after PCI compared with CABG in NA centres continued to increase more so among patients enrolled in NA compared with EU sites. This culminated in a significant difference in the risk of the 5-year composite of death, MI, stroke, or IDR after PCI versus CABG between EU and NA centres (pinteraction=0.02) (Central illustration). The higher rates of IDR after PCI noted at both 3 and 5 years in both the EU and NA cohorts were predominantly driven by increased target vessel revascularisations, the majority of which were target lesion revascularisations. Kaplan-Meier analyses of both the primary composite endpoint of death, MI and stroke and major secondary endpoint of death, MI, stroke, and IDR, assessed by region and by revascularisation modality, are shown in Figure 1A-Figure 1D and Figure 2A-Figure 2D, respectively.
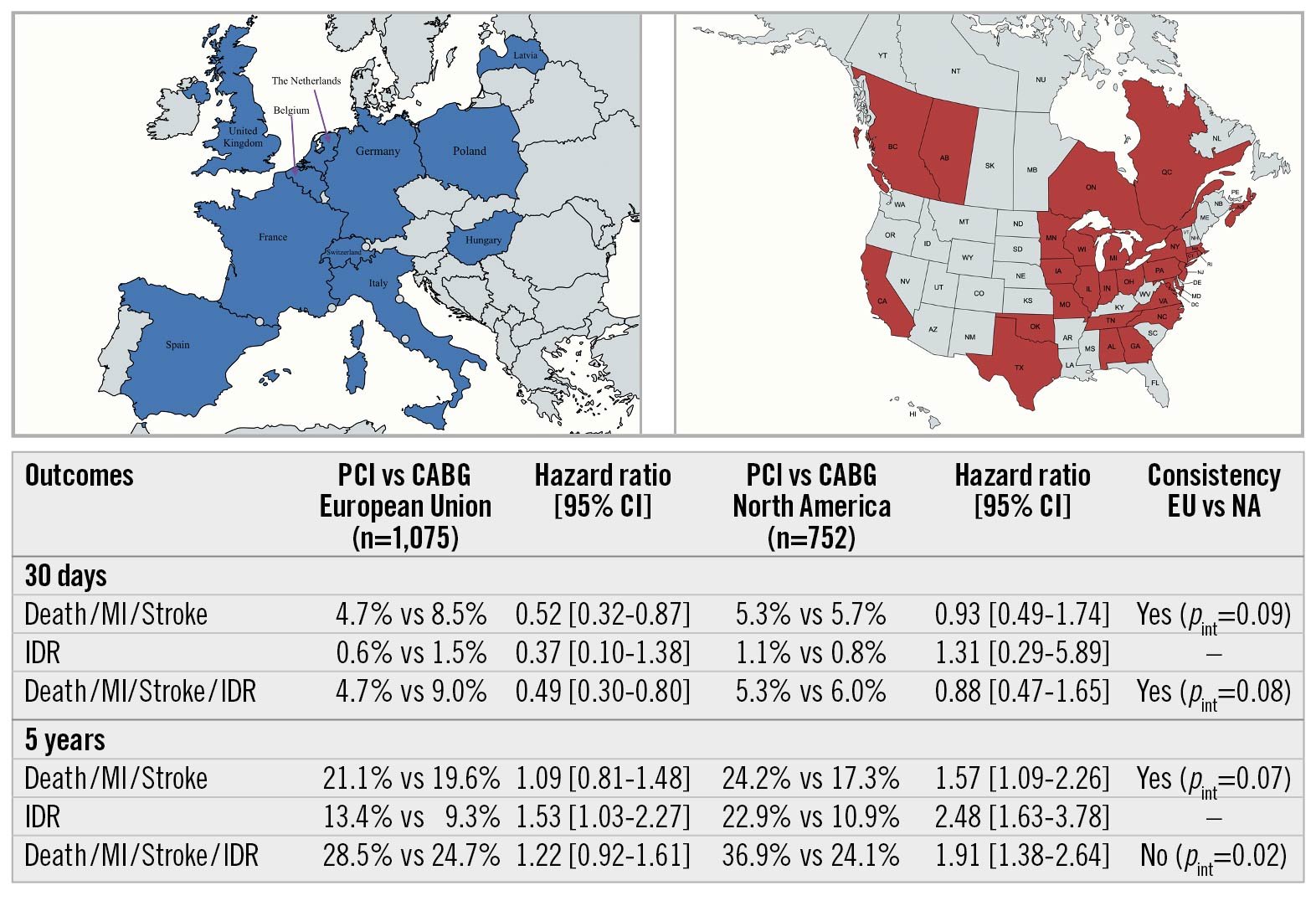
Central illustration. Geographical variations in left main coronary artery revascularisation outcomes from the EXCEL trial. CABG: coronary artery bypass graft; CI: confidence interval; EU: European Union; IDR: ischaemia-driven revascularisation; MI: myocardial infarction; NA: North America; PCI: percutaneous coronary intervention; pint: p-value for interaction
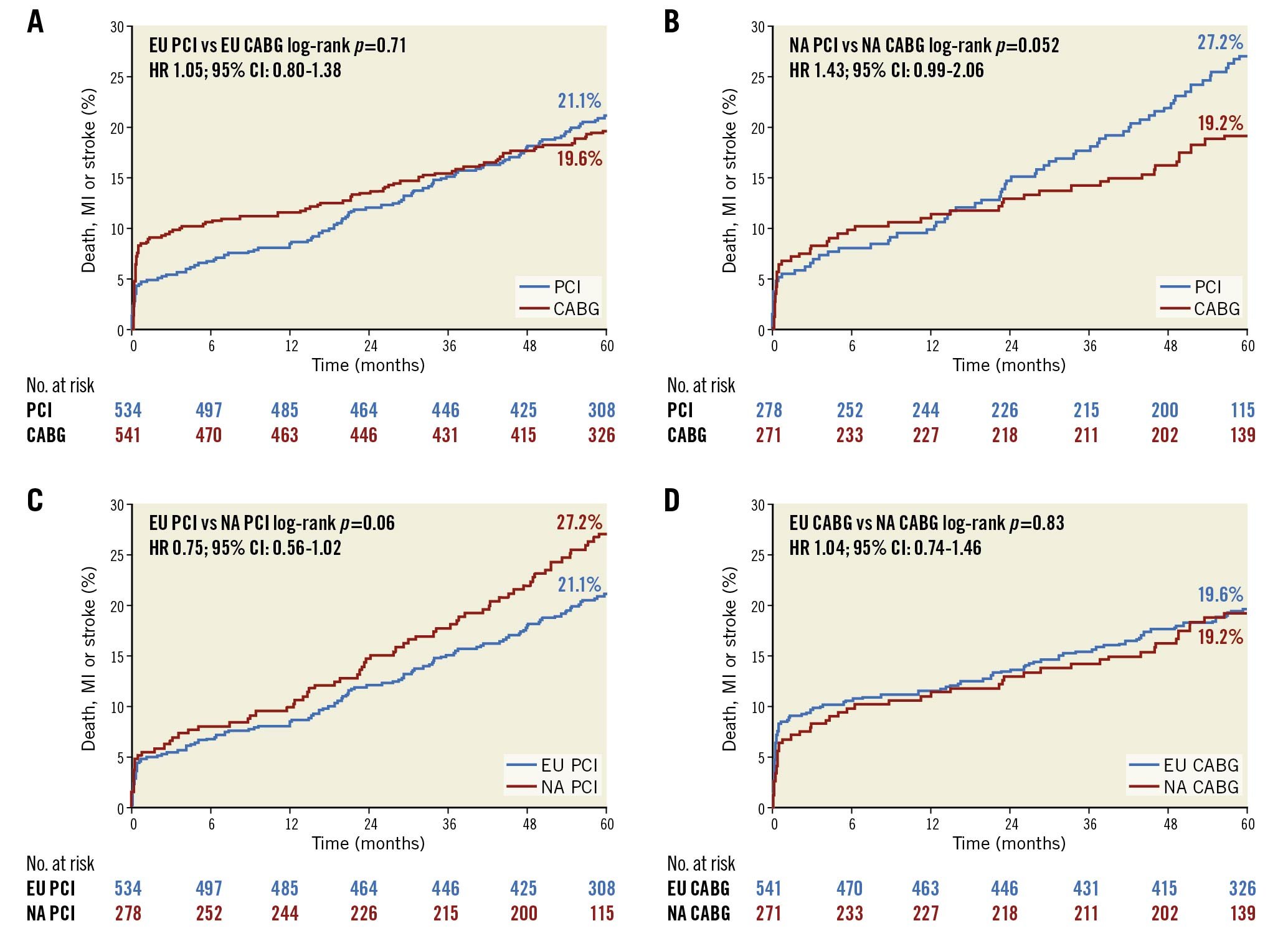
Figure 1. Rates of death, myocardial infarction, or stroke up to 5 years. A) PCI versus CABG at EU sites. B) PCI versus CABG at NA sites. C) PCI at EU versus NA sites. D) CABG at EU versus NA sites. CABG: coronary artery bypass grafting; CI: confidence interval; EU: European Union; HR: hazard ratio; MI: myocardial infarction; NA: North American; PCI: percutaneous coronary intervention
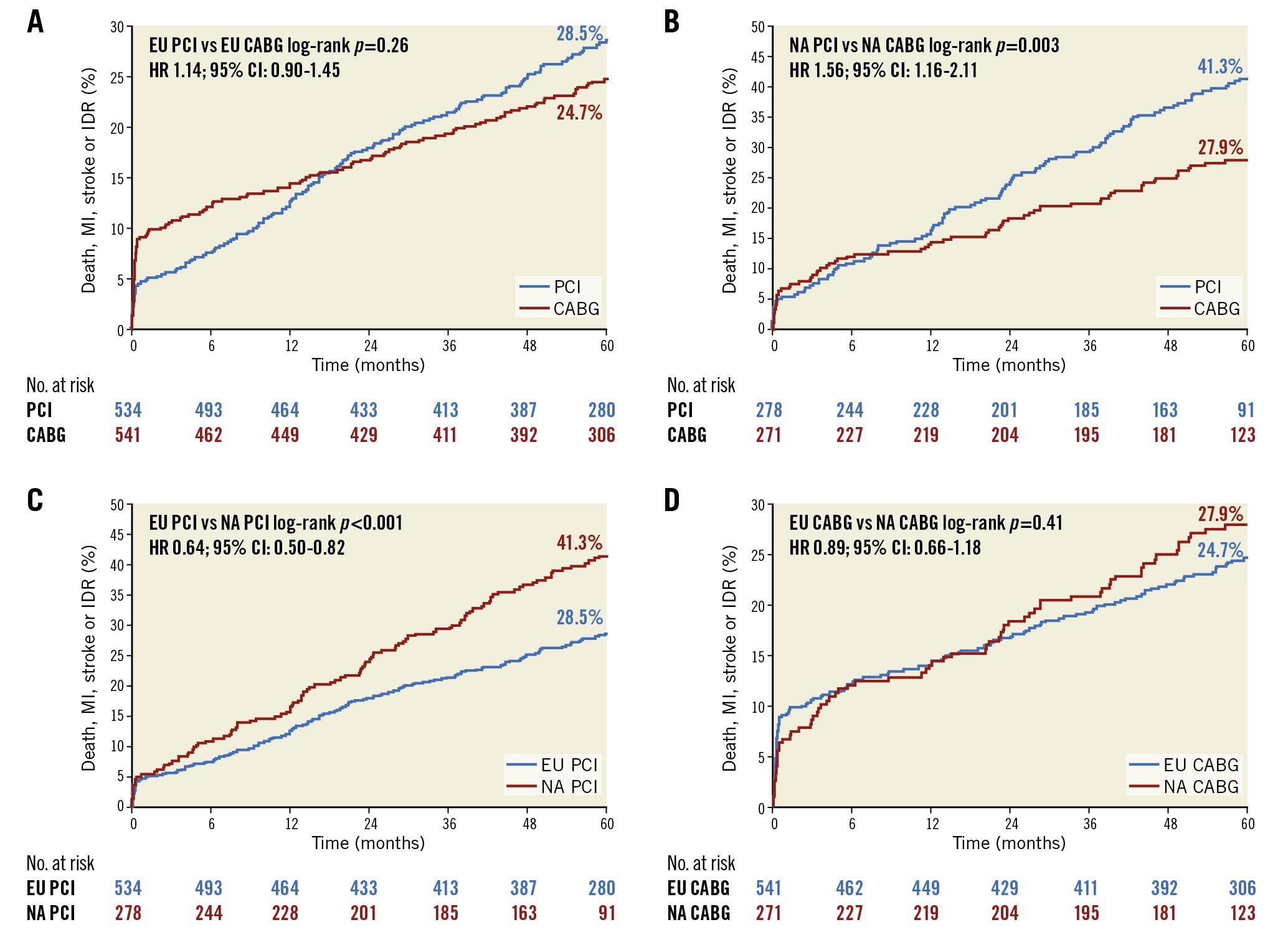
Figure 2. Rates of death, myocardial infarction, stroke, or ischaemia-driven revascularisation up to 5 years. A) PCI versus CABG at EU sites. B) PCI versus CABG at NA sites. C) PCI at EU versus NA sites. D) CABG at EU versus NA sites. CABG: coronary artery bypass grafting; CI: confidence interval; EU: European Union; HR: hazard ratio; MI: myocardial infarction; NA: North American; PCI: percutaneous coronary intervention
Discussion
The impact of geographic variation in the management of obstructive LMCAD has been incompletely characterised6. Patient risk factors and methods and outcomes of PCI and CABG are known to vary within and between the EU, NA, and other geographies789101112; therefore, an analysis of participant and procedural characteristics in the regions contributing patients to the EXCEL trial was considered important to contextualise the results of this landmark study.
A recent systematic review and meta-analysis of 701 PCI trials reported that NA centres tended to recruit a greater percentage of hypertensive and hyperlipidaemic patients, whereas Asian centres included more diabetics and smokers. EU centres enrolled a greater proportion of male participants whilst NA recruited more women10. The population breakdown from EXCEL was consistent with this analysis. Differences in patient characteristics by geography may also impact on outcomes. In a meta-analysis of the SYNTAX, PRECOMBAT, and BEST trials, a sex-treatment interaction was observed between the Asian (PRECOMBAT and BEST) and Western (SYNTAX) trials11. Female sex favoured CABG in the SYNTAX trial (pinteraction=0.02) but not in the Asian studies. There was no sex-treatment interaction in EXCEL, with equitable rates of PCI and CABG in the EU versus NA comparison (Supplementary Table 1). Substantial diversity in cardiovascular risk profile, adjunct pharmacotherapy, and aspects of the surgical and percutaneous approaches used between individual countries also contributed to varying event rates in SYNTAX12, illustrating the potential variability inherent in global multicentre clinical trials1314.
EXCEL is the largest trial to date comparing PCI versus CABG for treatment of LMCAD. While the protocol mandated some restriction on the types of patients enrolled (e.g., low and intermediate SYNTAX scores with equipoise for revascularisation by PCI or CABG), the procedural techniques and adjunct pharmacotherapies employed were largely left to investigator discretion, which has the presumed benefit of reflecting real-world practice but inevitably gives rise to variation at regional, national, and continental levels. Designating a strictly proscriptive homogenous approach to LMCAD revascularisation for every recruiting centre would in theory enhance capture of patient- and procedure-related differences in outcome and diminish operator-related confounding, but in practice it would be almost impossible to achieve given the differences in demographics, healthcare funding infrastructure, and operator expertise.
Of note, the higher absolute and relative rates of IDR after PCI in NA compared with EU centres were associated with a significant difference between treatments in the prespecified secondary composite endpoint of death, MI, stroke, or IDR at 3 and 5 years in NA (Table 4). The difference in IDR was driven by repeat revascularisation of the target lesion and vessel. The reasons for this are probably multifactorial. NA centres recruited more patients with diabetes, hypertension, and those who had prior PCI or MI (within the last two months) at the time of randomisation. This might indicate a relatively higher-risk cohort more prone to restenosis. Less operator experience with LM PCI in NA compared with EU sites may also have had an impact; unfortunately, while all sites were pre-qualified as having a minimum expertise in LM PCI and CABG procedures, detailed site and operator procedural experience was not collected to afford further volume-outcomes analysis. There were significant differences in the prescription of statins and antagonists of the renin-angiotensin axis and the duration of dual antiplatelet therapy administration following PCI between the EU versus NA, but these variations, although potentially contributory, cannot wholly account for the disparity in IDR rates. The threshold for reintervention after PCI may also be lower at NA compared with EU centres, driven either by more frequent assessments, greater rates of stress testing, reimbursement, or by concern about the implications of restenosis after LM intervention. The EXCEL study was not designed to assess which of these possibilities drove the differences in IDR rates between the geographies.
Nonetheless, despite these differences, the major primary 5-year composite endpoint of death, MI or stroke occurred with similar relative frequency after treatment with PCI versus CABG across the different geographies. This constancy may be a reflection of the experience of the sites participating in EXCEL and a testament to the favourable outcomes after EES and internal mammary artery use in nearly all patients undergoing PCI and CABG, respectively.
Limitations
Enrolling site location was not a stratification variable and, although the baseline characteristics of patients randomised to PCI versus CABG within each geographic region were well balanced, differences in unmeasured confounders cannot be excluded. The regions of enrolment are subgroups, and as such were individually underpowered for hypothesis testing. We thus relied on tests for interaction to examine whether relative outcomes for PCI versus CABG varied across the geographies; however, interaction testing is also inherently underpowered. We cannot, therefore, exclude small differences in outcomes between regions. This is particularly true for the individual components of the primary endpoint. Of note, however, by inspection, late MI rates tended to be greater after PCI compared with CABG, more so in NA sites compared with EU sites. It is unknown whether the greater rate of late repeat revascularisation after PCI in NA contributed to this difference or was a consequence (to treat late spontaneous MIs or MIs due to restenosis). The use of stress testing during follow-up and the exact reasons for reintervention were not collected in detail in the EXCEL trial. Further study is warranted to understand the greater utilisation of IDR in NA. Similarly, the greater 30-day mortality observed in the EU in the present study (which was attenuated with long-term follow-up) cannot entirely be explained by EU centres recruiting individuals with more complex coronary anatomy since patients enrolled at NA centres had greater rates of comorbidities, previous interventions and more acute clinical presentations. Additional studies are required to determine whether the numerous patient and procedural differences between the EU and NA translate into differences in early mortality after complex intervention. Only 78 patients were enrolled from South America, Asia, and Australia, precluding meaningful assessment of outcomes from these regions. Finally, the present results do not apply to patients with operator-assessed high SYNTAX scores, in whom PCI is generally not recommended in operative candidates.
Conclusions
The present prespecified subgroup analysis demonstrates that, despite widespread variation in demographic and angiographic features of the enrolled patients and the PCI and CABG techniques and medications used, the 30-day, 3-year and 5-year results of the EXCEL trial were consistent across the enrolling geographies for the primary endpoint of death, stroke or MI in patients randomised to PCI versus CABG. As these are generally accepted as the most important endpoints after revascularisation and represented the powered primary composite outcome for the study, the principal results from the EXCEL trial that PCI and CABG offer comparable long-term outcomes for selected patients with LMCAD may be generalised to the EU and NA (the USA and Canada). However, a lower rate of adverse periprocedural events occurred within 30 days after PCI compared with CABG (a finding also consistent between the EU and NA), an outcome that was counterbalanced by a lower rate of adverse events during long-term follow-up after CABG. The decision to choose PCI versus CABG for patients with LMCAD at experienced centres should thus be made by a Heart Team and tailored to individualised risk factors and preferences. Whether the patient resides in the EU or NA need not be a major factor affecting this clinical decision. However, the substantial difference in the follow-up utilisation of IDR after PCI compared with CABG in NA centres (compared with a smaller increase in late IDR after PCI in EU centres) should be acknowledged and taken into account by physicians and patients when choosing between procedures.
Impact on daily practice
The international EXCEL trial demonstrated that percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) surgery have comparable 5-year rates of the composite primary endpoint of death, stroke, or myocardial infarction when used to treat obstructive left main coronary artery disease (LMCAD) with low to intermediate anatomical complexity. Despite the substantial geographical variation in adjunctive pharmacotherapy and anatomical and procedural characteristics, the relative 30-day, 3-year and 5-year primary composite outcomes after PCI versus CABG were consistent with respect to participating centre geography and mode of revascularisation. The principal finding from EXCEL of comparable 5-year rates of major adverse outcomes after PCI and CABG for left main disease thus apply to patients enrolled in EU and NA centres. Significantly, higher long-term rates of ischaemia-driven revascularisation after PCI versus CABG were observed among patients enrolled in NA centres. The reasons for this are probably multifactorial in origin and warrant further investigation.
Funding
The EXCEL trial was funded by Abbott Vascular (Santa Clara, CA, USA).
Conflict of interest statement
A. de Belder reports grant support from Abbott Vascular and Medtronic, and consultancy/honoraria from Boston Scientific Corporation. D.E. Kandzari reports institutional research/grant support from Abbott Vascular, Biotronik, Boston Scientific, Cardiovascular Systems, Medtronic, and Teleflex, personal consulting honoraria from Cardiovascular Systems, and Medtronic. N.J. Lembo reports being on the advisory board of Abbott Vascular, and on the speakers bureau of Abbott Vascular, Boston Scientific, Medtronic, and Abiomed. P.W. Serruys reports being a consultant for Biosensors, Sinomed, SMT, Balton sp, Philips/Volcano, Xeltis, HeartFlow, Novartis, and Meril Life, and being a DSMB member for PROSPECT ABSORB. A.P. Kappetein reports being an employee of Medtronic. J.F. Sabik III reports being a consultant for Medtronic, being on the advisory board of Medtronic Cardiac Surgery, and being Abbott North America Surgical PI for the EXCEL trial (no payments). G.W. Stone reports speaker or other honoraria from Terumo, Infraredx, and Cook, being a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Abiomed, Ancora, Elucid Bio, Occlutech, CorFlow, Reva, MAIA Pharmaceuticals, Vascular Dynamics, Shockwave, V-Wave, Cardiomech, and Gore, and equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, and MedFocus family of funds. In addition, Mount Sinai Hospital receives grants from Abbott for research, and the Cardiovascular Research Foundation receives payments to the institution from Abbott for biostatistics, clinical events committee and core laboratory work on the clinical trials and for support to attend meetings. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

