Abstract
Aims: The SYNTAX-LE MANS substudy prospectively evaluated 15-month angiographic and clinical outcomes in patients with treated left main (LM) disease.
Methods and results: In the SYNTAX trial, 1,800 patients with three-vessel and/or LM disease were randomised to either CABG or PCI; of these, 271 LM patients were prospectively assigned to receive a 15-month angiogram. The primary endpoint for the CABG arm was the ratio of ≥50% to <100% obstructed/occluded grafts bypassing LM lesions to the number placed. The primary endpoint for the PCI arm was the proportion of patients with ≤50% diameter stenosis (‘patent’ stents) of treated LM lesions. Per protocol, no formal comparison between CABG and PCI arms was intended based on the differing primary endpoints. Available 15-month angiograms were analysed for 114 CABG and 149 PCI patients. At 15 months, 9.9% (26/263) of CABG grafts were 100% occluded and an additional 5.7% (15/263) were ≥50% to <100% occluded. Overall, 27.2% (31/114) of patients had ≥1 obstructed/occluded graft. The 15-month CABG MACCE rate was 8.8% (10/114) and MACCE at 15 months was not significantly associated with graft obstruction/occlusion (p=0.85). In the PCI arm, 92.4% (134/145) of patients had ≤50% diameter LM stenosis at 15 months (89.7% [87/97] distal LM lesions and 97.9% [47/48] non-distal LM lesions). The 15-month PCI MACCE rate was 12.8% (20/156) and this was significantly associated with lack of stent patency at 15 months (p<0.001), mainly due to repeat revascularisation.
Conclusions: At 15 months, 15.6% (41/263) of grafts were at least 50% obstructed but this was not significantly associated with MACCE; 92.4% (134/145) of patients had stents that remained patent at 15 months, and stent restenosis was significantly associated with MACCE, predominantly due to revascularisation.
Introduction
Although angiographic long-term patency has been reported for various conduit types, there is little information relating to graft patency following left main revascularisation with CABG1-6. Similarly, although short-term (six to nine months) angiography results have been published following stenting with drug-eluting stents in the left main territory7-12, long-term (>1 year) angiography results are rare13-15.
The SYNTAX trial is a multinational randomised trial comparing revascularisation with PCI to CABG in 1,800 patients with left main and/or multivessel disease, with an additional 1,275 non-randomised patients enrolled in two parallel nested registries. The results from the primary endpoint at one year have been previously published16 and follow-up through five years is ongoing. This report describes the results from a predefined substudy of angiographic and clinical outcomes at 15 months in patients with left main disease (SYNTAX-LE MANS). The 15-month primary endpoint was specifically chosen for angiographic follow-up to ensure that results from the angiogram did not affect the clinically-driven primary endpoint of the overall trial at 12 months.
Methods
Patient selection and overall study design have been previously described16,17. In brief, a heart team conference, consisting of a cardiac surgeon and an interventional cardiologist, assessed patients with de novo LM and/or multivessel disease, and patients deemed by consensus to be eligible for equivalent revascularisation by either PCI or CABG were randomised 1:1 to one of the two treatment options. Patients not considered equally eligible for either technique were entered into one of two parallel nested registries (the PCI registry for CABG-ineligible patients and the CABG registry for PCI-ineligible patients). SYNTAX-LE MANS was a pre-defined substudy of patients from the randomised cohort who provided a separate written, informed consent for entry into the substudy. Eligible patients were those with LM disease enrolled in the overall SYNTAX trial who did not have renal dysfunction (defined as creatinine >2.0 mg/dL [150 μmol/L]) or hypersensitivity to contrast agents that could not be adequately pre-medicated. The protocol for the substudy was approved in September 2005; enrolment in the overall SYNTAX trial began in March 2005. Patients with LM disease who were already enrolled in the SYNTAX trial at the time of the SYNTAX-LE MANS substudy approval were asked to consent for the 15-month follow-up angiogram during their next follow-up visit; patients who were not enrolled at the time of substudy approval were asked to sign the informed consent for the substudy at the time of enrolment into the SYNTAX study. The study was conducted in accordance with the principles of the Declaration of Helsinki and all local regulations. The Institutional Review Committee on human research at each site approved the protocol before enrolment of the first patient. The trial has been registered (ClinicalTrials.gov number NCT00114972). A challenge in study design was that the contrasting techniques to improve revascularisation of the LM employ distinct approaches and success measures. PCI treats occlusion by mechanical scaffolding that is typically assessed by angiographic measurements of the stent post implant (e.g., minimum lumen diameter, percent diameter stenosis [%DS], late loss), whereas, with CABG, the narrowed/occluded segment is untouched and arterial or venous conduits are placed distal to narrowing/occlusion, which is measured by blood flow or perfusion of the downstream territories. Hence, from a study design standpoint, there was no single validated angiographic endpoint that would provide a continuous measure to compare the two techniques. Therefore, the study was deliberately designed with differing primary endpoints in each arm and the study protocol pre-specified that no statistical comparisons would be made between treatment arms.
As per the protocol, the time window for the 15-month angiogram was set between 14 and 16 months post-allocation, but definitely after the 12-month primary clinical endpoint to avoid introducing an “oculostenotic reflex”, which may have affected the primary endpoint. Patients who were enrolled in the study and who had a clinically-driven angiogram from nine to 13 months (inclusive) after treatment allocation could use the earlier angiogram to fulfil the 15-month angiographic requirement and were not required to undergo a second angiogram. A total of 22 CABG and 16 PCI analysed angiograms were performed earlier than 14 months (425 days). The earliest PCI angiogram was performed at 260 days post-procedure and the earliest CABG angiogram was performed at 294 days post-procedure; neither patient was recorded as experiencing a MACCE or ST/GO during the study.
CABG endpoints
The primary endpoint for the CABG arm was the graft obstruction/occlusion ratio for all treated lesions at 15 months, defined as the ratio of ≥50% to <100% obstructed or 100% occluded grafts/anastomoses (as measured by absence of opacification on an angiogram) to the number of grafts/anastomoses placed. For all grafts bypassing the LM, the degree of obstruction/occlusion was first based on visual assessment, and then was further evaluated by quantitative coronary angiography (QCA) if the visual assessment was ≥50% stenosis. QCA was analysed in independent corelab (Cardialysis BV, Rotterdam, The Netherlands) using CAAS 5.4 analysis software (PIE Medical BV, Maastricht, The Netherlands). The CAAS software has been described separately18. The obstruction/occlusion ratio was also expressed on a per-patient basis as the proportion of patients with at least one obstructed/occluded graft at 15 months. Additional secondary endpoints included the pattern and location of graft stenosis defined by qualitative coronary angiography, the proportion of patients with complete revascularisation at 15 months, the rate of major adverse cardiac and cerebrovascular events (MACCE, defined as all-cause death; MI; CVA; and revascularisation) at 15 months, and the association between graft obstruction/occlusion and MACCE at 15 months. The pattern of stenosis was analysed as ostial/proximal, distal, body of graft, or string sign (defined as diffuse narrowing throughout the graft with less than 1 mm in diameter). Complete revascularisation was defined post-procedure as the treatment of any lesion with more than 50% DS in vessels ≥1.5 mm diameter as estimated on the diagnostic angiogram during the local heart team conference. Completeness of revascularisation was assessed post-procedure by the operator. Complete revascularisation at 15 months was defined as unimpaired flow to all distal beds of the LM (i.e., vessels showing a significant lesion that was grafted with no significant graft lesions at follow-up, nor new significant lesions in the left main or vessels in continuity with the left main), and was assessed by the core laboratory.
PCI endpoints
The primary PCI endpoint was the proportion of patients with ≤50% DS (‘patent’ stents) of treated lesions in the LM at 15 months, as assessed by QCA. Secondary PCI endpoints (by QCA) included the reference vessel diameter and minimum lumen diameter at pre-procedure, immediately post-procedure, 15 months post-procedure, and post-procedure acute gain. Other secondary 15-month endpoints included the percentage of patients with distal and non-distal LM lesions that had %DS ≤50; thrombus or aneurysm; late loss; and the percentage of patients with complete revascularisation of the LM and LM territory, defined as successfully treated lesion(s) at index and no restenosis (%DS≤50) or new significant lesion in vessels with at least 1.5 mm reference diameter supplying the LM territory. The angiographic analysis of distal (bifurcated) LM lesions was performed according to the method defined by the European Bifurcation Club19. Finally, MACCE rates at 15 months, and the association between MACCE at 15 months and the primary endpoint, were also calculated.
Statistics
The expected stent patency rate at 15 months post-allocation was approximately 80% (range: 74% to 97%); 100 patients in the PCI arm with angiographic follow-up data would provide a 95% confidence interval with a width of ±7.8% for an 80% patency rate. The expected graft obstruction/occlusion rate at 15 months post-allocation is 5% to 12%; 100 patients in the CABG arm with angiographic follow-up data would provide a 95% confidence interval with a width of ±6.4% for a 12% obstruction/occlusion rate or ±4.3% for a 5% obstruction/occlusion rate, respectively. With an expected attrition rate of 30%, a minimum of 143 patients per treatment group needed to be enrolled for the preceding statements to be relevant in order to have 100 patients per group with angiographic follow-up.
As previously described, per protocol, no between-groups statistical comparison was performed. Data are presented as summary statistics within each study arm. Binary variables were expressed as percent/count and continuous variables as means ± standard deviation. The association between MACCE and the primary endpoint for each arm was assessed by regression modelling with angiographic values as independent variables and 15-month post-allocation MACCE as the dependent variable in a series of univariate logistic regression models; each covariate was considered separately in a univariate model. Statistical significance was set at an alpha level of 0.05. All statistical analyses were performed using the SAS System version 8 (or above) software (SAS Institute Inc., Cary, NC, USA).
Results
A total of 115 patients were enrolled in the CABG arm and 156 patients were enrolled in the PCI arm (Figure 1). Of these, three patients in the PCI arm died (one non-cardiac death, one cardiomyopathy, and one sudden cardiac death); no patients died in the CABG arm. All other patients who provided informed consent underwent angiography. Available 15-month angiograms were suitable for analysis in 114 (99.1%) CABG patients and 149 (95.5%) PCI patients. Patients in this substudy represent a subset of the LM patients enrolled in the SYNTAX trial. Baseline characteristics for the entire LM cohort have been previously published20 and were generally similar to the SYNTAX-LE MANS subset, with a few exceptions. More patients in the SYNTAX-LE MANS subset were male compared to the overall LM cohort (LE MANS: 84.3% [97/115] CABG and 75.0% [117/156] PCI vs. LM: 75.6% [263/348] CABG and 72.0% [257/357] PCI), and fewer SYNTAX-LE MANS patients had medically treated diabetes (LE MANS: 20.9% [24/115] CABG and 15.4% [24/156] PCI vs. LM: 22.4% [78/348] CABG and 21.8% [78/357] PCI). There were no differences in the SYNTAX score or any of its components between the SYNTAX-LE MANS subset and the overall LM cohort.
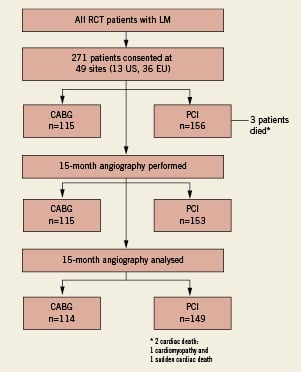
Figure 1. Patient flow in each arm. Patient disposition in the SYNTAX-LE MANS substudy. CABG: coronary artery bypass surgery; LM: left main disease; PCI: percutaneous coronary intervention; RCT:randomised controlled trial
Patients In the CABG arm, 84.3% (97/115) were male, the mean age was 65.0 years, 20.9% (24/115) had medically treated diabetes (Table 1), and 13.9% (16/115) patients were treated off-pump. Nearly two-thirds of patients (66.1% [76/115]) had LM+2-vessel disease or LM+3-vessel disease, with only 14.8% (17/115) of patients having isolated LM disease. According to baseline SYNTAX score calculations (which includes all lesions), the post-index procedure rate of complete revascularisation, as determined by the physician, was 65.2% (75/115) and the mean baseline SYNTAX score was 31.6±13.1. The mean number of conduits, including those placed to the RCA, was 2.6±0.7 (range: 1 to 4) with a mean number of arterial conduits of 1.4±0.6 (range 0 to 3). A total of 99.1% (114/115) patients had at least one arterial graft, 97.4% (112/115) of patients had an arterial graft to the left anterior descending artery and double LIMA/RIMA was used in 27.8% (32/115). All grafts were solely arterial in 27.0% (31/115) of patients, and only 1/115 (0.9%) patients had solely venous grafting. The mean number of distal anastomoses per patient to the LM territory only was 2.3±0.7 (range: 1 to 4), 41 grafts were jump grafts, and 12 were Y-grafts. In total, 263 LM grafts were used in 114 patients; of these, 115 (43.7%) were LIMA (of these, three were free LIMA), 34 (12.9%) were RIMA (of these, 11 were free RIMA), 17 (6.5%) were radial artery, 93 (35.4%) were venous grafts, and four (1.5%) were unknown.
Outcomes at 15 months On a per-graft basis, 15.6% (41/263) of LM grafts were at least 50% obstructed at 15 months; 9.9% (26/262) were 100% occluded; and 5.7% (15/263) were ≥50% to <100% obstructed/occluded (Figure 2A). On a per-patient basis, 27.2% (31/114) of patients had at least one obstructed/occluded graft (Figure 2B), and five (4.4%) had at least 50% obstruction of all LM grafts. Among arterial grafts only, 11.5% (19/165) of grafts were 100% occluded and an additional 5.5% (9/165) were 50% to 100% obstructed. Among venous grafts only, 7.1% (7/98) grafts were 100% occluded and an additional 6.1% (6/98) were 50% to 100% obstructed. At baseline, 65.2% (75/115) of patients were determined by the investigator to have complete revascularisation of all LM territory; by 15 months, this value had dropped to 53.5% (61/114), as assessed by the corelab. The predominant pattern of occlusion among all CABG patients was ostial/proximal (51.2% [21/141]), followed by distal (34.1% [14/41]) (Figure 3A), but when the pattern of obstruction/occlusion was analysed by type of graft, arterial grafts were equally likely to demonstrate proximal or distal (with 17.9% [5/28] string sign) stenosis (Figure 3B), but venous grafts predominantly exhibited ostial/proximal stenosis (Figure 3C). Among arterial grafts, the highest proportion of obstructed/occluded grafts were placed to the left circumflex obtuse marginal (35.0% [7/20]), followed by the intermediate branch (30.8% [4/13]) (Figure 4A); notably, of 110 arterial grafts placed to the left anterior descending artery, 10.9% (12) had obstruction/occlusion at 15 months. Among obstructed/occluded venous grafts, the majority were anastomosed to the left circumflex obtuse marginal (17.6% [3/17]), followed by the intermediate branch (12.1% [4/33]). Few venous grafts were anastomosed to the mid- or distal left anterior descending artery (n=4) and of these, 50.0% (n=2) occluded. However, of 19 venous grafts placed to the left anterior descending artery diagonal, none had obstruction/occlusion by 15 months (Figure 4B).
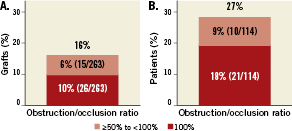
Figure 2. Primary endpoint at 15 months in the CABG arm. A) Ratio of obstructed/occluded segments per graft (primary endpoint); and B) Ratio of obstructed/occluded grafts per patient.
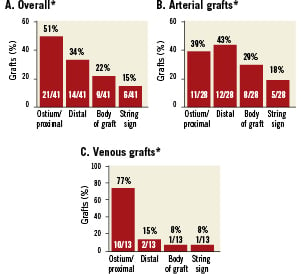
Figure 3. The pattern and location of graft stenosis as determined by qualitative coronary angiography (per graft). A) Results for the overall CABG cohort; B) Results for arterial grafts only; and C) Results for venous grafts only. *Patients may be counted in >1 category. Note that the presence of ostial/proximal occlusion would mask the detection of downstream stenosis.
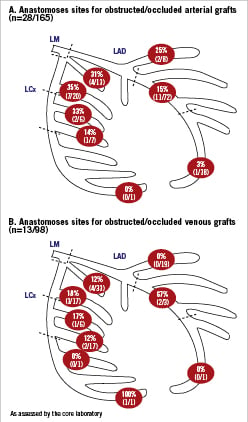
Figure 4. Distal anastomoses sites for obstructed/occluded and total grafts (core laboratory). A) arterial grafts; and B) venous grafts. Values are the numbers and percentages of obstructed/occluded grafts over total grafts placed for each location. LAD:left anterior descending artery; LCx: left circumflex artery; LM: left main coronary artery
The overall 15-month MACCE rate in this population was 8.8% (10/114), with repeat revascularisation comprising the largest component of MACCE (4.4% [5/114]), no death, and CVA and MI each comprising 2.6% (3/114) (Table 2). Of five patients in this group who underwent repeat revascularisation, four were localised in the left main territory. Two of these had occurred by 12 months post-procedure, and the remaining three at 380 days, 444 days, and 453 days post-procedure. Three CABG patients had repeat revascularisations (all via PCI) that occurred within 14 days of the 15-month angiogram: a re-PCI that occurred on day 430 post-procedure following an angiogram on day 429, a re-PCI that occurred on day 453 following an angiogram on day 440, and a re-PCI that occurred on day 444 following an angiogram on day 438. In addition, five patients were recorded as having a graft occlusion within 14 days of undergoing a 15-month angiogram (with angiogram dates ranging from day 443 to day 454 post-procedure); none of these patients underwent revascularisation. MACCE at 15 months was not significantly associated with graft obstruction/occlusion (p=0.85), with the MACCE rate unchanged regardless of whether patients had graft occlusion/obstruction (Figure 5).
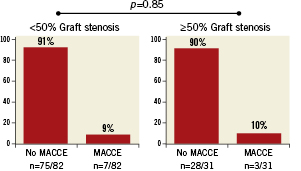
Figure 5. Graft stenosis versus MACCE. Graft occlusion/obstruction was not statistically significantly associated with major adverse coronary and cerebrovascular events at 15 months. Data were not available for one patient.
Results in the PCI arm
Patients Of the enrolled patients, 75.0% (117/156) were male, with a mean age of 65.4 years, 15.4% (24/156) had medically-treated diabetes, and the mean total SYNTAX score was 29.9±13.6 (Table 1). More than two-thirds of patients (70.5% [110/156]) had LM+2-vessel disease or LM+3-vessel disease, and 97 (62.2%) had a LM distal lesion by angiography. According to baseline SYNTAX score calculations (which includes all lesions), the mean number of stents implanted was 3.4±2.0 (range: 1 to 9); only 15.4% (24/156) of patients had one stent implanted and 17.3% (27/156) had ≥6 stents implanted. The physician-reported procedural success rate was 87.8%, and the rate of complete revascularisation post-procedure was 67.3% (105/156). The mean total length of implanted stents was 61.4±40.8 mm (range: 8.0 to 200.0 mm), and 19.2% (30/156) of patients had long stenting (>100 mm per patient). Provisional T-stenting was the most common bifurcation stenting technique (63.8% [90/141]), followed by classic T-stenting, main branch first (11.3% [16/141]), culotte or trousers technique (8.5% [12/141]), crush technique (6.4% [9/141]), and classic T-stenting, side branch first (5.7% [8/141]). Final dilation of bifurcation stenting with kissing balloons was performed in 70.3% (102/145) of patients.
Of 97 patients with a left main bifurcation lesion, the side branch was treated with a stent in 50 (51.5%) patients. Acute gain post-procedure was highest in non-distal lesions (1.79 mm), noticeably lower in distal main vessel lesions (1.15 mm), and lowest of all among stented side branch lesions (0.85 mm) (Table 3).
Outcomes at 15 months For the primary endpoint, 92.4% (134/145) of patients had patent lesions (%DS ≤50) at 15 months; distal LM lesions were slightly less likely to still be patent (89.7% [87/97]) compared with non-distal lesions (97.9% [47/48]) (Figure 6). The difference between distal and non-distal lesions trended toward –but did not reach– statistical significance (p=0.08 by the Chi-square test). At 15 months, 53.0% (79/149) had complete revascularisation of the LM and LM territory as assessed by the corelab, 1.3% (2/149) had experienced a thrombus, and no aneurysms were observed.
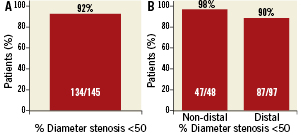
Figure 6. Diameter stenosis at 15 months in the PCI arm. A) Percent of patients who had stents with diameter stenosis ≤50% (primary endpoint); B) Percent of patients with non-distal and distal LM lesions who had stents with diameter stenosis ≤50%.
Percent diameter stenosis at 15 months was similar post-procedure among all lesion types, but at 15 months, %DS in distal LM bifurcation lesions was 16.0±15.1% versus 12.3±7.7% for non-distal or proximal LM bifurcation lesions (Figure 7), and 23.2±12.8% for stented side branch lesions. In contrast, late loss was similar across all types of lesions at 0.22 to 0.34 mm for distal and non-distal main vessel lesions and 0.47 mm for distal side branch lesions (Table 3).
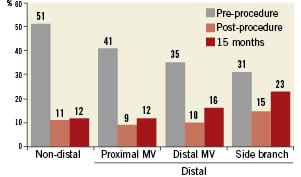
Figure 7. Percent diameter stenosis – for left main stents at pre-procedure, immediately post-procedure, and 15 months post-procedure for non-distal and distal lesions. MV: main vessel
The overall MACCE rate 15 months was 12.8% (20/156) of patients, of which the major contributor was repeat revascularisation (9.0% [14/156]), followed by MI (3.8% [6/156]); the combined safety endpoint of death/CVA/MI was 6.4% (10/156) (patients may have had >1 MACCE event) (Table 2). Repeat revascularisation specific to the left main territory occurred in eight of 14 patients who underwent repeat revascularisation (57.1% of total patients revascularised). Five PCI patients had repeat revascularisation (all via PCI) that occurred within 14 days of the 15-month angiogram. Of these, three occurred within the 60-day window for the 15-month angiogram and two occurred earlier: one patient had a MI and re-PCI on day 412 following an angiogram on day 408, and one patient had an angiogram on day 399 followed by a re-PCI on day 403. Stent restenosis at 15 months was significantly associated with MACCE at 15 months, with 45.5% (5/11) of patients with %DS <50 experiencing a MACCE event (Figure 8). Of these, 2/11 had a death, CVA, or MI, and 4/11 had a repeat revascularisation.
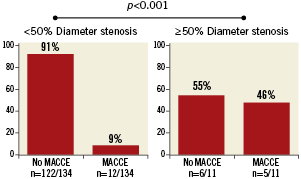
Figure 8. Stent restenosis versus MACCE. Stent restenosis was statistically significantly associated with major adverse coronary and cerebrovascular events at 15 months. Data were not available for four patients.
Discussion
The use of surgery or stenting for the treatment of left main stenosis has been a continuing topic of debate for many years. Although CABG is still recognised as the gold standard of treatment, recent changes to the ACC/AHA PCI recommended treatment guidelines have upgraded PCI of the LM coronary from Class III to Class IIb21. The use of evidence-based medicine, as represented by treatment guidelines, depends on the publication of studies, such as SYNTAX, that provide objective results for unanswered treatment questions. To date, very little has been published for either surgery or stenting regarding long-term (>1 year) angiographic outcomes specifically for LM interventions, making evaluation of the optimal technique for LM revascularisation difficult22. The SYNTAX-LE MANS substudy was designed to provide such long-term angiographic information for the LM in the context of a prospective, controlled, randomised trial. As previously noted, because of the differing endpoints between the two treatment arms, direct comparisons were not made between treatments, but the information presented here provides outcomes from current treatment practices in a pre-specified, balanced, randomised, multinational cohort of patients.
Patients in this study were a subset of the previously-reported SYNTAX left main cohort20. Baseline patient and lesion characteristics were generally similar between the overall cohort and the SYNTAX-LE MANS subset, with the exception of a higher proportion of males in the SYNTAX-LE MANS subset, particularly in the CABG arm, and a lower proportion of patients with medically-treated diabetes, particularly in the PCI arm of the SYNTAX-LE MANS subset. Additionally, the one-year MACCE rates were slightly lower in the SYNTAX-LE MANS subset compared with the overall LM cohort (LE MANS: 6.1% [7/115] CABG and 10.3% [16/156] PCI vs. LM: 13.7% [46/336] CABG and 15.8% [56/355] PCI). Because patients in the overall LM cohort were prospectively asked to participate in the SYNTAX-LE MANS substudy, we believe that these differences are unlikely to be due to selection bias, but rather represent a lack of statistical power in the smaller SYNTAX-LE MANS subset.
It is important to note that in our study, the rate of complete revascularisation post-procedure was determined by the site investigators, and thus may be affected by subjective operator variability, but completeness of revascularisation at 15 months was determined by the corelab. The lack of operator consistency in determining complete revascularisation at these two time points is a limitation of our study. Moreover, the rate of complete revascularisation in this study is lower than reported for other trials7,8,11, primarily because of a more restrictive definition of complete revascularisation in our study. In SYNTAX, all lesions were scored at baseline, regardless of intent to treat the lesion or not. Complete revascularisation was only achieved if all lesions scored at baseline were also treated. This is in contrast to other studies, which typically consider only lesions that were intended to treat when calculating the rate of complete revascularisation.
CABG
In the CABG arm, 10% of LM placed grafts/anastomoses were 100% occluded at 15 months, and an additional 6% were ≥50% obstructed. At 15 months post-procedure, a slightly larger proportion of arterial grafts (17%) were obstructed/occluded compared with venous grafts (13%). At 15 months, 54% of patients still had complete revascularisation of the LM territory (from a post-procedure value of 65%). Graft occlusion rates in our study are somewhat higher than those reported in the literature for the same time period. Single and multicentre studies of LIMA/RIMA grafting (ranging from 109 to 2,400 patients) that have been published in the last ten years have generally shown 6- to 18-month graft patency rates of 92% to 98%, with a much larger variety in patency rates of vein grafting over the same period (46% to 97%), but with the majority of rates from 80% to 90%1,2,6,23,24. A large recent study of on- versus off-pump CABG demonstrated significantly worse graft patency at one year in patients who underwent off-pump surgery compared with on-pump (82.6% off-pump versus 87.8% on-pump; p<0.01)25.
Venous grafts demonstrated primarily ostial occlusion, which may mask additional downstream occlusion/obstruction; slightly more arterial grafts showed distal occlusion than proximal occlusion. It is likely that venous grafts thrombose retrograde to the aortic anastomosis because there is no run-off to preserve patency of even a segment of the graft. At least two recent studies have suggested that internal mammary artery grafts may be particularly subject to graft occlusion when there is only moderate stenosis of the native coronary vessel, likely because of competitive flow3,26. A study by Arima et al5 has further suggested an increased development of string sign occlusion (diffuse narrowing throughout the graft with less than 1 mm in diameter) in bypass grafting of non-distal left main lesions, which they hypothesised is due to withering of the graft after reversal of left main stenosis following bypass grafting. Correspondingly, identification of LM flow-limiting lesions before surgery using fractional flow reserve has been shown to improve outcomes following CABG27,28.
In our study, graft occlusion or obstruction was not associated with clinical MACCE at 15 months. The majority of graft occlusions/obstructions were only noted after routine angiography, implying that these graft occlusions/obstructions were largely asymptomatic. In comparison, in the PREVENT IV trial, 46% of venous grafts were occluded at one year in the placebo group, and 8% of patients had experienced a MACE (defined as death, non-fatal MI, or revascularisation)2.
PCI
In this substudy, relatively more patients with PCI underwent a pre-specified angiogram at 15 months than in the CABG arm. Although the intent of this analysis is not to compare different treatment arms, this result is likely due to the greater ease of obtaining consent from PCI patients for a control angiogram. At 15 months, 92% of treated LM lesions had ≤50% in-stent stenosis, and stenosis was both more frequent and more advanced in the LM distal lesions than in the LM non-distal lesions. A total of 53% of patients still had complete revascularisation of the LM territory at 15 months, from a post-procedure rate of 67%.
The Intracoronary Stenting and Angiographic Results: Drug-Eluting Stents for Unprotected Coronary Left Main Lesions (ISAR-LEFT MAIN) study previously reported more frequent restenosis in the distal portion of LM lesions compared with the ostial9. Among the most clinically important findings in the PCI arm of our study was the discovery that late loss was similar in distal and non-distal LM lesions. This indicates that the difference in %DS between distal and non-distal lesions at 15 months is primarily due to insufficient acute gain post-procedure in distal lesions, suggesting there may be technical issues during stent implantation that create the difference in acute gain. Interventional cardiologists must be sure to focus on the quality of treatment in the distal LM, including the use of more aggressive post-dilatation29, the use of intravascular ultrasound guidance30, kissing with noncompliant balloons at high pressure post-procedure31,32, and so on, to help to improve this apparent disparity. Our results contrast with those of the French Multicentre Registry for Stenting of Unprotected LMCA Stenosis (FRIEND)12, which observed greater acute gain in distal LM lesions than in non-distal lesions and late loss in distal lesions that was nearly double that of non-distal lesions at 12 months. This registry treated a much higher proportion of distal lesions compared with non-distal lesions than in our study, which may have affected the results. In the FRIEND registry, the percentage of final kissing balloons was 93% (compared with 70% in the current study), which may have additionally improved the acute gain in distal lesions12. Also, differences in the methodology of QCA may account for variation in results; specifically, three-dimensional QCA has been reported in the SYNTAX left main population and suggests that the bifurcation angle may affect the outcome of distal left main lesions treated with two stents15.
Lack of stent patency at 15 months was significantly associated with the occurrence of MACCE in patients treated with PCI. However, there are some important caveats to note about this analysis. First, the number of patients with stent restenosis at 15 months is small (n=11). Second, 5/11 of patients who had stent restenosis had a MACCE event; four of these patients underwent repeat revascularisation with PCI. Unlike a redo CABG, which is performed less frequently, a repeat PCI can re-treat a restenosis more easily, often at the time of detection of the problem, and may therefore be undertaken more readily than a surgical reintervention. Additional follow-up would be needed to determine the long-term consequences of repeat PCI after restenosis.
Study limitations
Our substudy has an important limitation that must be noted. The overall number of patients enrolled in this substudy is relatively small and not sufficiently powered statistically. Therefore, results should be considered descriptive and not definitive. Larger studies will be needed to confirm the observational results presented here. In our study of graft disease location, not only would the presence of ostial occlusion mask the presence of downstream disease, but a graft may also thrombose entirely in the presence of distal disease. Therefore, the analysis of disease location can be limited if the graft has become occluded rather than simply stenosed. Finally, MACCE in our study was not adjudicated according to target vessel versus non-target vessel location, and MACCE rates may therefore have been affected by the presence of RCA disease.
Conclusions
At 15 months post-procedure, graft occlusion/obstruction was reported in more than a quarter of patients who underwent LM revascularisation with CABG, but graft occlusion/obstruction was not significantly associated with MACCE. In patients treated with PCI, 92% of patients who had stents placed in the LM territory had ≤50% in-stent stenosis at 15 months, but among the low number of patients who experienced in-stent restenosis, there was a significant association with MACCE at 15 months. Additional follow-up will be needed to determine the long-term clinical consequences of graft or stent failure; patients in the SYNTAX trial will continue to be followed for five years.
Acknowledgements
This study was sponsored by Boston Scientific Corporation. The authors thank Jian Huang, MS, and Peggy J. Pereda, MS (Boston Scientific Corporation) for statistical analysis; and Vicki M. Houle, PhD (Boston Scientific Corporation) for assistance in manuscript preparation.
Conflict of interest statement
Drs. Morice, Ståhle, and Kappetein have received research/grant support from Boston Scientific Corporation. Dr. Mack reports being a consultant or member of the Speaker’s Bureau for Boston Scientific, Cordis, and Medtronic. Dr. Feldman has received grant support from Abbott, Atritech, Boston Scientific, Cardiac Dimensions, Edwards, Evalve, and St. Jude; he is a consultant for Abbott, Boston Scientific, Cardiac Dimensions, CoAptus, Coherex, Cordis, Intervalve, Qantum-Cor, Square One, and WL Gore; and is a member of the Speaker’s Bureau for Boston Scientific Corporation. Dr. James has received lecturing honoraria from Boston Scientific. Dr. Fournial has received research/grant support from Boston Scientific Corporation and has served as chairman in a symposium organized by Edwards Lifesciences Company. Drs. Leadley and Dawkins are full-time employees of and have stock options in Boston Scientific Corporation, Inc. Ms. Morel is a full-time employee of Cardialysis, Inc BV. All other authors report no disclosures or conflicts of interest.

