
In 2009, Serruys et al published the SYNergy Between PCI With TAXUS and Cardiac Surgery (SYNTAX) trial in which 1,800 patients with left main or three-vessel coronary artery disease (CAD) were randomly assigned to undergo coronary artery bypass surgery (CABG) or percutaneous coronary intervention (PCI) to determine if PCI performed with drug-eluting stents (DES) was non-inferior to CABG1. The trial was a prospective randomised trial conducted in 85 sites and in 17 countries in Europe and the USA. The primary endpoint was a composite of major adverse cardiac and cerebrovascular events (MACCE), defined as death from any cause, stroke, myocardial infarction, or repeat revascularisation throughout a 12-month period after randomisation. In addition, rates of MACCE were analysed on the basis of the SYNTAX score, a comprehensive anatomical assessment of the extent of disease based on the location, severity, and extent of coronary stenoses, with a low score indicating less complicated CAD and higher scores indicating more complex CAD2.
At 12 months, the incidence of MACCE was lower in the CABG group (12.4%) than in the PCI group (17.8%), p=0.002. Up to five years, the Kaplan-Meier curves for MACCE continued to diverge with estimates of 26.9% in the CABG group and 37.3% in the PCI group, p<0.00013. In patients with low SYNTAX scores, there was no difference in MACCE between groups, 28.6% in the CABG group vs. 32.1% in the PCI group, p=0.43; in patients with intermediate or high SYNTAX scores, MACCE was significantly higher with PCI, 25.8% vs. 36.0%, p=0.008, and 26.8% vs. 44.0%, p<0.0001, respectively.
In this issue of EuroIntervention, Roy et al4 report that geographic variability had no significant treatment effect on MACCE rates at five years in the SYNTAX study.
The authors had the unenviable task of demonstrating that results across countries were the same. Most of our statistical methods are designed to show that results are different. The basic premise of the classic hypothesis-testing paradigm considers a null hypothesis that results are the same, with the alternative hypothesis that they are different. One then gathers evidence to show that it is inconsistent with the null hypothesis and so rejects the null with the implication that the alternative is possible, i.e., the results are different. If we reject the null, we do so with a chance of being wrong; this is labelled as level of significance and is usually set at a probability of 0.05. We also select a power, often 80%, which is the probability of rejecting the null hypothesis when in fact the null is wrong.
The authors provide statistical test results of the homogeneity of odds ratios (ORs) across the countries with a p-value of 0.08 which exceeds the level of significance of 0.05, and claim, based partly on this result, that “no significant heterogeneity was observed between individual countries”. But, did they have the power to find a difference? How comfortably can one claim homogeneity? In meta-analysis we are routinely faced with the issue of examining heterogeneity of results across studies. Although tests and statistics are often used, power is often an issue and simply displaying the results across studies can be informative. As shown in Figure 1, the forest plot provides such a visual perspective. This plot depicts the ORs of MACCE at five years reported in the SYNTAX trial across the countries, with the order of the countries based on an increasing sample size and precision of results. What can we see in this plot? First, no country by itself has a statistically significant result favouring one of the interventions, which is expected given the sample size associated with any one country. Second, one could take comfort in the fact that most of the ORs are in the same direction favouring CABG. When the sample size is small, one would have less precise results and some of the ORs by chance could go in the opposite direction, which is the case for countries such as Portugal and Denmark. The one exception is France, with a relatively larger sample size but an OR that favours the PCI group. This artefact could be explored to uncover a potential source of heterogeneity.
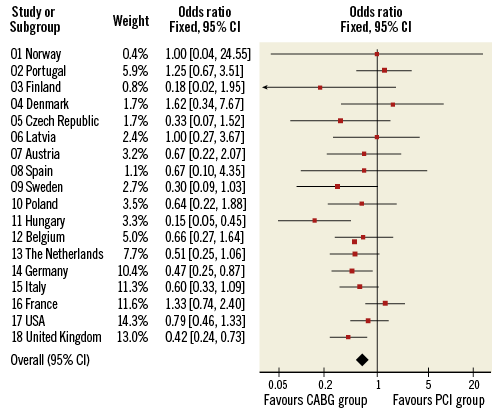
Figure 1. Forest plot depicting the ORs of MACCE at five years reported in the SYNTAX trial across the countries; the order of the countries is based on an increasing sample size and precision of results.
Although SYNTAX represents a comprehensive comparison of PCI with CABG, the study has important limitations. PCI was performed with paclitaxel-eluting stents, a first-generation DES. Second-generation sirolimus-eluting stents are associated with fewer cardiac events as compared with paclitaxel-eluting stents5. In SYNTAX, the rate of stent thrombosis within one year was 3.2% and accounted for approximately one fourth of the clinical events in the PCI group6. In comparison, the Evaluation of XIENCE vs. Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial comparing PCI with second-generation everolimus-eluting stents vs. CABG reported definite stent thrombosis in only 0.7% of patients within three years after PCI7. In addition, studies now show that a PCI strategy guided by measurement of fractional flow reserve (FFR) is superior in lowering clinical events as compared to a strategy guided by angiography alone8,9. The ongoing Fractional Flow Reserve vs. Angiography for Multivessel Evaluation (FAME-3) trial plans to demonstrate that FFR-guided PCI with second-generation DES is non-inferior to CABG in patients with multivessel CAD (ClinicalTrials.gov Identifier: NCT02100722).
Guidelines currently recommend a Heart Team approach to revascularisation in patients with unprotected left main or complex CAD10. In these patients, calculation of the Society of Thoracic Surgeons (STS) and SYNTAX scores is practical. However, as shown in this study, the mean calculated SYNTAX scores by highly selected sites and region are lower than that by the core laboratory by greater than four points. This could impact on the revascularisation strategy chosen by the team. The generalisability of the SYNTAX trial hinges on the meticulous calculation of the SYNTAX score which requires local validation and acceptable inter-observer variability. Knowledge of one’s institution’s clinical outcomes from CABG and PCI is also essential for the institution to adopt the recommended practice.
Conflict of interest statement
The authors have no conflicts of interest to declare.

