
Introduction
In patients with multivessel disease (MVD) and/or unprotected left main disease (UPLMD), clinical decision making for the appropriate revascularisation strategy entails a personalised approach, ideally by the Heart Team1. A treatment recommendation based on individual risk assessment will further improve patient selection for a given strategy of revascularisation.
The SYNTAX score II includes six clinical and two anatomical variables to improve prognosis estimation and ultimately provide a treatment recommendation2. Clinical variables (age, creatinine clearance [CrCl], and left ventricular ejection fraction [LVEF]) were initially incorporated into the anatomical score to develop the logistic clinical SYNTAX score3, which enhanced one-year mortality forecasting. Subsequently, this core model was expanded, and variables based on interaction effects for the prediction of long-term mortality after percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG) were incorporated. Diabetes was not included in the SYNTAX score II because it was not an independent predictor of mortality and did not have an interaction effect with CABG and PCI for long-term mortality2. The SYNTAX score II, therefore, predicts four-year mortality for patients with complex coronary artery disease undergoing PCI and CABG. The foremost feature of the SYNTAX score II is that it refines classification, which provides a real individualised assessment.
SYNTAX score II calculator
Since the publication of the SYNTAX score II, an online calculator has been largely expected. Clinical characteristics, such as gender, age, peripheral vascular disease (PVD; defined as aorta or arteries other than coronaries, with exercise-related claudication, or revascularisation surgery, or reduced or absent pulsation, or angiographic stenosis of more than 50%), chronic obstructive pulmonary disease (COPD; defined as the long-term use of a chronic bronchodilator or steroids for lung disease), LVEF (as percentage), and CrCl (defined by the Cockcroft-Gault formula, expressed in ml/min) combined with the angiographic presence of unprotected left main coronary disease and the anatomical SYNTAX score are computed to obtain the SYNTAX score II with the corresponding predicted four-year mortality for PCI and CABG.
The factors that drive adverse events after PCI and CABG are not always the same. For instance, angiographic anatomic complexity has a major impact on PCI results while it does not affect the results after CABG. On the other hand, advanced age and clinical comorbidities like COPD have a more negative impact on CABG than PCI. Independent predictors of mortality were identified using the baseline characteristics of CABG and PCI cohorts of the SYNTAX trial and validated in a large external cohort (i.e., DELTA registry)2. On the basis of the interaction effects between PCI and CABG, specific patient characteristics altered the mortality estimation of the anatomical SYNTAX score. For example, in a 60-year-old woman with a low anatomical SYNTAX score, a reduced LVEF impacted on the SYNTAX score II favouring CAGB compared to PCI (Figure 1). In contrast, in a 73-year-old male with a high anatomical SYNTAX score, left main disease and COPD (with normal LVEF), the SYNTAX score II favoured PCI compared to CABG (Figure 2). Nevertheless, if the same patient presented without COPD, the treatment recommendation would be PCI or CABG based on the similar mortality risk prediction (Figure 3). This accurate individual risk prediction provided by the SYNTAX score II enhances clinical decision making for the appropriate revascularisation strategy.
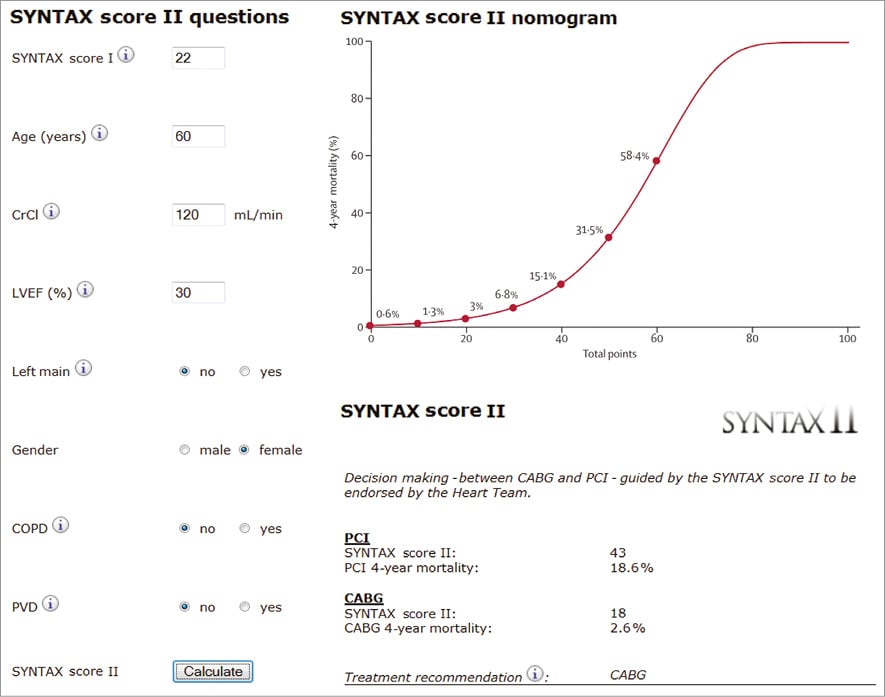
Figure 1. Case example 1. Syntax score II calculation of a 60-year-old female patient with an anatomical SYNTAX score of 22, normal renal function (CrCl 120 ml/min) and left ventricular dysfunction (EF 30%), and without left main disease, COPD or PVD. The calculated SYNTAX score II for PCI is 43 with a predicted four-year mortality of 18.6%, and a SYNTAX score II for CABG of 18 with a predicted four-year mortality of 2.6%, favouring CABG over PCI. CrCl: creatinine clearance; EF: ejection fraction; COPD: chronic obstructive pulmonary disease; PVD: peripheral vascular disease
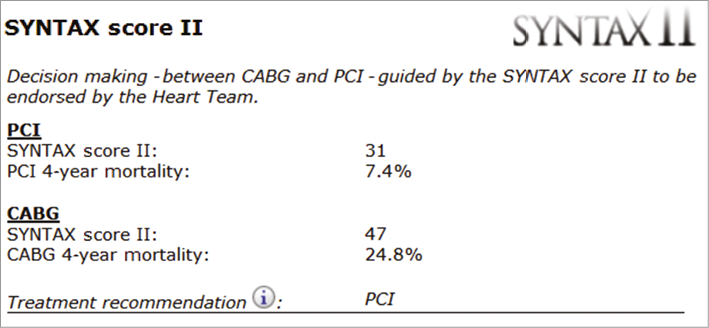
Figure 2. Case example 2. SYNTAX score II calculation of a 73-year-old male with an anatomical SYNTAX score of 33, normal renal function (CrCl 100 ml/min) and normal LVEF (60%), with left main disease and COPD. The calculated SYNTAX score II for PCI is 31 with a predicted four-year mortality of 7.4% in contrast to a SYNTAX score II for CABG of 47 with a predicted four-year mortality of 24.8%. Therefore, the treatment recommendation is for PCI over CABG. CrCl: creatinine clearance; LVEF: left ventricular ejection fraction; COPD: chronic obstructive pulmonary disease
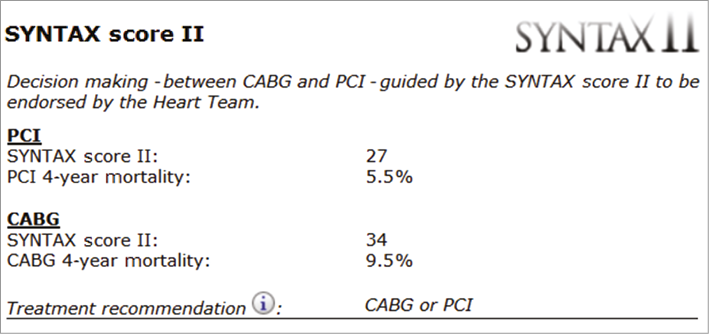
Figure 3. Case example 3. SYNTAX score II calculation of a 73-year-old male with an anatomical SYNTAX score of 33, normal renal function (CrCl 100 ml/min) and normal LVEF (60%), with left main disease without COPD. The calculated SYNTAX score II showed no significant differences for PCI (27 with a predicted four-year mortality of 5.5%) or CABG (34 with a predicted four-year mortality of 9.5%). The treatment recommendation shows equipoise between CABG and PCI. CrCl: creatinine clearance; LVEF: left ventricular ejection fraction; COPD: chronic obstructive pulmonary disease
Clinical application
The SYNTAX score II has been evaluated highly on its clinical utility for decision making in several validation studies and has already been applied to large randomised trials (EXCEL trial and SYNTAX II trial)2,4,5. External validation in the DELTA registry revealed that the SYNTAX score II had a fair discrimination ability with a concordance index (c-index) of 0.716 with reference to that for the internal validation of 0.725 (SYNTAX trial). Moreover, in the CREDO-Kyoto registry, the SYNTAX score II discriminated well in both CABG (c-index: 0.70) and PCI (c-index: 0.75) patient groups. The EXCEL trial (Evaluation of the XIENCE Everolimus Eluting Stent vs. Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) is an international, prospective, unblinded, randomised multicentre trial that enrolled 1,905 subjects in 131 centres (ClinicalTrials.gov Identifier: NCT01205776). It was designed to establish the safety and efficacy of the everolimus-eluting stent in patients with UPLMD. The SYNTAX score II was prospectively applied to the EXCEL trial4, and this year we will learn how accurate the predictions provided by the SYNTAX score II are.
The SYNTAX II trial is a multicentre, three-vessel disease, all-comers, open-label, single-arm trial of 450 patients in 25 interventional cardiology centres in Europe (ClinicalTrials.gov Identifier: NCT02015832). All patients were treated with the SYNERGY™ everolimus-eluting platinum chromium coronary stent system (Boston Scientific, Marlborough, MA, USA). The SYNTAX II trial strategy consists of contemporary PCI treatment of de novo three-vessel disease following the Heart Team patient selection applying the SYNTAX score II with pressure wire functional assessment and intravascular ultrasound (IVUS) guidance for optimisation of drug-eluting stent implantation. Patient enrolment was completed in November 2015, and the estimated primary completion date is December 2016 (final data collection date for primary outcome measure). The SYNTAX score II will also be implemented in the upcoming SYNTAX III trial.
Limitations
Some limitations need to be mentioned. First, because of the rarity of complex coronary artery disease in premenopausal women, mortality predictions with the SYNTAX score II in younger women are predominantly based on the linear association between age and mortality. The differences in mortality predictions in younger women between CABG and PCI will therefore be affected by larger 95% confidence intervals. Second, the interobserver variability of the anatomical SYNTAX score can potentially affect the mortality predictions. Lastly, the possibility of improved mortality with newer-generation drug-eluting stents could result in overestimation of the predicted mortality by the SYNTAX score II model. Further external validation in a large randomised population would be warranted.
Conclusion
Several validation studies and ongoing clinical trials may support the clinical utility of SYNTAX score II. The online calculator of SYNTAX score II provides an important tool to help guide the Heart Team decision-making process regarding the selection of the best revascularisation strategy for patients with MVD and/or UPLMD in daily clinical practice. To access the online SYNTAX score II calculator go to http://www.syntaxscore.com/.
Guest Editor
This paper was guest edited by Andreas Baumbach, MD, Bristol Heart Institute, University Hospital Bristol, Bristol, United Kingdom.
Conflict of interest statement
Y. Sotomi is a consultant for Goodman and has received a grant from the Fukuda Memorial Foundation for Medical Research and Sunrise Labs. Y. Onuma and P.W. Serruys are members of the Advisory Board for Abbott Vascular. M.A. Morel is an employee of Cardialysis. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

