As compared with bare metal stents, the use of drug-eluting stents (DES) has been associated with a significant reduction of angiographic and clinical restenosis and has become the default device in the contemporary practice of percutaneous coronary intervention (PCI). However, despite a remarkable improvement in PCI efficacy with DES, coronary artery bypass grafting (CABG) is still the preferred revascularisation strategy for patients with multivessel coronary artery disease (CAD), particularly those with diabetes and higher anatomic complexity1. Over the last decade, advances in DES technology, technical refinement, and adjunctive antithrombotic therapy have led to progressively improved PCI outcomes for complex CAD. In addition, there has been an evolution in invasive techniques that allow detailed assessment of both function and anatomy, which facilitates integrated use of fractional flow reserve (FFR) and intravascular ultrasound (IVUS) as adjunctive tools in complex PCI2.
The angiographic SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) score is a parameter to reflect the anatomic complexity and has a key role in decision making for optimal revascularisation in multivessel CAD3. Besides, PCI has substantially evolved since completion of the SYNTAX trial, in which the first-generation DES was used and the disease severity was assessed according to the angiographic assessment alone. Until recently, the long-term clinical effect of the most up-to-date contemporary PCI concept (i.e., decision making with more comprehensive risk assessment including anatomic and clinical factors, physiology guidance for PCI appropriateness, imaging guidance for PCI optimisation, and smarter DES and adjunctive drugs) has been lacking.
In this issue of EuroIntervention, this issue is addressed by Serruys and colleagues, who performed a two-year follow-up of the SYNTAX II study4.
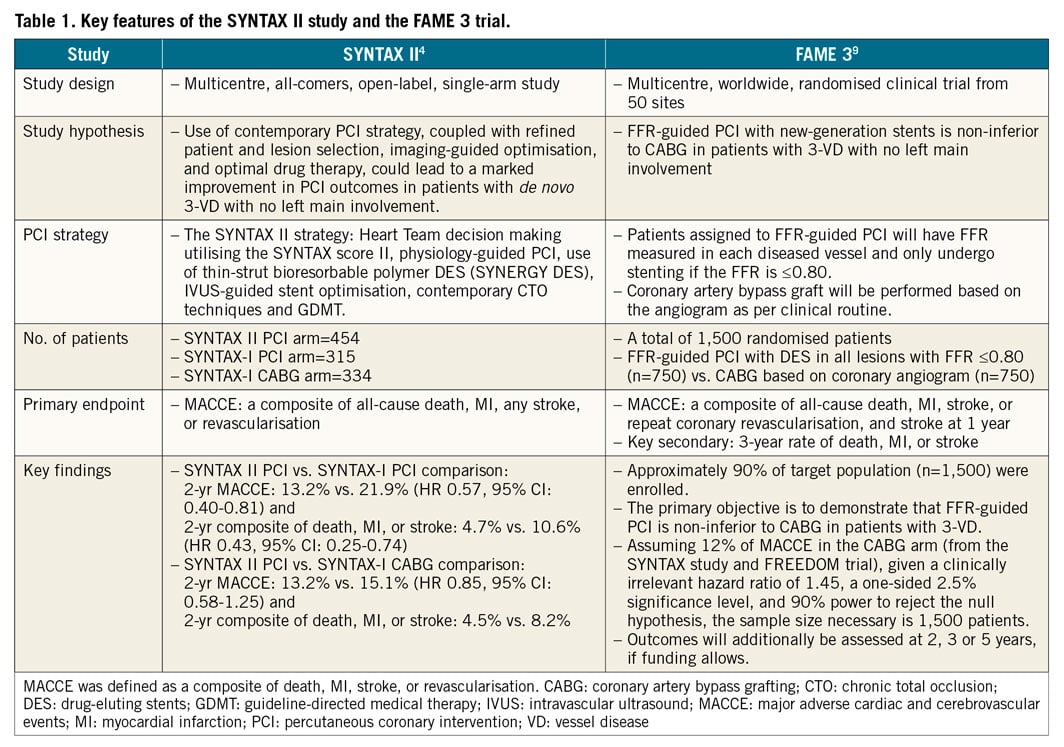
The SYNTAX II strategy includes Heart Team decision making utilising the SYNTAX score II, physiology-guided PCI, IVUS-guided stent implantation, use of thin-strut bioresorbable polymer DES, contemporary chronic total occlusion PCI techniques and guideline-directed medical therapy. The outcomes in the SYNTAX II cohort (n=454) were compared with predefined PCI (SYNTAX-I PCI) (n=315) and CABG (SYNTAX-I CABG) (n=334) cohorts from the SYNTAX-I trial. Physiological assessment (instantaneous wave-free ratio [iFR]/FFR) was performed in 76% of the lesions; this measurement deferred stenting in 25% of the target lesions. Post-implantation IVUS was performed in 76% of the lesions leading to a further optimisation in 30% of the stented lesions. At two years, PCI with the SYNTAX II strategy was associated with a significant reduction of major adverse cardiac and cerebrovascular events (MACCE: a composite of death, MI, stroke, or revascularisation) compared to the SYNTAX-I PCI cohort (13% vs. 22%; hazard ratio [HR] 0.57, 95% confidence interval [CI]: 0.40-0.81), mainly driven by a reduction in MI of 66% and in revascularisation of 38%. There were few revascularisation events and no MI in the initially deferred lesions. Interestingly, PCI with the SYNTAX II strategy was similar to the SYNTAX-I CABG cohort with respect to the two-year rate of MACCE (13% vs. 15%; HR 0.85, 95% CI: 0.58-1.25).
The favourable outcomes using state-of-the-art PCI with the SYNTAX II approach might be attributable to several integrated aspects rather than one single diagnostic or therapeutic aspect. First, beyond anatomical complexity alone, patient characteristics and comorbidities should be taken into consideration for the optimal choice of revascularisation method. Thus, individual risk assessment according to the integrated anatomic and clinical risk algorithm (i.e., the SYNTAX score II) optimises the decision-making process5. Second, the use of physiology-guided revascularisation might significantly reduce the rate of MACCE in patients with multivessel CAD. In the FAME trial, physiology-guided PCI for multivessel CAD resulted in a significant reduction of a composite of death or MI compared to angiography-guided PCI6. Third, post-procedural IVUS guidance might contribute to stent optimisation and be related to a reduction in device-related ischaemic events. In contemporary PCI practice, there has been an increased utilisation of IVUS and several trials have demonstrated better PCI outcomes with IVUS guidance compared to angiographic guidance7,8. Lastly, together with optimised decision making with regard to patients, lesion selection, and implant technique, the thin-strut bioresorbable polymer DES might in part be associated with the observed low rates of stent thrombosis, MI, and revascularisation.
The SYNTAX II study might suffer from inherent limitations. This is a single-arm study comparing a contemporary PCI strategy with an historical control in SYNTAX-I. There has been an almost 10-year lapse of time between the SYNTAX-I and II studies, with a remarkable paradigm shift in medical practice and patient care. Therefore, the key findings of the SYNTAX II study should be confirmed or refuted through randomised clinical trials (RCT). The FAME 3 trial is designed to investigate whether FFR-guided PCI with contemporary DES is non-inferior to CABG in patients with three-vessel disease9. The key features of the SYNTAX II and the FAME 3 studies are summarised in Table 1. In contemporary PCI practice, it should be realised that further improvement of PCI outcomes can be achieved by “best PCI practice”, with better decision making for patients and treatable lesions, and imaging-guided PCI optimisation. In this respect, the SYNTAX II approach combined all the technical and conceptual advances in the field of PCI. Finally, both SYNTAX II and FAME 3 will provide clinical evidence regarding the advantages and limitations of state-of-the-art PCI for multivessel CAD as compared to CABG.
Conflict of interest statement
The authors have no conflicts of interest to declare.

