
HOW DID A PERCUTANEOUS CORONARY INTERVENTION/CORONARY ARTERY BYPASS GRAFTING RANDOMISED TRIAL GENERATE THE ANATOMIC SYNTAX SCORE?
Following the CABRI1 and ARTS2 trials on multivessel disease, a challenging new trial was proposed to surgeons, namely the SYNTAX (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial, sponsored by Boston Scientific as an IDE FDA trial on the use of the TAXUS™ stent (Boston Scientific, Marlborough, MA, USA) in three-vessel disease and left main. Actually, the left main component of the trial was a proposal made by Marie-Claude Morice and Antonio Colombo. A surgeon, Friedrich Mohr (Leipzig), one of the key surgeons in European surgery, accepted the challenge on one condition: there should be no anatomic exclusion criteria and all patients should be included, in other words, he required an all-comers surgical trial and wanted to avoid any “cherry picking” selection from the interventional cardiology side3. Under the leadership of Mary Russell, the medical officer of Boston Scientific, a trial was designed with two components - the left main and the three-vessel disease cohorts. However, the sample size and the power calculation for non-inferiority (with reflex to superiority) of percutaneous coronary intervention (PCI) versus coronary artery bypass grafting (CABG) was based on the entire population. All patients had to be evaluated for eligibility for randomisation or for inclusion into either the CABG or PCI registry if only one treatment option was suitable. Prior to the start of the trial, the trial designers’ concern was the proper, adequate, semi-quantitative assessment of the anatomic complexity of the coronary circulation by the surgeons and the interventional cardiologists before any inclusion of patients either in the randomised arms or in the registry. Originally, the anatomic SYNTAX score was simply a tool, to force the physician to inspect meticulously and in a structured way the cineangio-graphy and the anatomic complexity. To create that tool, different scores existing in the literature at that time were combined - the Leaman score, chronic total occlusion (CTO) score, Medina score, thrombus score, calcification score, and so on… (Figure 1). As previously mentioned, the Leaman score weighted the theoretical physiologic impact of a 50% stenosis or a total occlusion in each coronary segment of the American Heart Association (AHA) classification according to the amount of blood supply delivered to the heart4. The hierarchical logistics between these different scores were quite a complex task, but this was finally mastered by Marie-Angele Morel and, prior to the start of the trial, a trainee at the Thoraxcenter, Georgios Sianos, was asked to report on the SYNTAX score in the newly created EuroIntervention journal in 20055. Today the paper has been cited 1,613 times according to Google Scholar and 341 publications contain the word SYNTAX score in their titles.
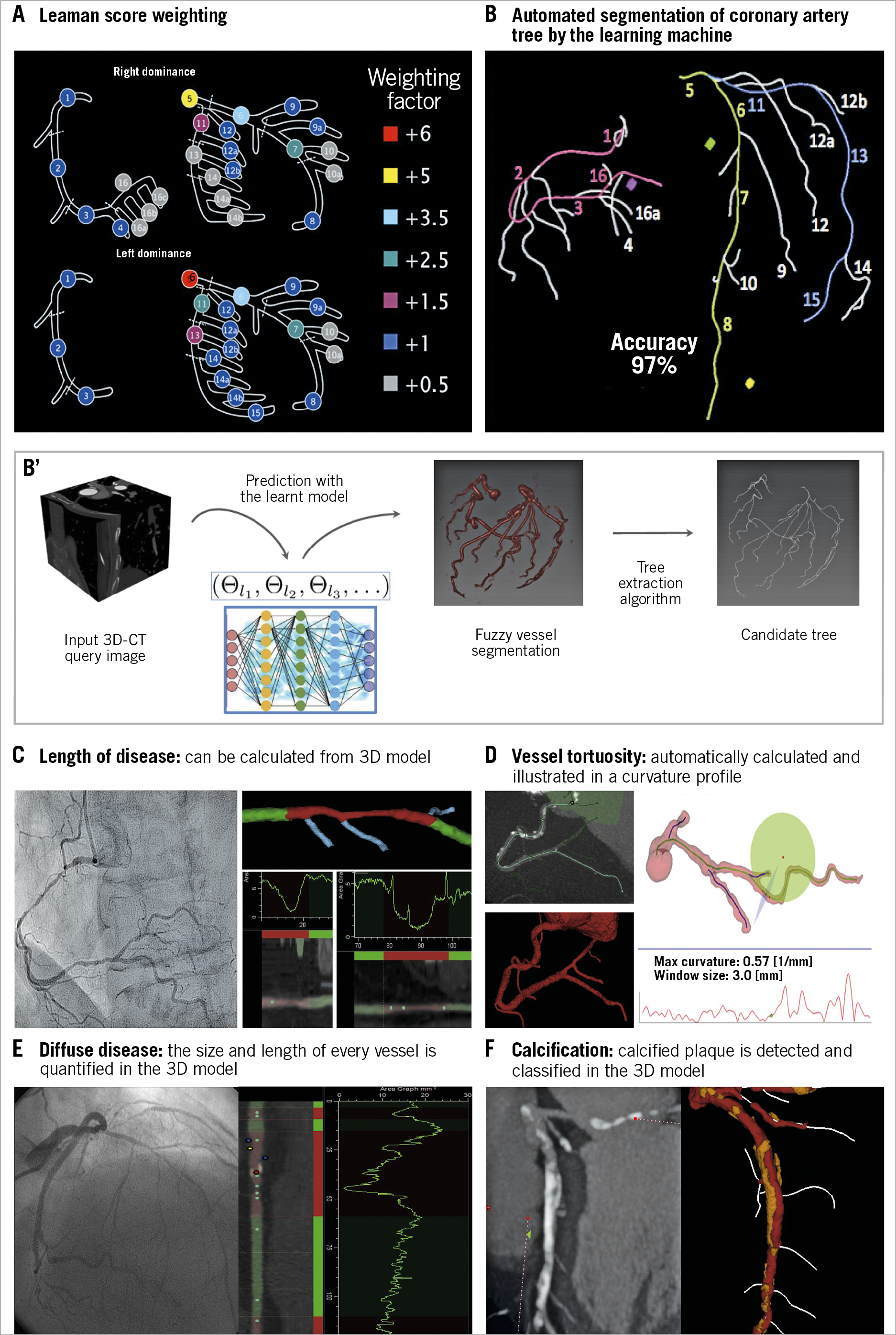
Figure 1. Anatomic SYNTAX score evaluated from the multislice CT scan with automated segmentation produced by the learning machine.
During the enrolment for the SYNTAX trial, the possible prognostic value of the anatomic SYNTAX score was tested in the population of the ARTS II trial (with the help of Marco Valgimigli)6.
However, during the SYNTAX trial recruitment, the investigators had not the slightest idea of the significance of the score outcome recorded in the case report form. At the time of the result analysis, the prognostic value of the score in predicting the rates of major adverse cardiac and cerebrovascular events (death, stroke, myocardial infarction, revascularisation) in substrata of patients treated by PCI was realised7. However, the prognosis of surgical cases was not affected by the anatomic SYNTAX score since the surgeon “bypasses” all these anatomic complexities8. The anatomic SYNTAX score with its prognostic value had a Gaussian distribution with a first tertile corresponding to a value of less than 22 and the third tertile starting at a value of 33 (lucky numbers easy to remember by a busy practitioner…). In the New England Journal of Medicine7,9, the fact that, beyond a score of 22, the prognosis for major adverse cardiac and cerebrovascular events was not favourable after PCI was emphasised. At the same time, it was realised that the prognosis of a surgical case was (as expected) related to the EuroSCORE10 and ACEF score11, scores that are related to the clinical characteristics and comorbidities. The conclusion was reached that age, gender, left ventricular ejection fraction, creatinine clearance, peripheral vascular disease and chronic obstructive pulmonary disease affected differently the vital prognosis of patients undergoing surgical or percutaneous intervention12. Davide Capodanno had previously come to the same conclusion and elaborated the global risk classification13.
IN 2011, THE ANATOMIC SYNTAX SCORE BECAME FUNCTIONAL
The use of the fractional flow reserve (FFR) in patients with three-vessel disease gave another twist to the story of the anatomic SYNTAX score, since only physiologically significant stenoses would be retained in the score14. The functional SYNTAX score better predicts adverse events and reclassifies a substantial number of patients with multivessel disease from high risk (high anatomic SYNTAX score) to lower risk (low anatomic SYNTAX score) of adverse events after PCI. Angiography-derived FFR has recently been analysed in the SYNTAX II trial15. Angiographically derived FFR was analysable in 71.0% of lesions (836 lesions) in a post hoc analysis. The diagnostic performance of angiographically derived FFR to predict binary wire-based ischaemia was substantial (area under the curve 0.81, accuracy 73.8%), with a positive predictive value of 85.9%. Independent predictors of diagnostic discordance were lesions in side branches, involvement of bifurcations or trifurcations, and small vessels. According to the two-year patient-oriented composite endpoint, the functional SYNTAX score derived from angiographically derived FFR (fSSQFR) reclassified 26.1% of the patients (36 of 138) in the high- to intermediate-risk group into the low-risk group appropriately (net reclassification improvement 0.32; p<0.001) (Figure 2). The area under the curve for fSSQFR to predict the two-year patient-oriented composite endpoint was higher than that of the classic anatomic SYNTAX score (0.68 vs 0.56; p=0.002).
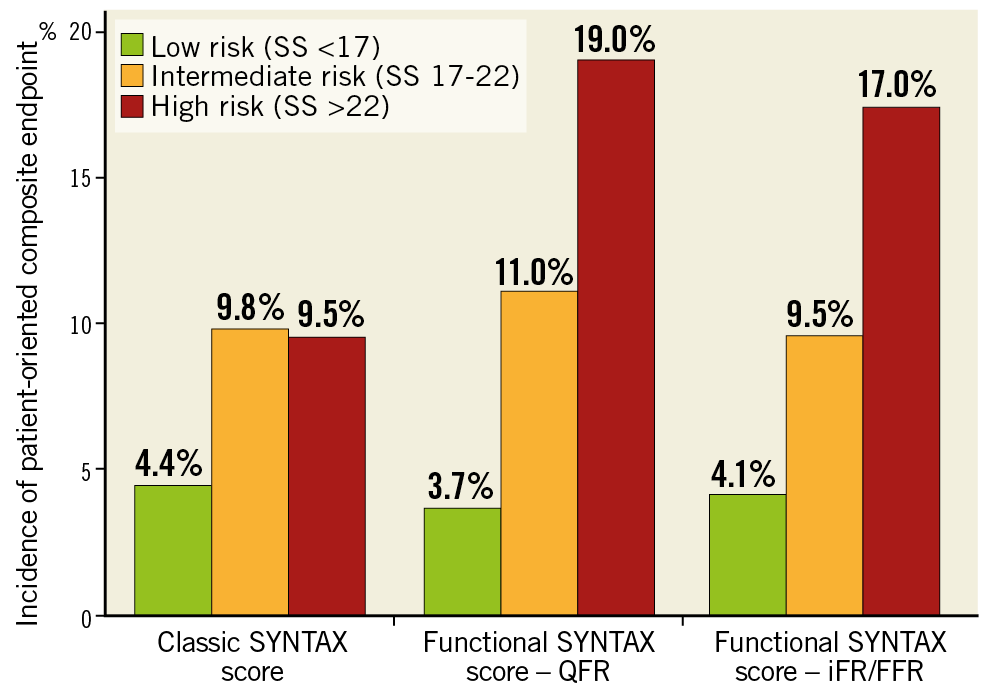
Figure 2. Incidence of patient-oriented composite endpoint stratified according to the classic anatomic SYNTAX score, the functional SYNTAX score derived from QFR, and the functional SYNTAX score derived from iFR/FFR. FFR: fractional flow reserve; iFR: instantaneous wave-free ratio; QFR: quantitative flow ratio; SS: SYNTAX score. Adapted from Asano et al15, with permission.
IN 2013, THE SYNTAX SCORE II: INDIVIDUALISED VITAL PROGNOSIS DERIVED FROM COMBINED ANATOMY AND COMORBIDITY
With the outcome of the SYNTAX study, it became evident that the anatomic complexity derived from the anatomic score with comorbidities derived from surgical scores such as ACEF11,16,17 or EuroSCORE10 had to be combined and merged, if we wanted to predict the vital prognosis of a patient revascularised by either surgery or percutaneous intervention. Individualised prognosis became an obsession with the desire to predict the ultimate outcome not affected by any adjudication process, i.e., all-cause mortality. Why all-cause mortality specifically at four years? Simply in order to precede the publication of the last follow-up of the trial reported at five years under the first authorship of Friedrich Mohr18.
The SYNTAX score II was developed by applying Cox proportional hazards models to the results of the SYNTAX trial and identified a combination of independent clinical and anatomical predictors of four-year all-cause mortality. Seven co-variables were identified according to the interactions with the anatomic SYNTAX score in the surgical and percutaneous case - age, gender, creatinine clearance, peripheral vascular disease, chronic obstructive pulmonary disease, left ventricular ejection fraction and left main stem disease. In other words, the anatomic SYNTAX score was merged with comorbidities and clinical characteristics. Obviously, the combination of these parameters affected in a different way the four-year all-cause mortality of the surgical and PCI patients. This statistical work was completed around the time of the four-year follow-up of the SYNTAX trial and was called the SYNTAX score II19,20,21. The SYNTAX score II is only valid in the context of three-vessel disease with or without left main, specifically using the TAXUS stent (Table 1). However, using the external validation cohort of patients from the PRECOMBAT and BEST trials, it was demonstrated that the SYNTAX score II performed similarly in patients both with and without diabetes22. Recently, the score has been applied erroneously for prognosis of simple PCI treatment in registries with types of stent other than the TAXUS23,24.
In summary, the SYNTAX score II calculator provides the clinician with an individual four-year vital prognosis (risk of all-cause death in percentage) (Figure 3) for a patient undergoing either a surgical or a percutaneous revascularisation25.
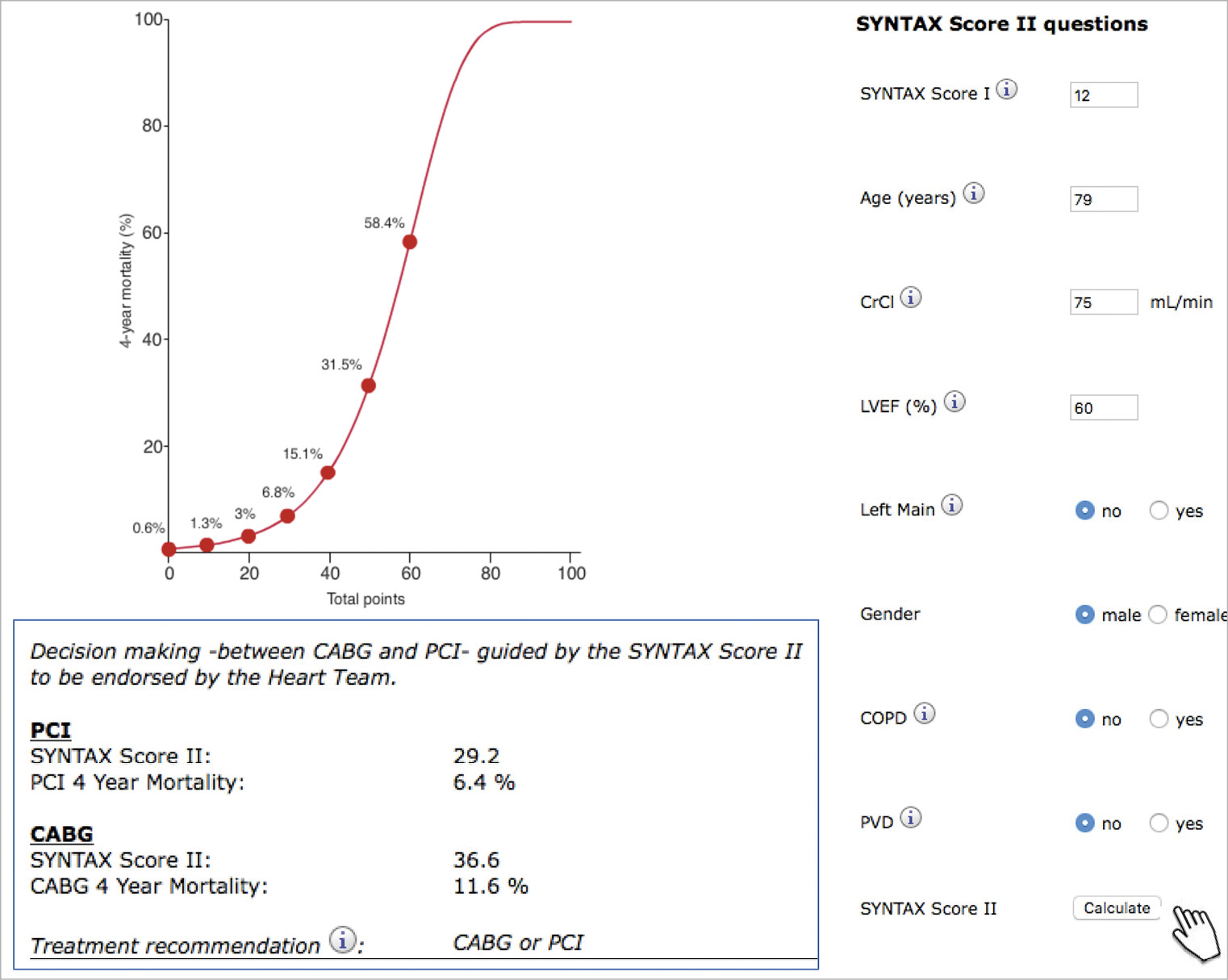
Figure 3. Web calculator of the SYNTAX score II. Nomogram depicting predicted four-year mortality as a function of the SYNTAX II score for patients who are candidates for myocardial revascularisation (CABG or PCI). The SYNTAX score II can be calculated from the web calculator to guide revascularisation strategy. After input of the anatomic SYNTAX score and patient’s comorbidities, predicted four-year mortality from PCI and CABG and treatment recommendation are provided25.
Recently, in a Serbian centre without surgery on site, the fact that the non-respect of the treatment recommendation (PCI only, CABG only, equipoise CABG/PCI) derived from the SYNTAX II impacts negatively on the four-year all-cause mortality of PCI-treated patients when the recommendation was CABG only was documented26. A similar observation was made in the EXCEL trial (Figure 4).
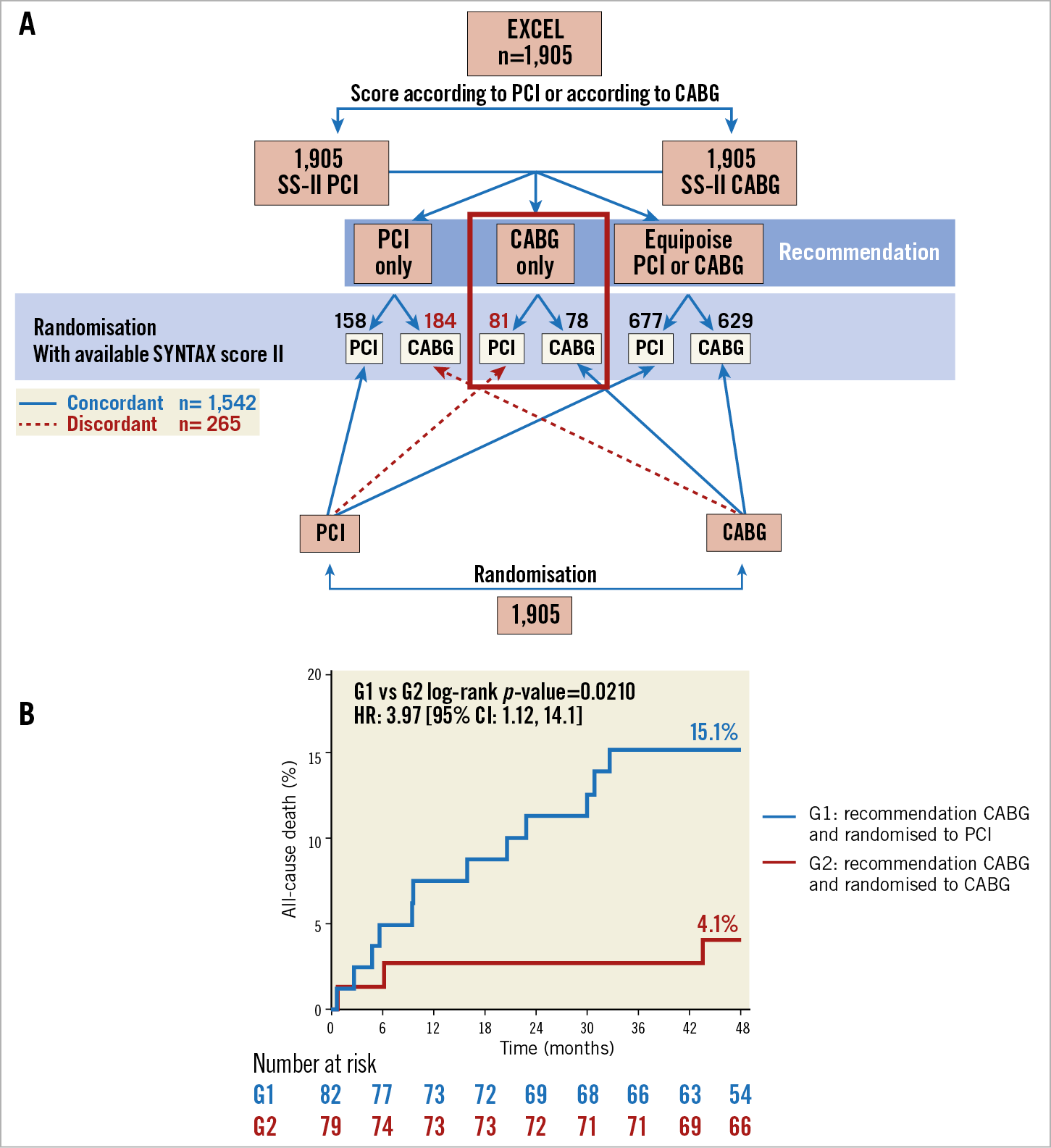
Figure 4. The EXCEL study. A) Flow of the patients in the EXCEL study according to allocated revascularisation strategy and treatment recommendation derived from the SYNTAX score II. B) The time to all-cause mortality curves of the patients who received a SYNTAX score II recommendation to undergo CABG and underwent PCI (blue line) versus CABG (red line). CABG: coronary artery bypass grafting; CI: confidence interval; HR: hazard ratio; PCI: percutaneous coronary intervention
2013, THE SYNTAX II TRIAL: AIMING AT BEST PRACTICE
After the initial and seminal SYNTAX trial, it was not possible in the short term to design and generate another randomised trial in the field of three-vessel disease. Therefore, with a kind of propensity selection, this time using mortality at four years predicted by the SYNTAX score II as a matching and inclusion criterion, the SYNTAX II trial was conducted. This was a registry with an “objective performance index”, based on the population of the original SYNTAX study27.
Indeed, by adopting the anatomic SYNTAX score in their guidelines and taking into account the results of the original SYNTAX trial, the European Society of Cardiology (ESC) and the American College of Cardiology recommended PCI of three-vessel disease in a subset of patients with an anatomic SYNTAX score less than 22 and the field of PCI treatment in three-vessel disease suddenly became very restricted.
However, in the meantime (after the report of the one-year outcome of the SYNTAX trial in 2009) PCI treatment evolved with a) the identification of physiologically significant lesions that had to be treated or deferred, b) the development of the second-generation drug-eluting stents, c) the use of intravascular ultrasonography (IVUS) to improve post-stenting results and mastering CTO treatment thanks to the involvement of a dedicated expert operator in CTO15,27. In other words, the best practice became 1) to select patients carefully on the basis of equipoise predicted mortality between surgery and PCI at four years applying the SYNTAX score II, 2) to judge the functionality of the stenosis and apply the lessons learned from the functional SYNTAX score, 3) to use IVUS to optimise the intervention28, 4) to use contemporary CTO revascularisation techniques, and 5) to apply optimal medical treatment29. This best practice has resulted in four major changes in outcomes, as described in the Figure 5 legend.
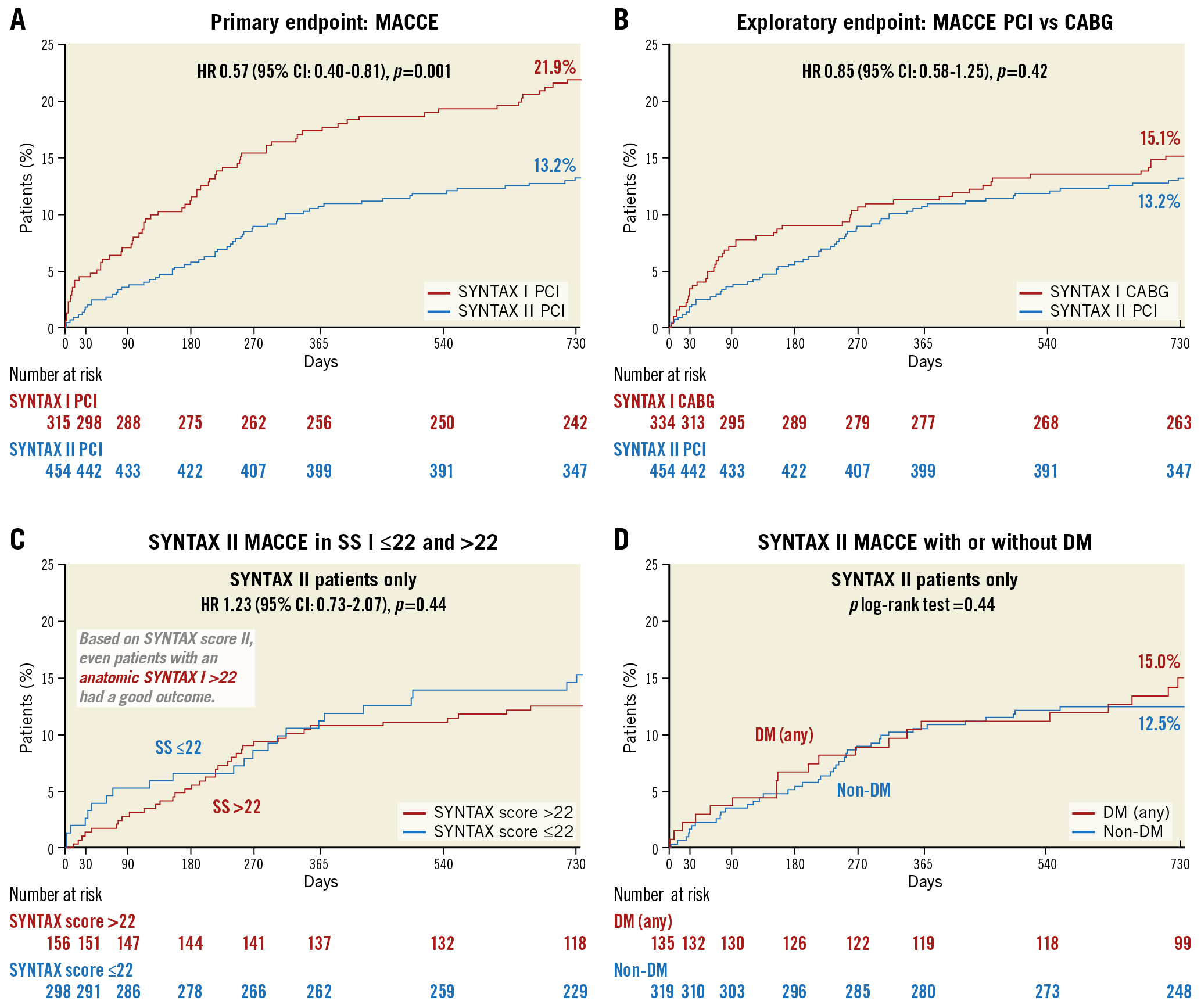
Figure 5. Four major improvements in patients with multivessel disease resulting from the best PCI practice (SYNTAX II trial). Patients with multivessel disease treated with contemporary PCI strategy in the SYNTAX II study had significantly lower rate of major adverse cardiac and cerebrovascular events at two years than patients in the SYNTAX I study (A). The major adverse cardiac and cerebrovascular events at two years in the SYNTAX II population treated with PCI was not different from the SYNTAX I population treated with CABG (B). There was no difference in the rate of major adverse cardiac and cerebrovascular events at two years in the SYNTAX II population with anatomic SYNTAX score >22 versus ≤22 (C). The rate of major adverse cardiac and cerebrovascular events at two years was not different between diabetic and non-diabetic patients with multivessel disease treated with contemporary PCI strategy (D).
As a substudy, 77 patients of the SYNTAX II trial (otherwise including a total of 454 multivessel disease patients) were scanned using multislice computed tomography (MSCT), and their anatomic SYNTAX score and FFR derived from computed tomography angiography (FFRCT) were analysed30. Following the encouraging results of this substudy and with the support of an unrestricted grant from General Electric Healthcare and HeartFlow, it was hypothesised that the SYNTAX score III (anatomy, plus functionality, plus comorbidity) would be the next step in the attempt to introduce more “precision medicine” in the field of myocardial revascularisation selecting the best treatment for our patients with three-vessel disease.
IN 2013, THE SYNTAX SCORE BECAME NON-INVASIVE AND WAS TESTED IN THE SYNTAX III REVOLUTION TRIAL
In a JACC Cardiovascular Imaging issue in 2013, the application and adaptation of the anatomic SYNTAX score to MSCT imaging was described for the first time31.
The last chapter of the story has indeed been written in the era of the MSCT scan with the emergence of the assessment of FFRCT to generate (in our jargon and scientific publication) the SYNTAX score III31,32.
The SYNTAX III Revolution trial is the first trial randomising two Heart Teams; evidence level C is so far non-existing since the benefit of the Heart Team conference has never been scientifically tested. In the trial, enrolling only patients known as having three-vessel disease diagnosed by conventional angiography, the agreement on treatment decision (surgery or percutaneous coronary intervention [PCI]) of two Heart Teams who received –in order to make their decision– either a conventional cineangiography or an MSCT scan of cases with three vessels with or without left main disease was evaluated and tested (Figure 6),32. The decision making of “CABG only”, “PCI only” or “equipoise CABG/PCI” was concordant between the two Heart Teams in 86% with a Cohen’s kappa of 0.82, qualifying the agreement as almost perfect according to the statistical Cohen’s kappa categorisation32. This was a virtual trial since, after signing off the decision making, both Heart Teams were unblinded so that they had all the information available prior to the real clinical treatment either in the interventional suite or in the operating room.
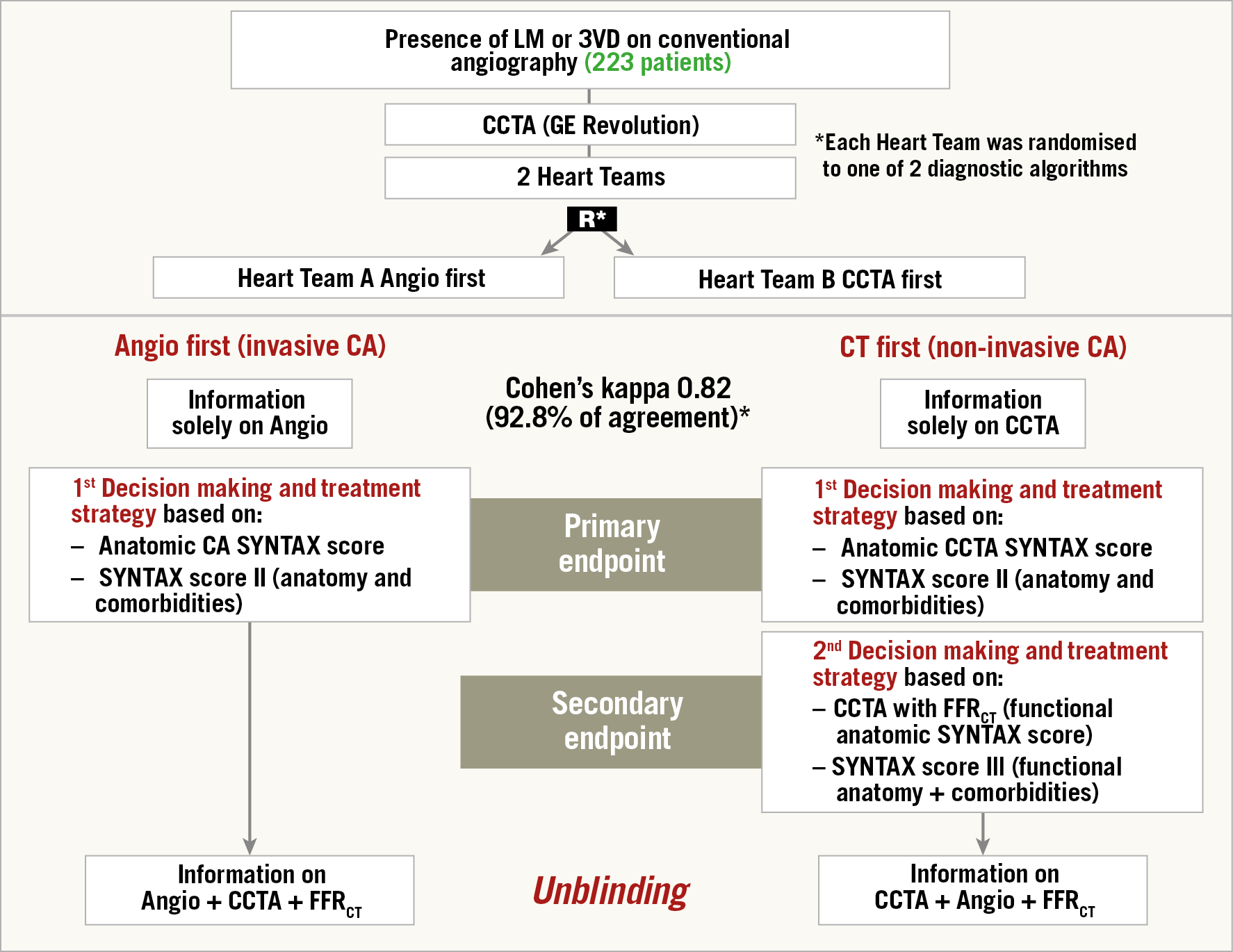
Figure 6. Design, flow diagram and main result of the SYNTAX III Revolution study. Heart Teams agreed on the coronary segments to be revascularised in 81.1%. FFRCT changed the treatment decision in 7% (14/196) of the patients. In 13 patients the surgical procedure was changed to a percutaneous approach.
During the conduct of the trial, every Wednesday evening at 6 pm –together with the surgeon, interventional cardiologist and radiologist– a video conference review session of the cases which had been signed off by the two Heart Teams was held33. The format of the presentation was always identical and extensively illustrated by maximum intensity projection and multiplanar reconstruction. For the surgeons, unaware of the diagnostic capabilities of the MSCT scan for coronary artery assessment and unacquainted of the existence of the colour-coded FFRCT, it was a moment of discovery and … ecstasy. During the long course of the trial (20 months), they started to express the following opinions: according to them, it would be possible, feasible, reasonable and safe to plan and execute surgery with the sole guidance of the MSCT scan. The radiologists and interventional cardiologists allowed them to elaborate freely on the topic but, at the very end of the SYNTAX III Revolution trial, decided to challenge them34,35. “Would you really be willing to perform surgery without prior cineangiography, using MSCT as the sole guidance of coronary grafting?” Indeed, the concept had to be tested first in a theoretical feasibility survey before attempting the real thing … and therefore the five surgeons of the SYNTAX III Revolution trial were invited to participate in a review of 20 MSCT scans of 20 patients who had indeed been operated previously by them during the course of the SYNTAX III Revolution trial. Each surgeon had to declare for himself whether the planning and the execution of the surgery would be feasible... and safe with, as guidance, the sole anatomic and functional assessment of the MSCT scan. The results of this “live survey” were quite impressive35. Eighty-four percent of the cases would be eligible for surgery without the preview of conventional angiography. Considering their “mature and responsible enthusiasm” (sic), the “real thing” was proposed to them –the first-in-man proof of concept, a feasibility and safety trial called CABG Revolution in 100 patients. In this upcoming trial, the surgeons will not have access to the conventional cineangiography before operation. The outcome of their bypass surgery will be assessed by MSCT scan 30 days following bypass surgery in order to judge the patency and correct anatomic location of their anastomoses. The CABG Revolution trial is due to start and, if it appears that the policy of surgical treatment without prior cineangiography guided solely by an MSCT scan is feasible and safe, then a major paradigm shift could be envisioned.
THE UNEXPECTED IMPACT OF THE NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE (NICE) IN THE UNITED KINGDOM ON THE ROLE OF FFRCT
The role of FFRCT in the chest pain diagnostic pathway was recommended by NICE in a publication dated February 2017. The best way to describe the possible major changes in the diagnostic approach of patients with new-onset chest pain is to examine Figure 7,36 and Figure 8 carefully. Normally, a patient with new-onset chest pain would be quickly interviewed on his/her case history, his/her risk profile, and would be submitted for physical examination, and electrocardiography (ECG) at rest would be performed with assessment of cardiac biomarkers, sometimes accompanied by an accessory chest film. The presence of ST-segment elevation would be the determinant reason to rush to the cath lab in the world of emergency care that can save a life by performing primary PCI. Whenever the ECG is non-diagnostic with normal troponin, patients with “stable angina” enter the world of non-invasive stress testing (stress ECG, stress echocardiography, adenosine single-photon emission computed tomography, positron emission tomography, Holter monitor for ST-segment analysis and so on…). Then the physicians wait for the results, postpone the decision and hesitate to move to coronary angiography since catheterisation means crossing a line to invasiveness. According to European and American statistical data, 40 to 60% of coronary angiography will reveal the absence of obstructive coronary disease which is far from being a benign syndrome37. In the lab, a second line of invasiveness will have to be “trespassed” by performing an ad hoc intervention, searching for the optimal angiographic view in which a diameter stenosis of more than 50% is visualised. In the case of complex three-vessel disease with or without left main stem lesion, patients will be referred to surgery or at least discussed by a Heart Team. Of note, the presence of troponin I or T was a major argument to keep the patient in the hospital and an effective argument to perform conventional cineangiography. Today, even patients with high-sensitivity troponin are first treated aggressively by specific and potent P2Y12 inhibitors and then deferred in their access to the cath lab. The United Kingdom guidance stated that CCTA would be the first line of diagnosis. “Based on the current evidence and assuming there is access to appropriate CCTA facilities, using HeartFlow FFRCT may lead to cost savings of £214 per patient. By adopting this technology, the NHS in England may save a minimum of £9.1 million by 2022 through avoiding invasive investigation and treatment”. The future vision of diagnosing patients with new-onset chest pain would indeed be the use of cardiac CT as a “one-stop shop” that would increase the quality and effectiveness of the diagnosis. Using cardiac CT as a one-stop shop would include CT angiography, perfusion, ejection fraction, wall motion analysis, wall thickness, FFRCT, SYNTAX score and calcification mapping, allowing a complete and detailed pre-planning of coronary intervention without prior conventional cineangiography. In other words, based on the SYNTAX score III, only one line of invasiveness would have to be crossed prior to therapy since the decision making based on non-invasive imaging using the SYNTAX score III would allow a careful dispatching of the patients towards either the interventional suite or the operating room of the surgeon. As mentioned above, carefully planned coronary intervention based on pre-existing information would certainly promote and improve the quality of our percutaneous interventional treatment.
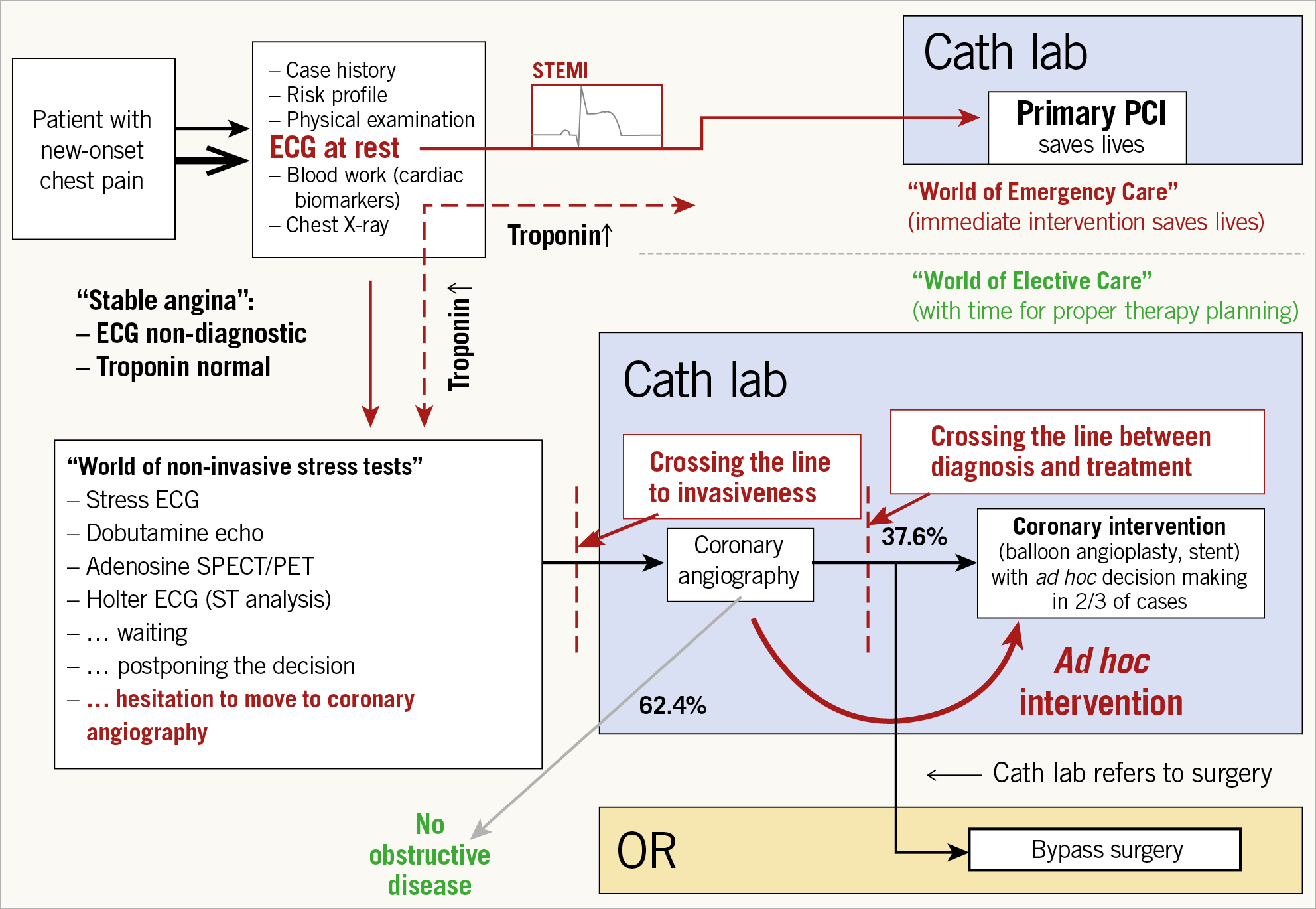
Figure 7. Chest pain diagnostic and treatment pathway.
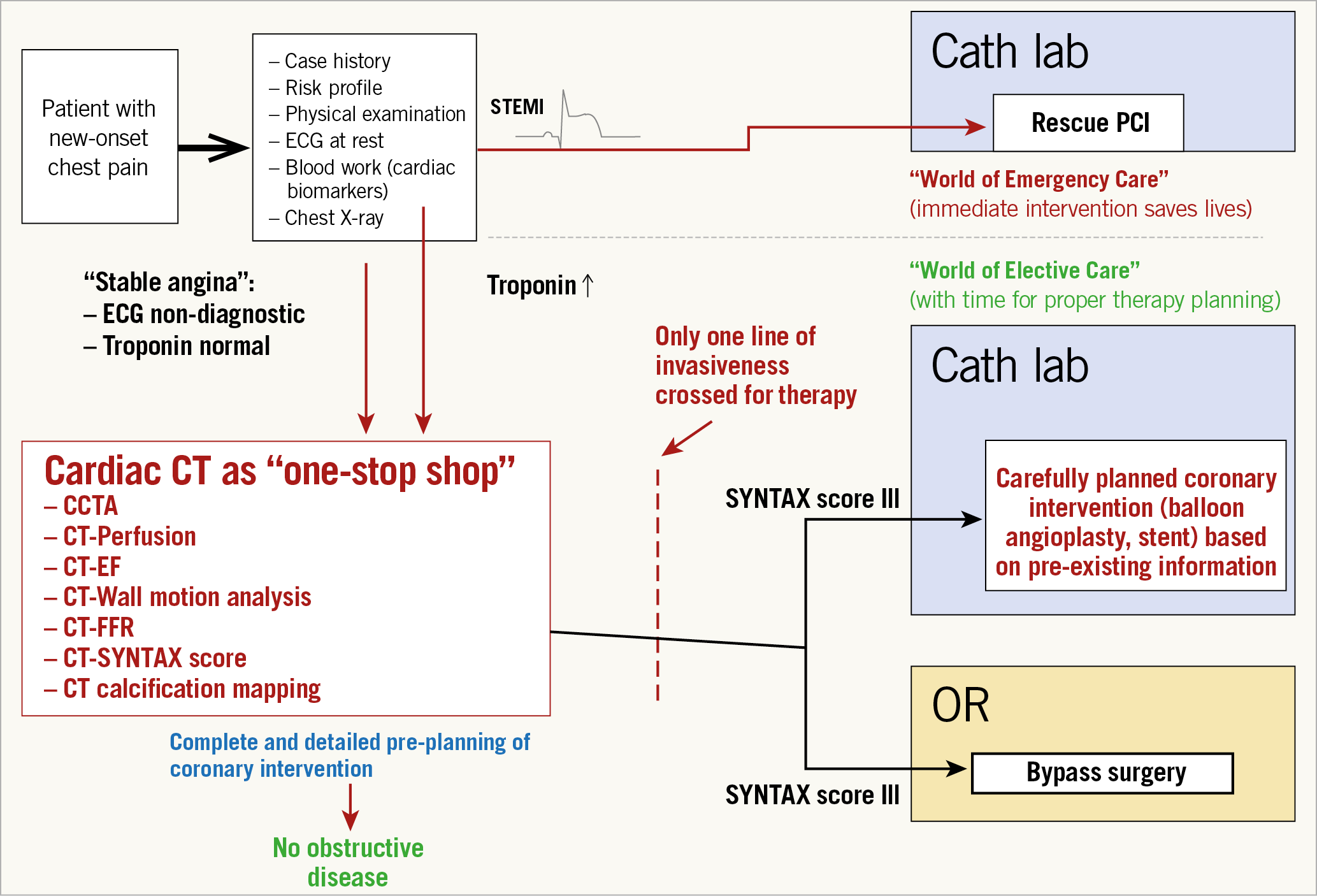
Figure 8. Future vision on cardiac computed tomography as a one-stop shop.
In the European Heart Journal, academic physicians and practitioners have opened a debate by raising the following question: should NICE guidelines be universally accepted for the evaluation of stable coronary disease38? Conversely, journalists and reporters have also raised the question of the role of CT angiography in stable chest pain “as either irrational exuberance or evidence-based medicine and they came to the inconclusive statement that some do not think a class I recommendation is relevant while others said CT angiography has consistently been shown to be superior to stress testing” (Michael O’Riordan, 11 January 2019, TCTMD)39. For those interested in estimated annual savings by putting NICE guidance into practice, Figure 9 is included showing the savings from five years onwards.
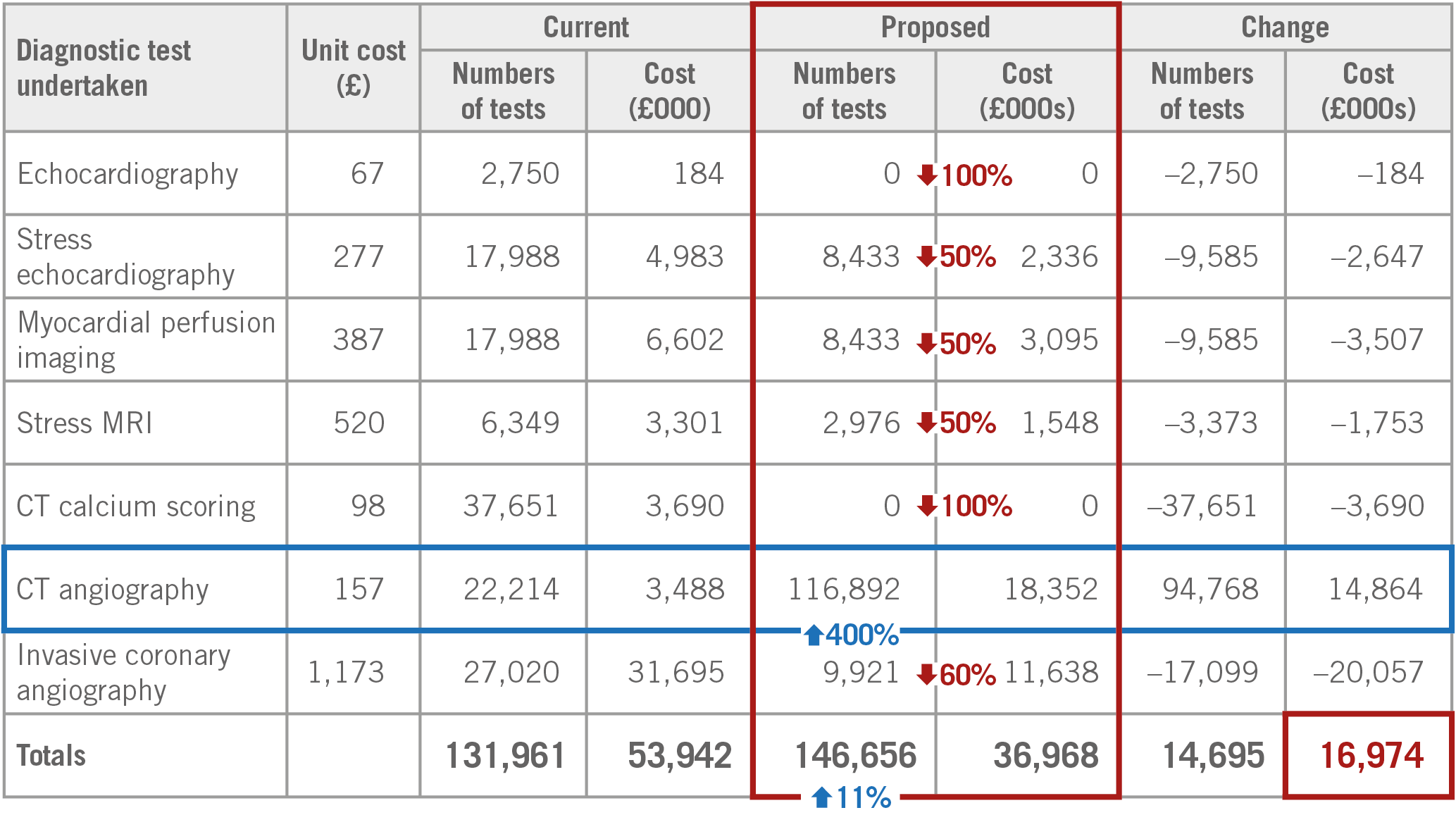
Figure 9. Estimated annual saving of implementing the NICE chest pain diagnostic pathway from year five onwards.
SYNTAX SCORE III: TOWARDS ARTIFICIAL INTELLIGENCE
One of the major limitations of the anatomic SYNTAX score lies in its 2D cine-fluoroscopic nature that is less than ideal for meeting the complexity of fractal tree subdivision and is associated with intrinsic difficulties to assess the severity of the stenosis, the length of the lesion, the diffuse character of lumen reduction, the tortuosity of the target vessel, the single, double or triple involvement of a bifurcation and the degree of calcification by fluoroscopy, etc. –in brief, all of the individual components of the anatomic SYNTAX score. The result is a lack of strong reproducibility due to subjective assessments of multiple observers40,41. Multiple attempts to simplify the score (by eminent statisticians) would have resulted in the loss of its prognostic value. The future of the score is not in its simplification… or discrediting it on the grounds of non-reproducibility or irrelevance from a physiologic point of view; its future resides in an automatic, highly reproducible assessment made objectively by intelligent, computerised algorithms using the power of machine learning for analysing a non-invasive, 3D image MSCT that has incorporated the physiologic characteristics of the whole coronary tree.
What next? Let us fantasise about the future. It is clear and evident that the sole MSCT guidance to plan and execute surgery in three-vessel disease is the ultimate challenge and constitutes the top of the hierarchical pyramid in the diagnostic and treatment guidance of our cardiovascular patients (Figure 10). If this strategy is safely achieved, then the MSCT with FFRCT in other patients with one- or two-vessel disease referred to the interventional suite will become the default diagnostic tool and planning strategy prior to PCI. For us interventional cardiologists, to know in advance, even before entering the interventional suite, the angiographic view in which the lesion is best visualised, the amount and localisation of calcium, the need and technique for treatment of a bifurcation lesion, the existence and characteristics of a chronic total occlusion (CTO), the length of the lesion, the 3D assessment of vessel tortuosity, etc., would be a tremendous diagnostic asset, giving us the opportunity to plan and prepare a rotablator, an instantaneous wave-free ratio, to alert our expert in CTO, to plan a two-stent strategy for a bifurcation lesion, to decide on a staged procedure, etc.
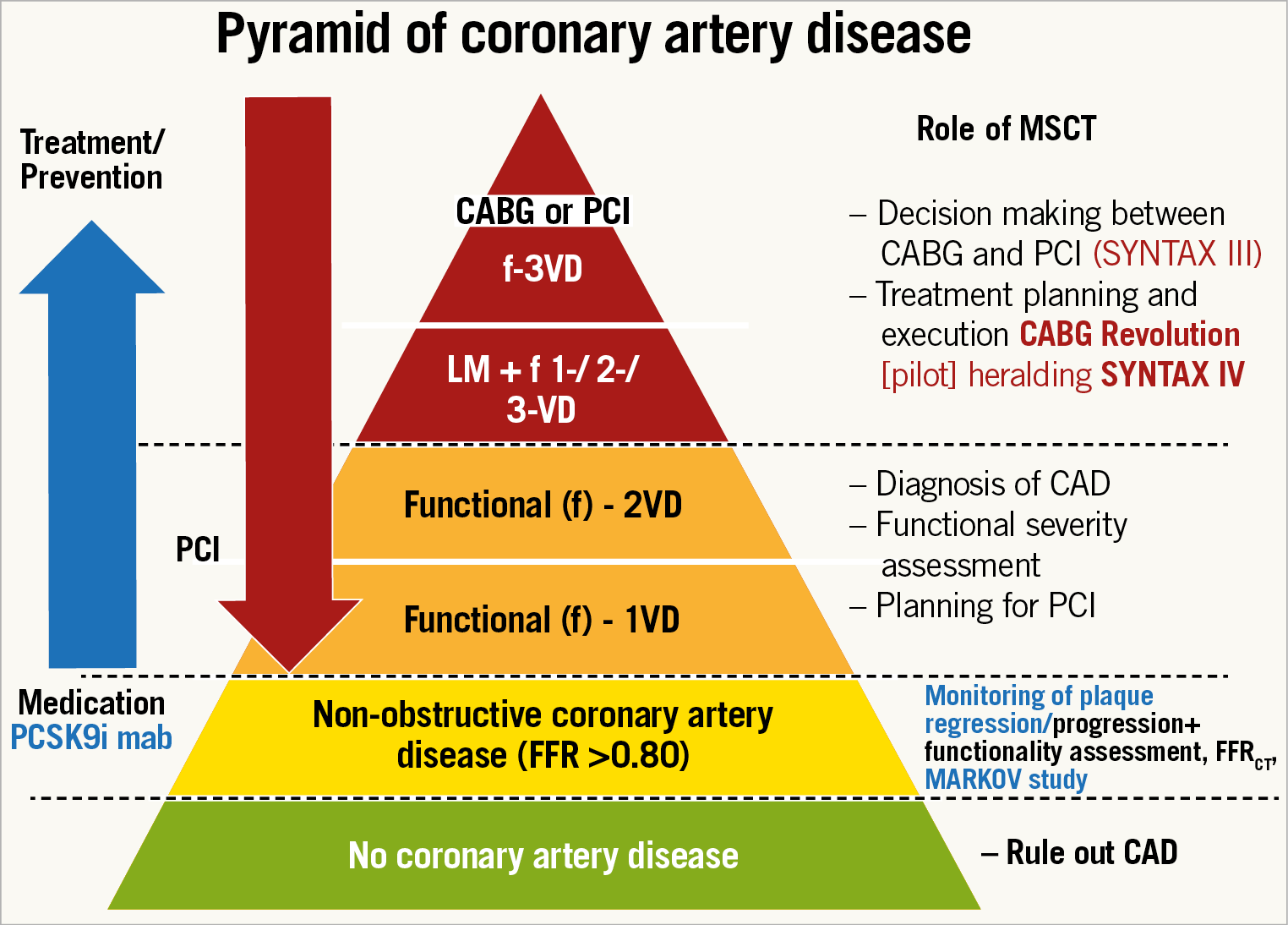
Figure 10. Role of the multislice computed tomography scan in different degrees of coronary artery disease. From the bottom of the pyramid up to its top: (1) in green, the patients without any obstruction of the coronary lumen with CAD ruled out by MSCT. (2) In yellow, those patients with mild anatomic obstruction of the coronary artery/arteries (<50%) without functional compromise of the coronary flow (FFR >0.80) – those subjects could benefit from pharmacological treatment, in particular from PCSK9 inhibitors (MARKOV study – NCT03851263). (3) In orange, patients with functionally significant 1- or 2-vessel disease, who may be referred to PCI based on the MSCT findings; and (4) in red, those patients with complex coronary artery disease (LM and/or 3-vessel disease) who can have the revascularisation strategy planned with the anatomic and functional results from the MSCT and could have the treatment executed based solely on this non-invasive information (CABG Revolution and SYNTAX IV). CABG: coronary artery bypass grafting; CAD: coronary artery disease; FFR: fractional flow reserve; MSCT: multislice computed tomography; PCI: percutaneous coronary intervention; PCSK9i: PCSK9 inhibitors; VD: vessel disease
In addition to an automatic and reproducible SYNTAX score III, the HeartFlow technology has focused on the 3D determination of virtual FFR in a colour-coded fashion along the entire coronary tree. Beyond this simple output, there is a complex analysis of shear stress analysis based on the Navier-Stokes equations and many other haemodynamic assumptions legally protected by an impressive number of software patents42. The good news is that all the parameters can be identified by machine learning and expressed in a metric approach. Today, the segmentation of the coronary tree according to the revised AHA nomenclature is provided by a deep learning algorithm with an accuracy of 97%. In addition, the HeartFlow technology offers the possibility to anticipate the physiological benefit of PCI thanks to an interactive planning program that makes possible the selection of one or multiple stents with a clear indication of the total stent length needed to normalise as much as possible the conductance of the major epicardial vessel (Figure 11),34.
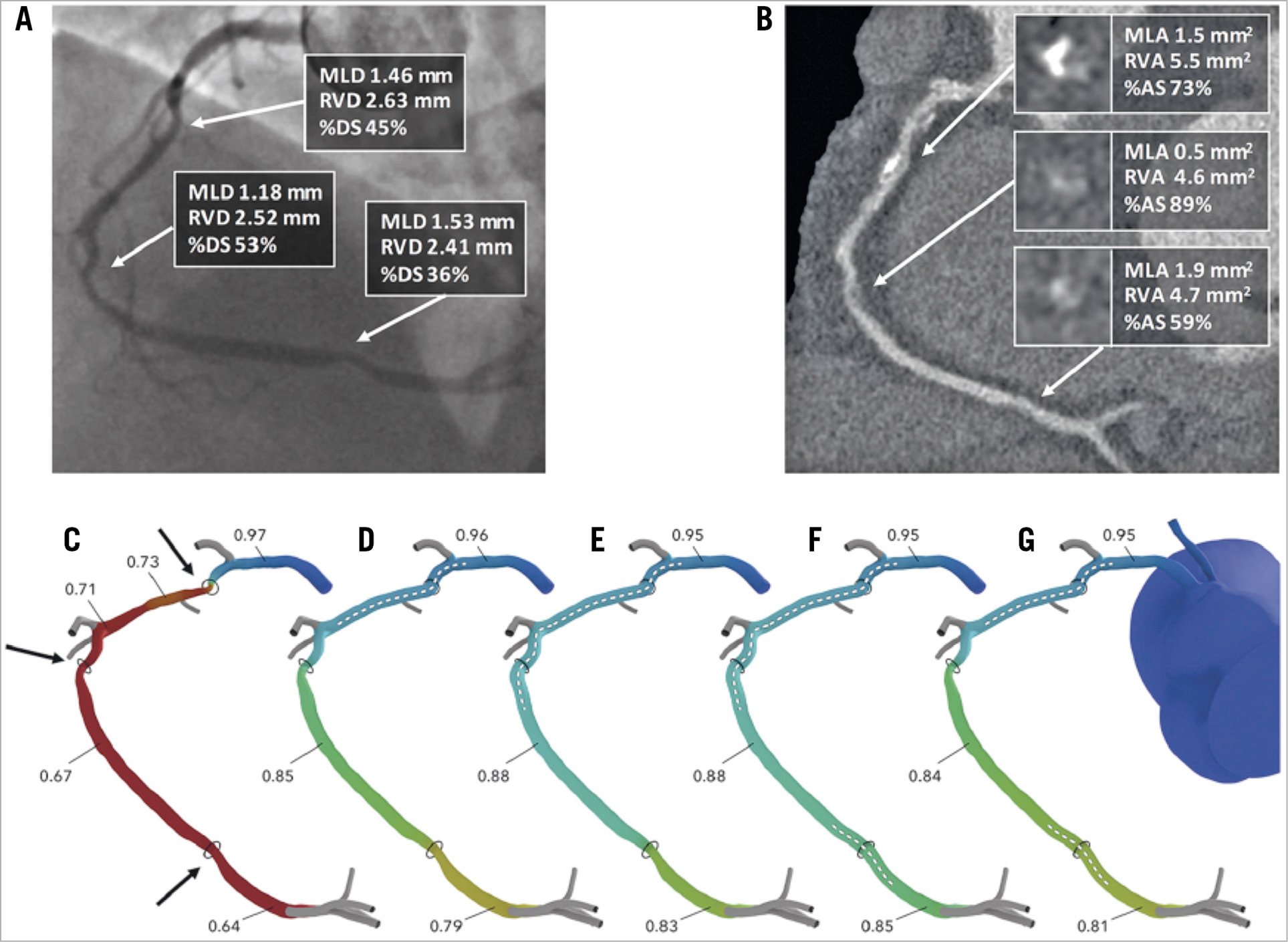
Figure 11. Assessment of PCI planner. A) Coronary angiography of right coronary artery. B) MSCT image of right coronary artery. C) Assessment of FFRCT. Target lesions are indicated by arrows. D) - G) Assessment of PCI planner for each PCI plan. Stent location marked by dotted line. AS: area stenosis; DS: diameter stenosis; MLA: minimum lumen area; MLD: minimum lumen diameter; MSCT: multislice computed tomography; PCI: percutaneous coronary intervention; RVA: reference vessel area; RVD: reference vessel diameter. Reprinted from Sonck et al34, with permission from Europa Digital & Publishing.
RISK SCORES AND ARTIFICIAL INTELLIGENCE
It is already obvious that a large collection of medical data stemming from multiple sources such as imaging, ECG, biomarkers, etc., and handled by machine learning will increase the speed and accuracy of diagnosis and recommendation of treatment. Telemedicine with automatic assessment of scores recommending specific treatment will be followed in a substantial fashion by a Heart Team that will inform the patients properly regarding the risks and benefits of the selected treatment, whenever the 95% confidence interval establishes the robustness of the medical decision43. Undoubtedly, statistical probability will be shared more and more with the patients. Physicians will use smartphones equipped with specialised applications connected by telemedicine to central organisations specialised in the treatment and handling of medical data. 3D animated descriptions of diseases, treatments and prognosis will become highly educational and understandable for the patient who is, by definition, a layman otherwise lost in this highly sophisticated field of medicine. As human beings, we will be confronted with precision medicine but, as emphasised in the book of Eric Topol “Deep Medicine”44, empathy and human dialogue with our patients will regain its central position and role in our relationship with our comrade patient. Machine learning and artificial intelligence will eventually have to discharge “the doctor” who is simply another human being, from all the long-lasting, tedious, cumbersome, mechanical, administrative tasks and functions that can be done much faster and much more accurately by machine learning, thereby restoring, at the highest level, the therapeutic relationship with our patient (therapeia, curing and healing in Greek) that we all need so intensively... and that is our raison d’être as medical doctors.
FROM SYNTAX SCORE TO EXCEL SCORE…?
Over the last ten years, the evolution of stent technology (from TAXUS to XIENCE), adjunctive therapy, operator’s skill, optimisation of the diagnosis and immediate assessment of the results (angiographically derived FFR, FFR, intravascular ultrasonography) have certainly improved the PCI outcomes of left main and three-vessel disease. Of note, our surgical colleagues have also improved the outcome of their treatment45. Therefore, other prognostic factors may be identified and their relative impact on all-cause mortality may change (Figure 12). So far, the classic illustration published in The Lancet has been revisited and important changes or disappearance of interaction have occurred that will have to be taken into account. Therefore, far from being declared obsolete or outdated, the SYNTAX score is more than ever alive and kicking –anatomy (SYNTAX score I) and physiology (functional SYNTAX score) of the human heart are permanent– but stent technology, evolving comorbidities of the patients and adjunctive pharmacological treatment periprocedurally and post-procedurally have changed and will change even further; the score has to be revitalised and revisited by the EXCEL trial analysis and will enter the era of artificial intelligence.
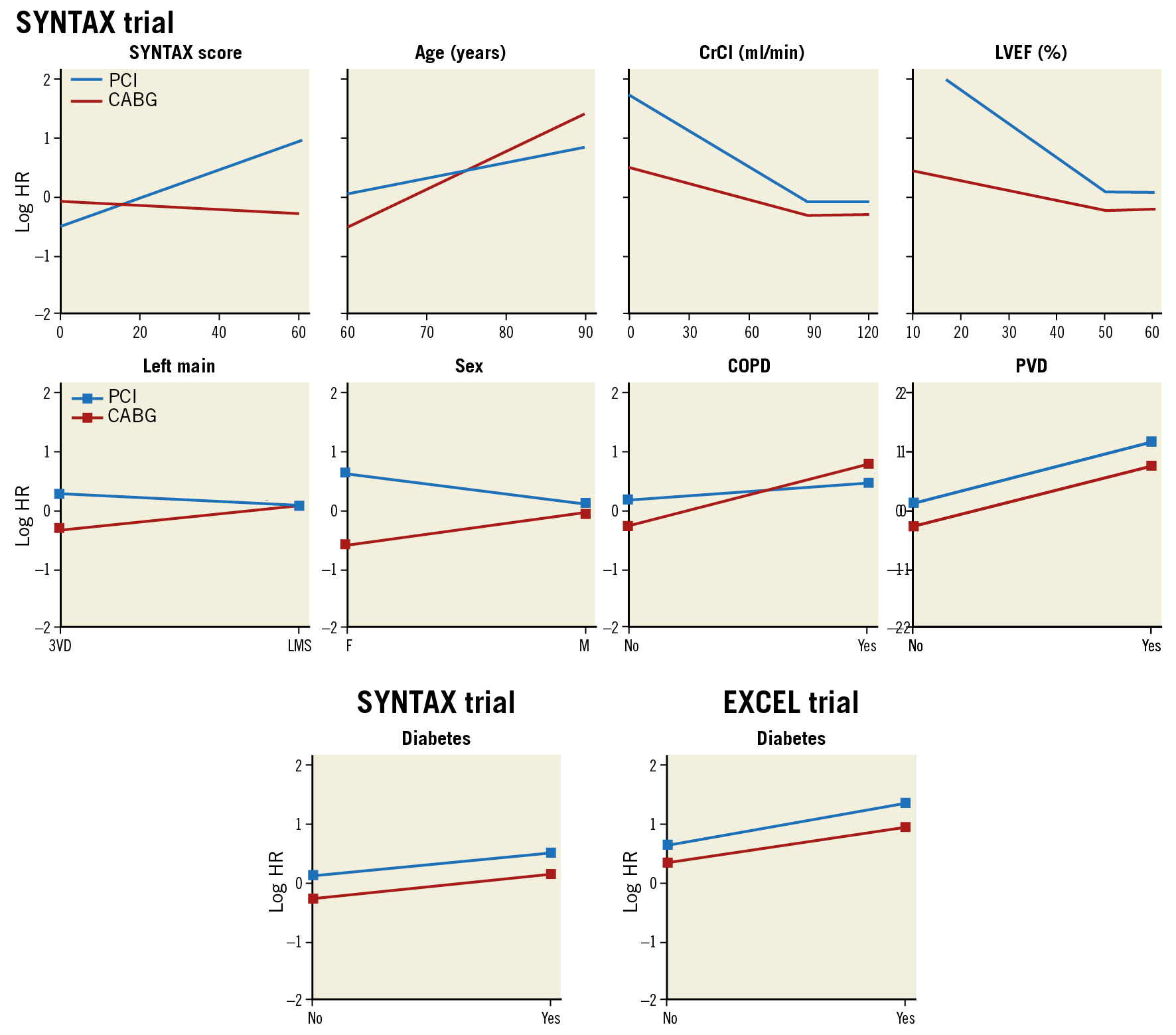
Figure 12. Predictor effects for CABG and PCI in SYNTAX score II in the SYNTAX trial and in the EXCEL trial. Predictor effects in the SYNTAX study are represented visually as a log HR for CABG and PCI on the y-axis for each predictor. Each predictor is expressed on the x-axis continuously or categorically, for a person of mean baseline characteristics. Note the differing gradients of the hazards for PCI and CABG in the SYNTAX trial, leading to the hazards crossing at an anatomic SYNTAX score of 15. At this crossover point of hazards, the mortality risk is much the same between CABG and PCI. This threshold of crossover of hazards will vary according to the level of other variables, namely being lower for female sex, reduced LVEF, and younger age, and higher for COPD, left main disease, and older age. Diabetes is included in the analysis to show its absence of interaction when included in the analyses. Similar methodological approach to the EXCEL trial, e.g., showing the absence of interaction between diabetes and revascularisation strategy. CABG: coronary artery bypass grafting. COPD: chronic obstructive pulmonary disease; CrCl: creatinine clearance; HR: hazard ratio; Left main: unprotected left main coronary artery disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; PVD: peripheral vascular disease; 3VD: three-vessel disease. Adapted from Farooq et al21, with permission.
WHAT GAPS MIGHT THE EXCEL SCORE FILL?
The SYNTAX II score had robust accuracy in both the CABG and PCI groups in the real world of a complex CAD population in the CREDO-Kyoto registry46. In a pooled cohort of patients in the BEST and PRECOMBAT randomised controlled trials, the SYNTAX score II had a good calibration but only moderate discrimination ability for long-term mortality prediction47.
On the one hand, the improved outcomes of PCI in patients with multivessel disease without left main but with the use of a contemporary PCI strategy have demonstrated essential progress in outcomes in the SYNTAX II trial27. On the other hand, significant improvement in prognosis after surgical revascularisation of the left main has been demonstrated between SYNTAX left main and the EXCEL trial, consistent with improved outcomes of cardiac surgery over time45. In that respect, the accuracy of the SYNTAX score II may no longer be up to date with the contemporary revascularisation and the recent guideline issue raised by the ESC48. The ESC guideline on myocardial revascularisation has indicated the issues in which the current scores are limited in their use for risk assessment in practice (Table 1).
The EXCEL score will be developed to predict the treatment benefit of PCI versus CABG in the setting of left main treatment associated with multivessel disease, i.e., the prediction of the risk difference between treatment assignment to CABG and PCI using conventional and modern statistical approaches, such as maximum likelihood (Cox regression) and penalised maximum likelihood (Lasso regression). The score will be based on the SYNTAX score II model with the possibility of incorporating potential predictors for long-term outcomes after CABG or PCI such as atrial fibrillation, brain natriuretic peptide (BNP), white blood cell count and haemoglobin level, etc.
The SYNTAX score II was developed to predict four-year all-cause mortality for PCI and CABG. Although all-cause mortality is a hard endpoint of cardiovascular disease and needs no adjudication, other relevant morbidity endpoints may be important for the score to predict. This is one of the limitations of the risk scores acknowledged by the 2018 ESC guideline on myocardial revascularisation48. Therefore, in the EXCEL score, separate models will be developed for the outcomes: (1) all-cause mortality; (2) death, stroke or myocardial infarction (primary endpoint of the EXCEL trial); and (3) death, stroke, myocardial infarction, or ischaemia-driven revascularisation (secondary endpoint of the EXCEL trial).
We believe that the EXCEL score will fill the gap of evidence that has evolved since the completion of the SYNTAX trial. In addition, the score will be developed using modern statistical approaches and will reflect the relevant mortality and morbidity outcomes observed in current practice.
Appendix. Authors’ affiliations
1. NHLI, Imperial College London, London, United Kingdom; 2. Amsterdam UMC, University of Amsterdam, Heart Center, Department of Clinical and Experimental Cardiology, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands; 3. Division of Cardiology, Department of Internal Medicine, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand; 4. Department of Internal Medicine, Cardiology Division, University of Campinas (UNICAMP), Campinas, Brazil; 5. Milton Hershey Medical Center, Penn State Heart and Vascular Institute, Hershey, PA, USA; 6. Department of Radiology, Division of Image Processing, Leiden University Medical Center, Leiden, the Netherlands; 7. Astra Charnwood Clinical R & D, Loughborough, United Kingdom; 8. Division of Structural Interventional Cardiology, Careggi University Hospital, Florence, Italy; 9. Department of Cardiology, Catharina Hospital, Eindhoven, the Netherlands; 10. Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven, the Netherlands; 11. Cardialysis BV, Rotterdam, the Netherlands; 12. Department of Cardiology, Bern University Hospital, Bern, Switzerland; 13. University Hospital of Wales, Cardiff, United Kingdom; 14. Department of Public Health, Erasmus University Medical Center, Rotterdam, the Netherlands; 15. Division of Cardiology, Cardio-Thoracic-Vascular Department, Azienda Ospedaliero Universitaria “Policlinico-Vittorio Emanuele”, Catania, Italy; 16. Centro Cardiologico Monzino, IRCCS, Milan, Italy; 17. Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy; 18. Department of Cardiology, Barts Heart Centre, Barts Health NHS Trust, London, United Kingdom; 19. Oxford Heart Centre, Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom; 20. Department of Cardiology, Hospital Clinico San Carlos, Madrid, Spain; 21. HeartFlow, Redwood City, CA, USA; 22. GE Healthcare, Waukesha, WI, USA; 23. Cardiovascular Center Aalst, OLV Clinic, Aalst, Belgium; 24. Department of Biomedical and Clinical Sciences “Luigi Sacco”, University of Milan, Milan, Italy; 25. Biomedical Image Analysis Group, Department of Computing, Imperial College London, London, United Kingdom; 26. Cardiovascular Research Foundation, New York, NY, USA; 27. The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York and the Cardiovascular Research Foundation, New York, NY, USA; 28. Department of Cardiology, Thoraxcenter, Erasmus Medical Center, Rotterdam, the Netherlands.
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Acknowledgements
We wish to acknowledge all those who contributed to the SYNTAX score.
Conflict of interest statement
P.W. Serruys reports personal fees from Abbott Laboratories, AstraZeneca, Biotronik, Cardialysis, GLG Research, Medtronic, Sino Medical Sciences Technology, Société Europa Digital & Publishing, Stentys France, Svelte Medical Systems, Philips/Volcano, St. Jude Medical, Qualimed, and Xeltis, outside the submitted work. P. Chichareon reports a research grant from Biosensors outside the submitted work. R. Modolo has received research grants from Biosensors outside the submitted work and from the Sao Paulo Research Foundation (FAPESP – grant number 2017/22013-8). N. Pijls has received institutional research grants from Abbott, Hexacath and HeartFlow, is a consultant for Abbott, Opsens, and GE Healthcare, and possesses equity in Philips, HeartFlow, and ASML. M. Valgimigli reports grants and personal fees from Abbott, Terumo and AstraZeneca, personal fees from Chiesi, Bayer, Daiichi Sankyo, Amgen, Alvimedica, Idorsia, Biosensors, CoreFlow, Vifor and Bristol-Myers Squibb, and grants from Medicure, outside the submitted work. J. Davies reports grants, personal fees and other from Philips Volcano, outside the submitted work; in addition, J. Davies has a patent iFR with royalties paid by Philips Volcano to Imperial College London. A. Banning is partially supported by the NIHR Oxford Biomedical Research Centre and reports grants from Boston Scientific and personal fees from Boston Scientific, Medtronic and Abbott Vascular, during the conduct of the study. J.J. Piek reports non-financial support from Abbott Vascular and personal fees and non-financial support from Philips/Volcano, outside the submitted work. B. Thomsen is an employee of GE Healthcare. B. Glocker reports grants from HeartFlow, and the European Commission, during the conduct of the study, personal fees from Kheiron Medical Technologies, Definiens, Axon Advisors, and GlaxoSmithKline, grants from UKRI, EPSRC, Innovate UK, and MRC/NIHR, outside the submitted work. C. Collet reports grants and personal fees from HeartFlow Inc, and personal fees from Philips and Abbott Vascular, outside the submitted work. G.W. Stone reports personal fees from Terumo, Amaranth, Shockwave, Valfix, TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Matrizyme, Miracor, Neovasc, V-wave, Abiomed, Claret, Backbeat, Sirtex and MAIA Pharmaceuticals, personal fees and other from Ancora, Qool Therapeutics and SpectraWave, other from Cagent, Applied Therapeutics, Biostar family of funds, and MedFocus family of funds, outside the submitted work; Columbia University, his employer, receives royalties from Abbott for sales of the MitraClip. Y. Onuma reports being a member of advisory board of Abbott Vascular. C. Taylor reports being a shareholder in and an employee of HeartFlow. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.

