Abstract
Aims: Coronary artery bypass graft (CABG) surgery is the standard of care for revascularisation of patients with left main coronary artery disease (LMCAD). Recent studies have suggested that percutaneous coronary intervention (PCI) with drug-eluting stents (DES) may provide comparable outcomes in selected patients with LMCAD without extensive CAD. We therefore designed a trial to investigate whether PCI with XIENCE cobalt-chromium everolimus-eluting stents (CoCr-EES) would result in non-inferior or superior clinical outcomes to CABG in selected patients with LMCAD.
Methods and results: The Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial is a prospective, open-label, multicentre, international study of 1,900 randomised subjects. Patients with significant LMCAD with a SYNTAX score ≤32 and local Heart Team consensus that the subject is appropriate for revascularisation by both PCI and CABG are consented and randomised 1:1 to undergo PCI using CoCr-EES or CABG. All patients undergo follow-up for five years. The primary endpoint is the three-year composite rate of death, stroke or myocardial infarction, assessed at a median follow-up of at least three years (with at least two-year follow-up in all patients), powered for sequential non-inferiority and superiority testing.
Conclusions: The EXCEL study will define the contemporary roles of CABG and PCI using XIENCE CoCr-EES in patients with LMCAD disease with low and intermediate SYNTAX scores.
Background and rationale
The left main (LM) stem of the coronary arterial tree is of vital importance as it supplies at least 70% of the myocardium of the left ventricle. Stenosis of the LM is one of the few specific coronary artery lesions in which coronary artery bypass graft (CABG) surgery definitively reduces the likelihood of death compared with medical therapy1-3. CABG has remained the gold standard for treatment of LMCAD for more than 30 years, and is recognised as such in international guidelines and appropriate use criteria4-6. Percutaneous coronary intervention (PCI) was developed almost 10 years later than CABG, and was initially applied principally in patients with single-vessel CAD, including the LM7. However, acute closure or late restenosis of the LM after balloon angioplasty was not infrequent and, given the amount of myocardium supplied, often resulted in large myocardial infarction (MI) or sudden death8. Moreover, LM disease frequently affects the distal bifurcation of the main stem2, adding to the complexity of PCI in this location. Nonetheless, the results of PCI of the LM have steadily improved with advances in technology and adjunct pharmacology. Specifically, the introduction of bare metal stents and adenosine diphosphate (ADP) receptor antagonists lowered the incidence of abrupt vessel closure and restenosis compared to balloon angioplasty, although mortality at one to two-year follow-up was still high, ranging from 3% to 31%9. Drug-eluting stents (DES) further lowered the risk of restenosis and, with technique refinement, PCI for unprotected LMCAD has become a safe procedure, at least for selected patients and in experienced hands10.
In the Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (SYNTAX) trial11, PCI with first-generation paclitaxel-eluting stents (PES) was inferior to CABG for the endpoint of major adverse cardiac and cerebrovascular events (MACCE: death, MI, stroke, or unplanned repeat revascularisation) in patients with three-vessel or LM disease. Among the 1,800 randomised patients in SYNTAX, 705 had LM disease. In the LM subgroup, MACCE at five years occurred in 36.9% of patients assigned to PES and 31.0% of patients assigned to CABG (hazard ratio [HR] 1.23, 95% confidence interval [CI]: 0.95-1.59; p=0.12)12. Stroke was less frequent with PES (1.5% vs. 4.3%; HR 0.33, 95% CI: 0.12-0.92; p=0.03) but repeat revascularisation was more common (26.7% vs. 15.5%; HR 1.82, 95% CI: 1.28-2.57; p<0.001). There were no significant differences in the rates of all-cause death or MI between CABG and PES. However, for LM patients with a low or intermediate SYNTAX score (≤32), all-cause death at five years was lower after PES than CABG (7.9% vs. 15.1%, p=0.02), whereas death tended to be increased with PES compared to CABG in patients with high (≥33) SYNTAX scores (20.9% vs. 14.1%, p=0.11). These results must be interpreted cautiously as the SYNTAX data were drawn from relatively small subgroups from a negative randomised trial, and thus should be considered hypothesis-generating only6. Consistent findings were noted, however, from the 600-patient randomised PRECOMBAT trial, in which the five-year composite rate of major adverse cardiac events (death, MI or stroke) was not significantly different between first-generation sirolimus-eluting stents and CABG in LMCAD, although revascularisation was less with CABG13. Of note, the mean SYNTAX score of patients enrolled in PRECOMBAT was only 25, consistent with most patients being of low to moderate complexity. These trials suggest that PCI may be an effective and durable treatment option in patients with LMCAD and low to intermediate anatomic complexity, perhaps even preferred over CABG given the lower risk of stroke.
To achieve adequate power, the primary endpoint of the SYNTAX trial included repeat revascularisation in the primary endpoint. Not only was unplanned revascularisation the most frequently encountered endpoint, but it also has a lesser impact on patient wellbeing than mortality, stroke and myocardial infarction7. Although the relevance of mortality, MI and stroke as clinically important endpoints is widely accepted, no previous trial has been adequately powered to examine whether CABG has superior, comparable, or non-inferior rates of the composite endpoint of mortality, MI, or stroke compared to PCI in patients undergoing LM revascularisation.
Since SYNTAX was performed, newer-generation DES have been introduced which have a substantially improved safety and efficacy profile compared to PES. In particular, compared to PES the fluoropolymer-coated cobalt-chromium everolimus-eluting stent (CoCr-EES) has been shown to result in lower rates of stent thrombosis, MI, target lesion revascularisation and possibly mortality compared to PES14. Use of CoCr-EES may therefore further improve outcomes in patients with LMCAD undergoing PCI. Two non-randomised registries have demonstrated superior clinical results with CoCr-EES in LM disease compared to first-generation DES15,16. These results have prompted some investigators to question whether the SYNTAX trial results would have been substantially different with contemporary DES17. In addition, PCI techniques have evolved (e.g., strategies to manage the bifurcation, use of intravascular ultrasound [IVUS], optical coherence tomography [OCT] and fractional flow reserve [FFR]), and adjunct pharmacology has improved (e.g., bivalirudin and more potent ADP antagonists), which may also influence the results of PCI.
At the same time, surgical methods have evolved, and postoperative care has continued to improve. These changes have led to improved outcomes after CABG with reduced hospital morbidity and mortality18. Furthermore, with the increased use of multiple arterial grafts, and bilateral internal mammary artery grafts in particular, bypass graft occlusion rates have further declined, which may lead to improved survival and enhanced quality of life19.
Given the evolution in both PCI and CABG, emerging randomised trial and registry data suggesting that PCI may have a role in the treatment of selected patients with LMCAD, and the fact that no prior study has been adequately powered to examine the differences between these revascularisation modalities for death, MI or stroke, a contemporary randomised trial was warranted. We therefore designed the Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial.
EXCEL trial design
STUDY OBJECTIVES
The primary objective of EXCEL is to compare the safety and effectiveness of the XIENCE CoCr-EES (Abbott Vascular, Santa Clara, CA, USA) with CABG in patients with LMCAD and a SYNTAX score ≤32 (low and intermediate-risk anatomic complexity). The study is designed to evaluate whether treatment of the LM stenosis±other significant coronary lesions with CoCr-EES compared to CABG using contemporary techniques results in non-inferior or superior rates of the primary composite measure of all-cause mortality, myocardial infarction or stroke at a median follow-up of three years.
TRIAL DESIGN
The EXCEL trial, registered at www.clinicaltrials.gov (identifier: NCT01205776), is a prospective, international, open-label, multicentre trial enrolling 2,900 subjects at up to 165 centres. Following diagnostic angiography demonstrating significant LM disease and local Heart Team consensus that the subject is appropriate for revascularisation by both PCI and CABG, approximately 1,900 eligible subjects will be consented and randomised 1:1 to undergo PCI using XIENCE CoCr-EES (N~950) or CABG (N~950). Randomisation will be stratified by medically treated diabetes mellitus (diabetic vs. non-diabetic), SYNTAX score (<23 vs. ≥23), and centre. The inclusion and exclusion criteria for randomisation appear in Table 1. Follow-up for all randomised subjects will continue for five years with an option for additional follow-up to 10 years. A universal screen failure registry of 1,000 consecutive patients with LM disease not randomised will be used to characterise the generalisability of the trial results.
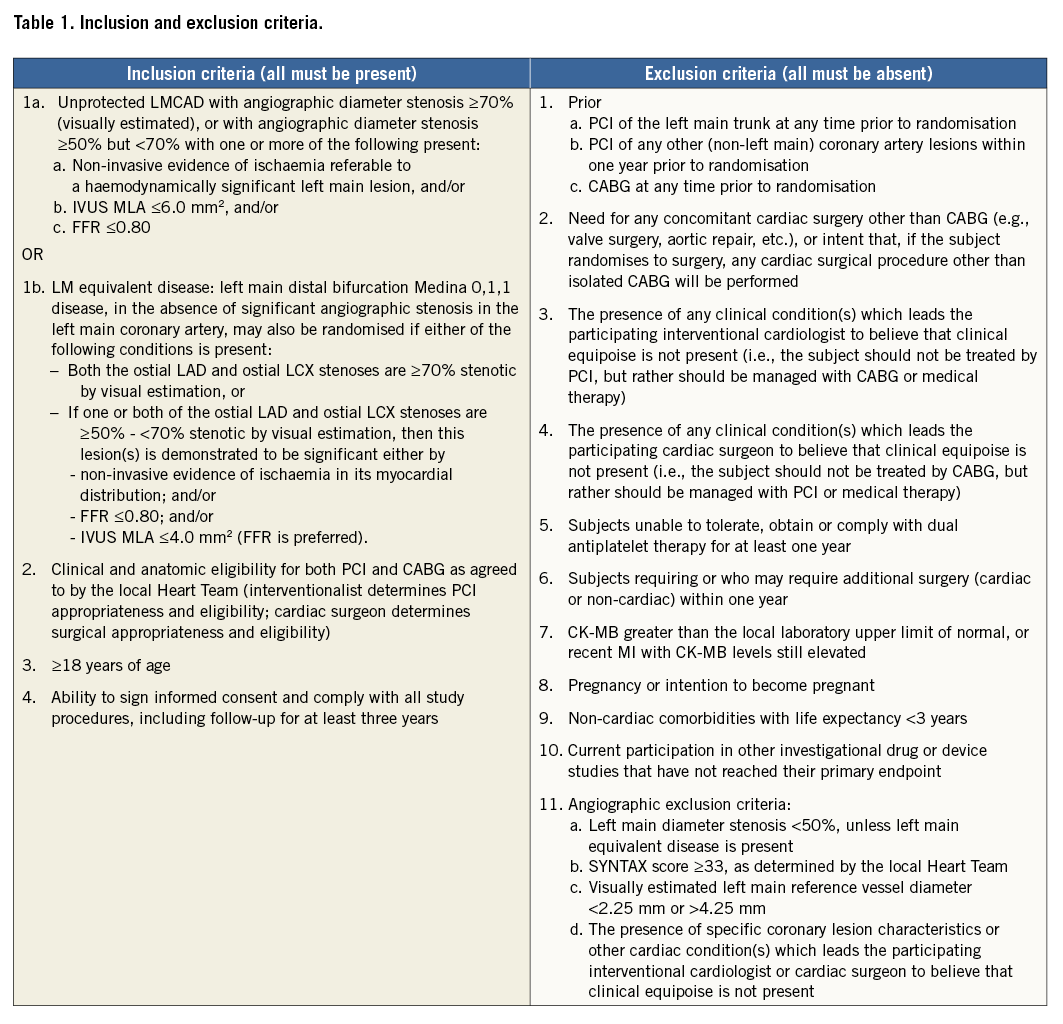
As the extent of CAD is more complex than just assigning the number of diseased vessels, Heart Team quantification of the coronary anatomy with the SYNTAX score is an essential part of the eligibility process20. Lesion location and characteristics are used to calculate the score, which is facilitated by using either a downloadable calculator or the SYNTAX score website (www.syntaxscore.com). As patients with LM disease in the highest SYNTAX score tertile have significantly worse outcomes with PCI5, only patients with less complex CAD, characterised by low and intermediate SYNTAX scores (≤32), as assessed by both the local interventional cardiologist and cardiac surgeon, will be eligible for randomisation. Acknowledging that local levels of experience may vary, prior to randomisation the interventional cardiologist must attest that the patient is an acceptable candidate for PCI using commercially available CoCr-EES, and the cardiac surgeon must attest that the patient is an acceptable candidate for CABG.
The LM stem is the most difficult coronary segment to characterise accurately by angiography, with the greatest degree of inter-observer variability21. A key principle of EXCEL is only to randomise clinically relevant LM stenoses. In prior studies, the prognosis of intermediate LM lesions with either an IVUS minimal luminal area (MLA) >6.0 mm2 or an FFR >0.80 was favourable with medical therapy alone22,23. Therefore, EXCEL inclusion criteria require an angiographic diameter stenosis of ≥70% or, if 50%-<70%, demonstration of either an MLA ≤6.0 mm2, an FFR ≤0.80, or non-invasive testing with evidence of ischaemia in multiple coronary distributions consistent with haemodynamically significant LM disease. Alternatively, patients may have left main equivalent disease (Medina classification 0,1,1 of the distal LM, with significant stenoses of the ostial left anterior descending and left circumflex arteries, as defined in Table 1).
UNIVERSAL REGISTRY
The first 1,000 consecutive screened subjects with LMCAD (≥50% angiographic diameter stenosis) who are either not eligible for randomisation or who are not randomised for other reasons (e.g., physician or patient refusal) will be consented for the universal registry. These subjects will be followed through the time of initial treatment per standard of care with either PCI, CABG or medical therapy. Considering the proportion of patients randomised during recruitment of the universal registry will provide insight as to the generalisability of the study results.
PROTOCOL PROCEDURES
After confirming that all inclusion and exclusion criteria are met and after obtaining written, informed consent, eligible patients are randomised (using an interactive voice response system) 1:1 to PCI with XIENCE CoCr-EES or CABG treatment. Randomisation is stratified by the presence vs. absence of medically treated diabetes, site-assessed SYNTAX score (low [<23] vs. intermediate [≥23-32], and study centre.
SPECIFIC PCI PROCEDURES
At the time of the PCI procedure, LM lesions which appear visually to be <70% stenotic should not undergo intervention unless there is evidence of ischaemia or morphologic severity as evidenced by either non-invasive functional evidence of ischaemia in the territory of the lesion (not explained by another coronary stenosis), and/or IVUS MLA ≤6.0 mm2, and/or FFR ≤0.80. In subjects with concomitant coronary disease outside of the LM complex, the following treatment sequences are recommended: 1) if LAD and/or LCX disease is present, treat the LAD and/or LCX first (distal to proximal, as per usual PCI practice), unless the severity of the LM stenosis requires primary treatment of the LM first; 2) if the LM lesion is critical (e.g., >90% visually assessed stenosis or clinical instability), treat the LM first, either with a balloon or definitive stenting to insure subject safety; 3) if the RCA has a severe culprit lesion in a large vessel and the LM stenosis is <70%, the operator may choose to treat the RCA before the LM lesion; otherwise, the LM lesion should usually be treated before the RCA; 4) chronic total occlusions (CTOs) should usually be treated after completion of the LM PCI (frequently as a planned second staged procedure).
LM lesion preparation, defined as pre-treatment with balloons or other approved devices (including rotational atherectomy for heavily calcified vessels) of the LM complex is left to the operator’s best judgement, but is strongly recommended. Direct stenting of the LM is strongly discouraged. If the distal LM bifurcation is involved, a provisional (crossover) technique is strongly preferred to two planned stents. If a provisional approach is chosen and a second stent is required, any of the following techniques may be used per operator discretion: T-stenting, T-stenting with small protrusion (TAP), mini-crush (reverse crush), or culotte. A planned two-stent technique strategy should be considered when the side branch (usually the LCX) is large (>3 mm), has significant disease (by angiographic or IVUS assessment) and/or lesion length >5 mm, or when there are other special anatomic considerations (e.g., heavy calcification). The planned two-stent technique may include T-stenting, TAP, crush, culotte or rarely V-stenting or Y-stenting. The use of kissing balloons after any two-stent technique is strongly recommended.
The goal of PCI is to achieve complete functional revascularisation of all ischaemic territories. For all “borderline or intermediate non-LM lesions” (40-70% diameter stenosis by angiographic visual estimate), it is strongly recommended to confirm physiologic lesion significance before treatment using FFR evaluation (preferred) or IVUS assessment. Non-ischaemia-producing lesions should not be treated. For all non-LM lesions, IVUS guidance pre-treatment and assessment post-treatment to optimise lumen dimensions is recommended (especially for LAD lesions).
All LM and non-LM lesions are treated only with XIENCE CoCr-EES. The choice of vascular access (femoral vs. radial), method of closure and whether to use haemodynamic support devices during the procedure are left to operator discretion. Planned staged procedures are liberally recommended for patients with extensive CAD, within two weeks (but in all cases within four weeks). The lesions planned for subsequent PCI are identified at the time of the initial procedure to differentiate planned from unplanned repeat revascularisation procedures.
PCI MEDICATIONS
Aspirin 300 to 325 mg po is administered to all patients at least two hours before PCI, followed by ≥75 mg per day indefinitely. Pre-PCI loading with an ADP antagonist is mandatory, with the choice of either clopidogrel, prasugrel or ticagrelor left to the discretion of the investigator. Chronic daily ADP antagonist therapy is mandated for at least one year after PCI in subjects who received an LM stent. Statin pre-loading with either 80 mg atorvastatin or 40 mg rosuvastatin is administered to reduce periprocedural myonecrosis. For procedural anticoagulation, bivalirudin is recommended, with unfractionated heparin or low molecular weight heparin also being acceptable. Glycoprotein IIb/IIIa inhibitors are strongly discouraged in subjects adequately pre-loaded with an ADP antagonist, but may be used for refractory ischaemic or thrombotic complications. Otherwise, optimal medical therapy is prescribed (Online Appendix).
SPECIFIC CABG PROCEDURES
CABG may be performed with or without the assistance of cardiopulmonary bypass (CPB), depending on the expertise of the centre. Use of a single cross-clamp technique for subjects undergoing CABG on CPB is strongly recommended and multiple applications of an aortic clamp are strongly discouraged. In off-pump and on-pump beating heart surgery, coronary stabilisation devices must be used. After completion of proximal and distal anastomoses, graft patency assessment is strongly recommended either with transit time Doppler flow measurements or with intraoperative angiography with fluorescence or standard radiographic angiography. Intermittent, cold blood cardioplegia is the preferred myocardial protection strategy for on-pump, arrested heart surgery. However, intermittent cold crystalloid cardioplegia or continuous warm blood cardioplegia is allowed if it is the local standard of care.
Per contemporary standards, all coronary arteries with ≥50% angiographic diameter stenosis and ≥1.5 mm in diameter should be revascularised. Arterial grafts are the preferred conduits for coronary revascularisation. The left internal thoracic artery should be used to graft the LAD in all subjects. The right internal thoracic artery (as an in situ graft or free graft) is the preferred second arterial graft, and should be used to graft the next most important and stenotic coronary artery. Other arterial grafts that may be used include the radial, in situ gastroepiploic, and free inferior epigastric arteries. Intraoperative transoesophageal echocardiography (TEE) is highly recommended prior to cannulation to assess left ventricular function, cardiac valves, and the ascending aorta (the ascending aorta may otherwise be assessed by epiaortic echocardiography). After coronary revascularisation, intraoperative TEE should be used to assess for new ventricular wall abnormalities, which, if identified, should prompt evaluation of the bypass graft supplying that myocardial territory for possible revision, if necessary.
CABG MEDICATIONS
It is strongly recommended that subjects are treated with aspirin and statins. Aspirin (≥75 mg) must be given within six hours after surgery intravenously, orally, rectally, or via a nasogastric tube if there is no important bleeding (≤50 cc/hr), and daily for the duration of the trial. If the patient is taking an ADP antagonist, it should be discontinued prior to surgery (at least five days prior for clopidogrel and ticagrelor and at least seven days prior for prasugrel). Post CABG, clopidogrel is not required but may be administered as per local standard of care in subjects with saphenous vein grafts or in those who underwent off-pump surgery. Subjects receiving amiodarone prophylaxis should be loaded prior to surgery, and have their treatment continued for a minimum of five days after surgery. ACE inhibitors are to be stopped before CABG to ensure they are no longer effective at the time of surgery. Otherwise, optimal medical therapy is prescribed (Online Appendix).
LABORATORY TESTS AND ELECTROCARDIOGRAPHY
The following tests are performed at baseline in all patients: HbA1c, CK, CK-MB, haemoglobin, white blood cell count, platelet count and serum creatinine. CK and CK-MB are measured post procedure in all patients at 12±2 hours and at 24±2 hours, or at discharge if sooner. CK-MB levels must be used to assess baseline entry criteria and post-procedure myonecrosis. Either CK-MB or troponin I or T levels may be used to assess myonecrosis >48 hours post procedure. Twelve-lead ECGs are performed pre-procedure, within 24 hours post procedure, at discharge, and at one-year follow-up. Additional biomarkers and ECGs are assessed for any evidence of ischaemia or other adverse cardiac events.
FOLLOW-UP
After hospital discharge, clinical follow-up is performed by office visit (preferred) or telephone visit at 30 days, six months, one year, two years, three years, four years and five years. Follow-up may be continued annually up to 10 years at the sponsor’s discretion (patients are pre-consented for this possibility). In addition, to minimise bias in the assessment of the primary three-year endpoint, at the time the last randomised subject reaches the two-year follow-up duration an additional follow-up visit will be performed for all patients in whom three-year follow-up has not otherwise been completed, unless the most recent follow-up was completed within 28 days. The following are assessed at each follow-up visit: the interval occurrence of any adverse events, including possible cerebrovascular events according to a National Institutes of Health Stroke Scale (NIHSS) validated TIA/stroke questionnaire; the modified Rankin Scale (mRS) score; and the results of anginal status and cardiac medication use. If the responses to the stroke questionnaire indicate a possible stroke or change in the mRS, a vascular neurologist, stroke specialist or mRS-certified personnel blinded to the treatment type will confirm the mRS score, determine whether a stroke has occurred and its severity. Routine angiographic follow-up in asymptomatic patients is not permitted in this study.
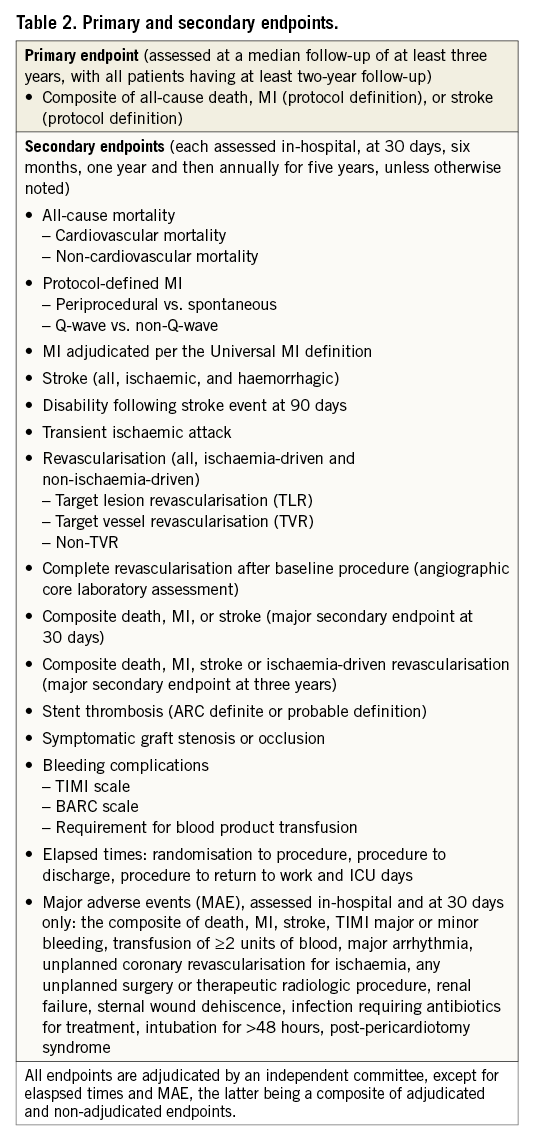
ENDPOINTS
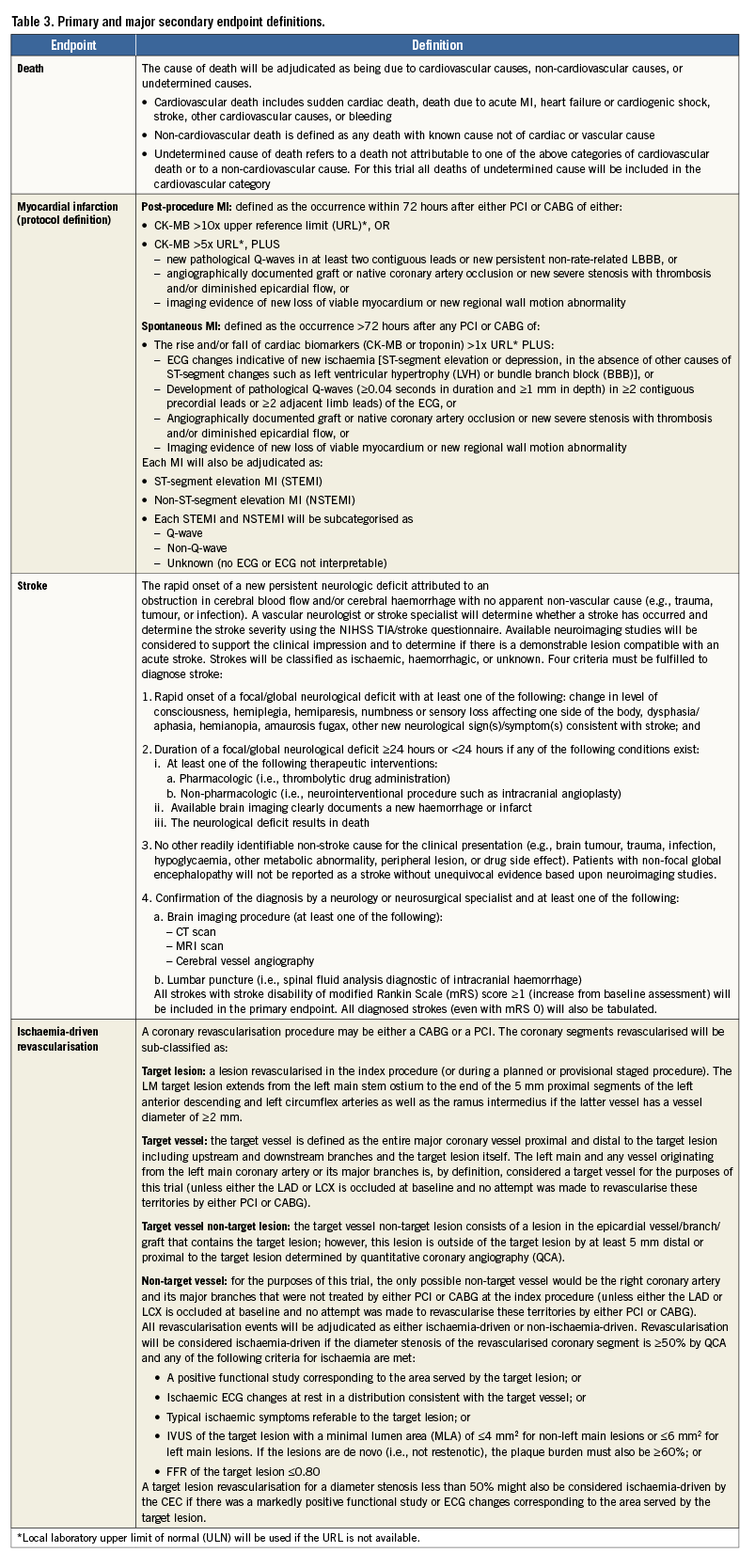
The primary endpoint of the randomised trial is the three-year composite rate of all-cause mortality, MI, or stroke (Table 2). The primary endpoint analysis will be conducted when at least 50% of patients have reached three-year follow-up and all patients have reached the two-year follow-up. All available data up to three-year follow-up will be used. Two major secondary endpoints are pre-specified: the 30-day composite rate of all-cause mortality, MI, or stroke, and the three-year composite rate of all-cause mortality, MI, stroke or ischaemia-driven revascularisation. Other secondary endpoints are listed in Table 2. The definitions of the primary and major secondary endpoints are detailed in Table 3. The primary endpoints, major secondary endpoints, revascularisation events, stent thromboses (Academic Research Consortium definite or probable criteria) and graft occlusions are adjudicated by an independent clinical events adjudication committee. An independent angiographic core laboratory is used to assess the extent of disease and the SYNTAX score at baseline, the residual SYNTAX score after PCI (and CABG, according to operative reports), and the degree of completeness of anatomic and ischaemic revascularisation.
Sample size and statistical considerations
ANALYSIS PLAN
All principal analyses will be performed in the intent-to-treat (ITT) population, defined as all subjects randomised, regardless of the treatment actually received. Secondary analyses will be performed in the per protocol population (defined as those subjects who received the intended randomised treatment as their first revascularisation within four weeks of randomisation, and had no violations of inclusion or exclusion criteria) and the as-treated population (according to the first revascularisation procedure performed). Hierarchical family-wise testing will be employed in the following order:
1. Primary endpoint: all-cause mortality, MI or stroke at three years, tested for non-inferiority.
2. First major secondary endpoint: all-cause mortality, MI or stroke at 30 days, tested for non-inferiority.
3. Second major secondary endpoint: all-cause mortality, MI, stroke or unplanned revascularisation for ischaemia at three years, tested for non-inferiority.
Formal hypothesis testing of the first major powered secondary endpoint will occur only if non-inferiority for the primary endpoint is met. Simultaneous hypothesis testing of the second major powered secondary endpoint and the superiority test of the primary endpoint will occur only if non-inferiority for the first major powered secondary endpoint is met.
PRIMARY ENDPOINT ANALYSIS
The primary endpoint sample size calculations were performed according to the Com-Nougue approach24, which utilises the difference in Kaplan-Meier estimates, derived from simulations. Assuming a primary endpoint event rate of 11.0% in both treatment arms at three years (based on three-year event rates from the SYNTAX trial13), with two-year minimum time to follow-up, median time to follow-up three years, 8% lost to follow-up at three years, a non-inferiority margin of 4.2%, and patient accrual time of 29 months, randomising 1,900 subjects (~950 per arm) provides 80% power to demonstrate non-inferiority of PCI to CABG with a one-sided alpha of 0.025. The non-inferiority margin of 4.2% for the primary endpoint was agreed upon as representing therapeutic interchangeability25 by the study leadership consisting of the principal investigators, executive committee, PCI and surgical committees, and country leaders of this protocol, comprising more than 100 physicians not related to the study sponsor, of whom approximately 50% are interventional cardiologists and 50% are cardiac surgeons. This margin was considered appropriate given the substantially lower periprocedural morbidity of PCI, and the likelihood of fewer strokes with PCI, especially in the first 30 days to one year, although the trial will not be powered to demonstrate a reduction in stroke.
The three-year incidence rates of the primary endpoint will be estimated using the Kaplan-Meier method to allow for censoring. The Greenwood formula will provide estimated standard errors. The 95% confidence interval for the difference between PCI and CABG in these rates will be calculated. If the upper 95% confidence limit is less than the non-inferiority margin of 4.2%, non-inferiority will be declared. If the upper 95% confidence limit is less than zero, then superiority for PCI will be declared. With a two-sided alpha of 0.05, the trial will have 80% power to demonstrate superiority with an absolute difference of 3.84% fewer events with PCI compared to CABG (e.g., 7.16% with PCI vs. 11.0% with CABG).
SECONDARY ENDPOINT ANALYSIS
Both major secondary endpoints will be evaluated using the difference in Kaplan-Meier failure rates. For the 30-day composite endpoint of all-cause mortality, MI or stroke, assuming a composite rate of 3.0% in each treatment arm, an accrual time of 29 months, and a non-inferiority margin of 2.0%, 1,900 randomised patients will provide 80% power to demonstrate non-inferiority of PCI to CABG with a one-sided alpha of 0.05. For the three-year composite endpoint of all-cause mortality, MI, stroke, or unplanned revascularisation for ischaemia, assuming the event rate is 22.0% in each treatment arm (using three-year event rates from the SYNTAX trial13), with a two-year minimum time to follow-up, median time to follow-up three years, 8% lost to follow-up at three years, a non-inferiority margin of 8.4%, and patient accrual time of 29 months, randomising 1,900 subjects provides 99% power to demonstrate non-inferiority of PCI to CABG with a one-sided alpha of 0.05. Hypothesis testing for the major secondary endpoints will be performed in a similar manner to that for the primary endpoint. Other secondary endpoint rates will be estimated by the Kaplan-Meier method and compared similarly.
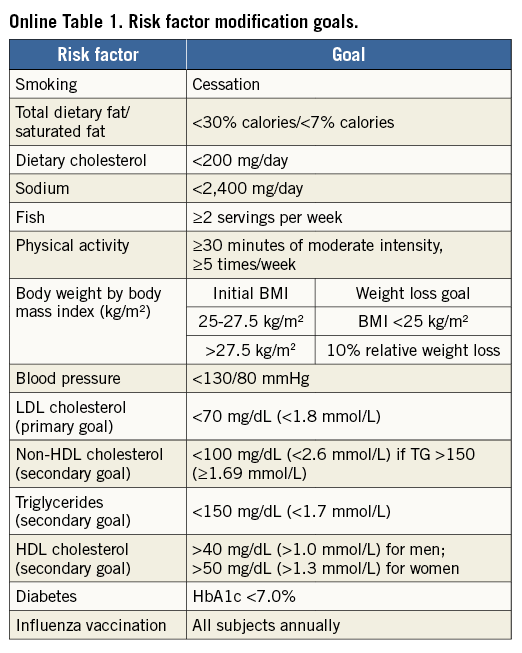
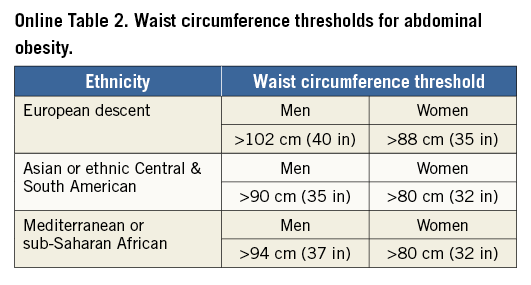
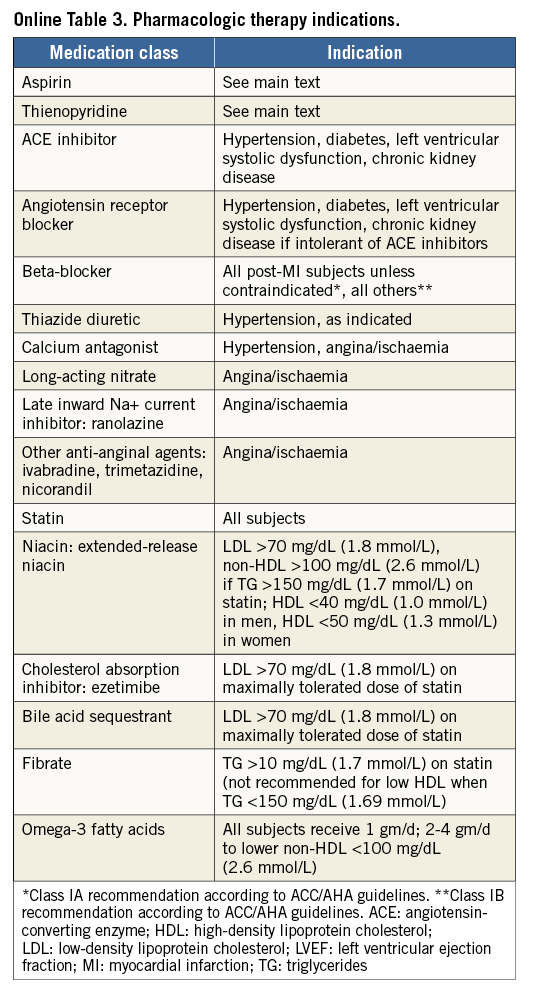
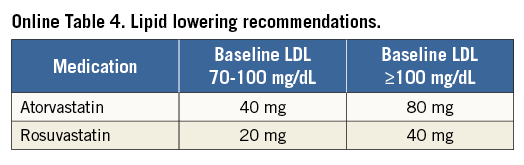
The primary and major powered secondary endpoints will be analysed in pre-specified subgroups. The treatment comparisons in these analyses are not powered for hypothesis testing and are descriptive in nature. For each covariate, two models will be run: one with just treatment and the covariate, and a second also containing the interaction term of covariate and treatment. Outcomes will be evaluated in the following subgroups: diabetes mellitus requiring medication vs. non-treated or no diabetes; age (≥ vs. < median and ≥ vs. <75 years); gender; body mass index (≥ vs. < median); prior MI; LVEF (≥ vs. < median and ≥ vs. <40%); chronic kidney disease (creatinine clearance from Cockcroft-Gault formula < vs. ≥60 ml/min); geographic location (USA vs. EU vs. other; USA vs. other; North America vs. EU vs. other; North America vs. other); number of diseased non-left main vessels (core lab assessed ≥3 vs. <3; ≥2 vs. <2); distal left main bifurcation involvement (core lab assessed); presence of one or more non-left main chronic total occlusion (core lab assessed); SYNTAX score (core lab assessed ≥ vs. < median; by tertiles: ≥23 vs. <23; ≥33 vs. <33); clinical SYNTAX score (core lab assessed ≥ vs. QUALITY OF LIFE AND HEALTH ECONOMICS To date, the only available data regarding the cost-effectiveness of PCI vs. CABG for LMCAD are derived from the SYNTAX trial26. The mean lifetime costs were $7,618/subject higher with CABG than with PCI, and quality adjusted life years (QALYs) also favoured PCI (by 0.29 QALYs), rendering PCI a highly dominant strategy. In the EXCEL study, health-related quality of life (HRQoL) and treatment costs will be assessed in 1,800 randomised patients alongside the core clinical trial to evaluate the impact of the PCI and CABG strategies on a range of relevant QoL domains and cost-effectiveness. HRQoL and functional status will be assessed using the following combination of generic and disease-specific measures selected to cover a broad range of health domains that may be affected by CAD, its treatment, and its complications. Disease-specific QoL will be assessed using the Seattle Angina Questionnaire (SAQ) and the London School of Hygiene Dyspnoea Questionnaire. Mental health and depression will be assessed using the Patient Health Questionnaire-9 (PHQ-9). Generic health status will be assessed using the Medical Outcomes Study 12-item Short Form (SF-12), and health utilities will be assessed using the EuroQoL (EQ-5D) with US-specific weights. These measures will be assessed using standardised, written questionnaires at baseline (prior to randomisation), one month, one year, three years, and five years. Data on cardiovascular-specific resource utilisation will be collected prospectively for the index hospitalisation and over the full follow-up period for all subjects using standardised case report forms. Procedural costs will be assessed using a resource-based approach to convert standard measures such as procedural duration and utilisation of specific supplies into costs. Other hospital costs will be assessed using an “event-driven” approach in which specific complications and outcomes are assigned standard costs based on external data. The cost and QoL data will be integrated to perform a formal cost-effectiveness analysis. The primary analysis will be performed from the perspective of the US healthcare system using a lifetime time horizon. Secondary analyses will be performed from the perspective of other healthcare systems with the collaboration of a local health economist. The primary cost-effectiveness and QoL analyses will be performed when all randomised subjects have completed a minimum of three years of follow-up. Although this time frame is slightly different from that for the main clinical endpoint, the longer follow-up duration for the economic analysis will minimise the need for extrapolation beyond the observed data. Conclusions Selecting the best revascularisation modality for patients with significant disease of the LM coronary artery is essential to optimise outcomes in this high-risk group. Data from previous studies suggest that DES may offer non-inferior or superior results for selected patients with LMCAD compared to CABG. However, prior randomised trials have had important limitations, including not being adequately powered for the meaningful composite endpoint of death, MI or stroke. In addition, prior studies have not evaluated contemporary PCI devices, drugs and techniques, nor state-of-the-art CABG therapies. The EXCEL trial has sufficient geographic representation and power to establish the roles of CABG and PCI using XIENCE CoCr-EES in the contemporary management of patients with LM disease with low and intermediate SYNTAX scores. Impact on daily practice The EXCEL study tests whether coronary bypass is still the gold standard for treatment of unprotected left main disease. When the primary endpoint is met and the trial shows that PCI using CoCr-EES is non-inferior to coronary bypass grafting, patients with left main disease and a SYNTAX score below 33 may opt for PCI as a less invasive management of left main disease. If non-inferiority cannot be established, CABG will remain the gold standard and only patients with severe comorbidities and increased surgical risk will be candidates for PCI. Guest Editor This paper was guest edited by John Pepper, FRCS; National Institute for Health Research (NIHR), Cardiovascular Biomedical Research Unit (BRU), Royal Brompton Hospital, London, United Kingdom. Funding The trial represents an academically led international investigation co-ordinated by approximately 50% interventional cardiologists and 50% surgeons, including four principal investigators (two surgeons and two interventional cardiologists, one from the USA and one from Europe for each subspecialty). The trial was designed by the principal investigators, in coordination with an executive committee, and speciality PCI, CABG, medical therapy and statistical committees. The trial is funded by Abbott Vascular. None of the study leadership accepted any compensation for their roles in this study, other than expenses. The principal investigators accept responsible for the design and conduct of this study, all study analyses, and the drafting and editing of all manuscripts. Conflict of interest statement The authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare. SUPPLEMENTARY DATA Online Appendix. Guidelines for optimal medical therapy Optimal medical therapy (secondary prevention plus angina therapy) in the EXCEL trial will be intensive and evidence-based, and will be applied equally to both treatment groups. Every subject should undergo individual risk assessment followed by aggressive risk factor reduction with tailored lifestyle intervention and pharmacological therapy to control risk factors, prevent future cardiovascular events, and manage symptoms (angina)27-29. These recommendations should be given in writing to all subjects, with a plan in place prior to discharge for close follow-up care to optimise long-term medical therapy. Online Table 1 illustrates the risk factor goals which are recommended. General recommendations and goals SMOKING All subjects who are smokers should enter a smoking cessation programme (or practice-based counselling with nurse co-ordinators) with a focus upon quitting, avoiding relapses, and minimising exposure to secondhand smoke27-29. DIETARY AND WEIGHT GOALS In subjects with an initial BMI of between 25-27.5 (kg/m²), the goal should be a BMI of less than 25 kg/m². If BMI is greater than 27.5, the goal is 10% relative weight loss. Online Table 2 lists waist circumference thresholds for abdominal obesity. Although these are not in themselves therapeutic targets, they are a useful screening tool for abdominal obesity as one of the components of the metabolic syndrome. An ideal diet should comprise less than 30% of calories as total fat and less than 7% of calories as saturated fat. Dietary cholesterol should be limited to less than 200 mg per day, sodium less than 2,400 mg per day, and at least two servings of fish per week are recommended. PHYSICAL ACTIVITY Physical activity goals are 30-60 minutes of moderate intensity exercise five or more times per week. Based upon evidence of contemporary cardiac rehabilitation programmes and taking into account that subjects in the EXCEL trial will have undergone coronary revascularisation, there should be little concern with regard to ischaemic risk from exercise training. An exercise prescription based upon the guidelines from the American Association of Cardiovascular Pulmonary Rehabilitation and the American College of Sports Medicine may be prescribed. Specifics are frequency of five or more times per week, an intensity based upon a resting heart rate plus 20 beats per minute, a Borg rating of perceived exertion (RPE) of 11-13 (“fairly light to somewhat hard”), or below the subject’s angina-ischaemia threshold. The duration should be 30-60 minutes and the modes include walking, treadmill, cycling, elliptical, rowing, stair climbing, or other30,31. INFLUENZA VACCINATION Influenza vaccination should be encouraged on an annual basis for all subjects. DIABETES The goals for diabetes management are to maintain fasting blood glucose levels between 80 and 125 mg/dL (4.44-7.49 mmol/L) and haemoglobin A1c levels of <7% in accordance with published recommendations32-34. More stringent goals, i.e., a haemoglobin A1c level of <6%, can be considered in individual subjects. All subjects with haemoglobin A1c levels of >7% should be referred to a diabetes clinic or a physician with expertise in the management of diabetes. Management will be in accordance with published guidelines and recommendations. LIPID GOALS Aggressive lipid-lowering therapy is advocated with the primary goal being LDL cholesterol of <70 mg/dL (<1.8 mmol/L)28,29,35,36. Secondary goals include increasing the levels of HDL cholesterol to >40 mg/dL (>1.0 mmol/L) for men and >50 mg/dL (>1.3 mmol/L) for women. Other secondary goals include maintaining triglyceride levels <150 mg/dL (<1.7 mmol/L), non-HDL cholesterol <100 mg/dL (<2.6 mmol/L), and total cholesterol/HDL cholesterol ratio of <4.0. Fasting lipid profiles should be analysed at baseline, six weeks after starting therapy, six months, and then annually throughout the trial but are not a protocol requirement. HYPERTENSION The goal is a blood pressure of less than 130/80 mmHg. All subjects with hypertension will receive lifestyle counselling focused on sodium restriction, weight loss, and exercise. Medications will be prescribed if necessary. Pharmacologic therapy Online Table 3 lists the recommended drugs to be used for each condition and the indications for therapy (subject to modification, pending finalisation and subsequent changes to ACC/AHA and ESC clinical practice guidelines for stable and unstable ischaemic heart disease). Goals of therapy are to achieve the desired level of risk factors and to control symptoms according to the subject’s individual tolerance of medications, so as to maintain an acceptable quality of life. TREATMENT OF HYPERTENSION The overall goal of therapy for hypertension is to provide maximal protection against cardiovascular consequences with minimal side effects. There remains, however, some uncertainty with regard to which drug should be used and in what order. Since all subjects in this trial have symptomatic coronary artery disease, initial therapy should be an angiotensin-converting enzyme inhibitor (ACE-I) or a beta-blocker. If the goal blood pressure is not reached, the next step is the addition of a diuretic or a calcium channel blocker. All subjects should be on a beta-blocker and an ACE-I prior to the addition of other agents. If there are contraindications to use, side effects, or blood pressure is not controlled, subjects should be referred to the principal investigator for further consultation. An ACE-I or angiotensin receptor blocker (ARB) should be administered to all subjects with diabetes, left ventricular systolic dysfunction, and/or chronic kidney disease. LIPID-LOWERING THERAPY After the procedure, all subjects should be started on a high-dose “statin” based on LDL level according to the recommended regimens included in Online Table 4. If lipid goals are not reached after the maximum tolerated dose of a statin, then the preferred option is to add Niacin ER 500 mg daily for four weeks to be titrated over a period of four weeks for each increase in dosage up to a maximum of 2,000 mg daily. Other options if the triglycerides are less than or equal to 200 mg/dL (2.3 mmol/L) are to add a bile acid sequestrant such as colesevelam six tablets daily or three tablets twice per day with a meal and liquid or ezetimibe 10 mg daily. If triglycerides are greater than or equal to 200 mg/dL (greater than 2.3 mmol/L) add either Tricor (fenofibrate) 145 mg per day or fish oils up to a dose of 4 gm daily. If the subject still does not reach lipid goals, the principal investigator should be consulted. ANTI-ANGINAL THERAPY All subjects should receive sublingual nitroglycerine for pain relief and prophylaxis. The choice between beta-blockers and calcium channel blockers for first-line anti-anginal therapy is not clear-cut but, in general, beta-blockers are advised as initial therapy, particularly in subjects with hypertension, left ventricular systolic dysfunction, and/or a history of myocardial infarction. Absolute contraindications to beta-blockers are severe resting sinus bradycardia, pre-existing second degree AV block, sick sinus node syndrome, asthma of at least moderate severity, or decompensated (class IV) heart failure. Most diabetics and subjects with chronic obstructive pulmonary disease but without frank bronchospasm will tolerate beta-blockers although close monitoring is recommended. Subsequent steps include the addition of drugs not already utilised, e.g., the addition of a calcium channel blocker, long-acting nitrate, or ranolazine in subjects already on a beta-blocker. Conversely, the addition of a beta-blocker to subjects who are already on a calcium channel blocker should be considered. New anti-anginal agents such as trimetazidine (approved for use in Europe) and ivabradine (approved for use in Europe) may be tried in selected subjects. VITAMIN SUPPLEMENTATION Vitamin supplementation with vitamin E, folic acid, vitamin B6, and vitamin B12 is not recommended. Although the evidence for vitamin D deficiency as a risk factor for CAD is growing, evidence for vitamin D supplementation as effective secondary prevention is lacking. Vitamin D supplementation is therefore not currently recommended, but may be considered if new evidence emerges during the course of the trial as appropriate, depending on results from two large ongoing trials.