Abstract
Unprotected left main coronary artery (ULMCA) stenosis has relatively high prevalence and exposes patients to a high risk for adverse cardiovascular events. The optimal revascularisation strategy (coronary artery bypass surgery [CABG] or percutaneous coronary intervention [PCI]) for patients with complex coronary artery disease is a topic of continuing debate. The introduction of the newer-generation drug-eluting stents (DES) –with documented improvements in both safety and efficacy– has prompted the interventional community to design two new dedicated randomised trials comparing CABG and PCI: the NOBLE (Coronary Artery Bypass Grafting Vs Drug Eluting Stent Percutaneous Coronary Angioplasty in the Treatment of Unprotected Left Main Stenosis) and EXCEL (Evaluation of XIENCE Everolimus Eluting Stent Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) trials. The aims of the present review are to describe the similarities and contrasts between these two trials as well to explore their future implications in ULMCA treatment.
Introduction
The relevance of an unprotected left main coronary artery (ULMCA) stenosis was first described more than 100 years ago1. James Herrick reported the story of a 55-year-old male who died in cardiogenic shock after a period of 52 hours. The autopsy found an extensive necrosis of the left ventricle associated with total occlusion of the left main coronary artery by a thrombus overlying an area of atherosclerotic narrowing1. The explanation for this massive necrosis is the large area of myocardium at risk in patients with ULMCA. It has been shown that, in a usual right dominant coronary anatomy, the left coronary artery supplies approximately 84% of the flow to the left ventricle2-4.
Currently, the prevalence of significant ULMCA disease –diameter stenosis greater than 50 percent– may vary from 4-6% of all patients who undergo coronary arteriography to 24% of patients with acute coronary syndrome5,6. Most of these patients are symptomatic and at high risk of cardiovascular events4,5,7. In that sense, for over 30 years, coronary artery bypass grafting (CABG) has been regarded as the standard of care for ULMCA by improving long-term prognosis when compared with optimal medical therapy8.
Since its clinical introduction in 19779, percutaneous coronary intervention (PCI) has gradually matured. The advent of drug-eluting stents (DES) has markedly improved the long-term outcomes in patients with complex coronary anatomy. Numerous studies have compared outcomes in subjects treated with either CABG or PCI10-12. Meta-analytic combinations of these studies have basically shown that PCI has similar five-year mortality and myocardial infarction, with a lower incidence of stroke and increased risk of repeat revascularisation when compared to CABG11,12.
The relatively high prevalence and substantial prognostic impact of ULMCA with an unclear optimal therapeutic option added to the introduction of the newer-generation DES –with proven improvements in both safety and efficacy13-19– has prompted the design of two new dedicated randomised trials comparing CABG and PCI. The aim of the present manuscript is to describe the design and future perspectives proposed by the ongoing EXCEL (Evaluation of XIENCE Everolimus Eluting Stent Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) and NOBLE (Coronary Artery Bypass Grafting Vs Drug Eluting Stent Percutaneous Coronary Angioplasty in the Treatment of Unprotected Left Main Stenosis) trials.
EXCEL and NOBLE: similarities and contrasts
The EXCEL trial (ClinicalTrials.gov identifier: NCT01205776) is an international, prospective, unblinded, randomised multicentre trial which enrolled 1,905 subjects in 131 centres (Figure 1). EXCEL was designed to establish the safety and efficacy of the everolimus-eluting stent (XIENCE PRIME™ or XIENCE V® or XIENCE Xpedition™ or XIENCE PRO™; Abbott Vascular, Santa Clara, CA, USA) in patients with significant ULMCA disease.
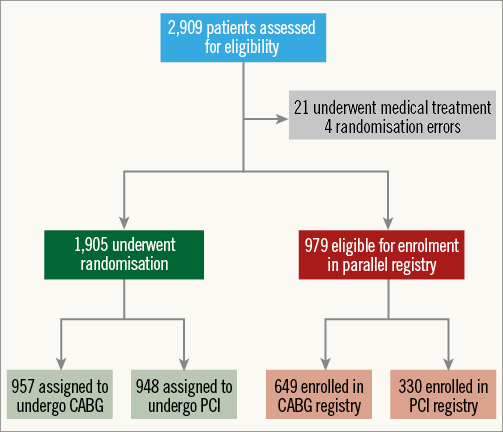
Figure 1. Enrolment and randomisation of patients with previously untreated left main coronary artery disease in the EXCEL trial.
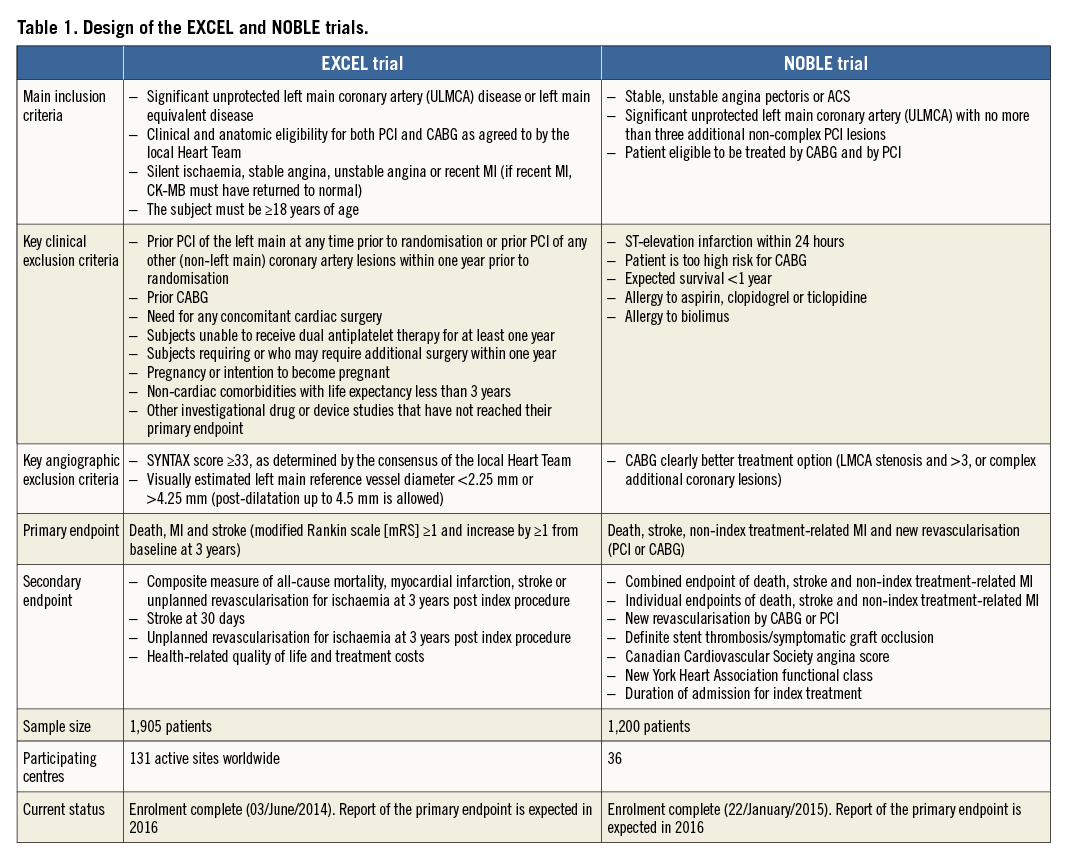
The NOBLE trial (ClinicalTrials.gov identifier: NCT01496651) is an international, prospective, unblinded, randomised multicentre trial which randomised 1,200 patients in 36 centres. The biolimus-eluting stent BioMatrix™ (Biosensors, Morges, Switzerland) is the recommended study stent but other CE-marked DES may be used at operators’ discretion. As shown in Table 1, although both trials aim to compare PCI versus CABG for ULMCA treatment, they do not have exactly the same design.
Comparison of anatomic selection criteria in the EXCEL and NOBLE trials
The EXCEL and NOBLE trials have, as core for inclusion, an equipoised treatment for PCI and CABG as assessed by the local Heart Team. It is mandatory in both trials that the interventional cardiologist and surgeon determine appropriateness and eligibility in their respective area of expertise.
The first difference between EXCEL and NOBLE is how the Heart Team assesses the ULMCA as being significant. The NOBLE trial adopted as significant an ULMCA with a visually assessed diameter stenosis (DS) >50% or fractional flow reserve (FFR) <0.80. The EXCEL trial defines significant ULMCA as one of the following: DS ≥70% (visually estimated) or DS ≥50% but <70% (requiring non-invasive or invasive [FFR ≤0.80] evidence of ischaemia or intravascular ultrasound [IVUS] minimal lumen area [MLA] ≤6.0 mm2). Additionally, the EXCEL trial has enrolled patients with left main equivalent disease defined as Medina classification 0,1,1 bifurcation disease with both the ostial left anterior descending artery (LAD) and ostial left circumflex artery (LCX) stenoses having ≥70% DS. If one or both of the ostial LAD and ostial LCX stenoses are ≥50% and <70% stenotic by visual estimation, then this lesion(s) is demonstrated to be significant either by non-invasive or invasive (FFR ≤0.80) evidence of ischaemia in its myocardial distribution or IVUS MLA ≤4.0 mm2. By protocol, in EXCEL, FFR was the preferred strategy to stratify lesion significance.
ULMCA disease should be regarded as a heterogeneous pathology when considering the choice of revascularisation modality. The anatomical complexity of the left main may vary from a single lesion in the shaft to distal trifurcation disease and its association with more complex downstream (three-vessel) disease, and may have been directly correlated to incomplete revascularisation and to late all-cause mortality following PCI20-22. The prevailing international revascularisation guidelines recommend revascularisation of ULMCA with CABG or PCI in subjects with SYNTAX scores which are low (SYNTAX score <23: class I recommendation for CABG or PCI [level of evidence B for both]) and intermediate (SYNTAX score 23-32: class I for CABG and class IIa for PCI [level of evidence B for both]). The same guidelines recommend against revascularisation with PCI of ULMCA disease with high SYNTAX scores (SYNTAX score ≥33: class I for CABG and class III for PCI [level of evidence B for both])7.
The EXCEL trial adopted an enrolment criterion of subjects with ULMCA disease up to intermediate anatomical complexity defined by a SYNTAX score <33 (assessed by the local Heart Team)23. On the other hand, the NOBLE trial has been enrolling patients with ostium, mid-shaft and/or bifurcation and with no more than three additional non-complex PCI lesions. Non-complex lesions in the NOBLE trial were defined as length <25 mm, non-chronic total occlusion, non-two-stent bifurcation, non-calcified and non-tortuous coronary lesions.
Study device
The EXCEL and NOBLE trials were designed to study the impact of revascularisation on ULMCA disease, incorporating changes in medical therapies, PCI technology and techniques, and advances in CABG which had been introduced since the completion of the SYNTAX and FREEDOM studies24-26. In EXCEL, the workhorse stent was the everolimus-eluting stent (EES) (XIENCE). The randomised comparisons of everolimus- versus paclitaxel-eluting stents were designed and powered for a combination of angiographic, ischaemic and safety outcomes, and have consistently shown the EES to be associated with more favourable outcomes compared to paclitaxel-eluting stents13-16. In addition, the largest patient-level meta-analysis (n=4,989) of the SPIRIT clinical programme has shown that EES were superior to paclitaxel-eluting stents in reducing all-cause mortality (3.2% vs. 5.1%, HR: 0.65, 95% CI: 0.49 to 0.86; p=0.003)17.
In NOBLE, the workhorse drug-eluting stent is a biolimus-eluting stent (BES) (BioMatrix) with bioabsorbable polymer. The stent was selected due to its high radial strength and expansion capacity, especially cell opening27. Furthermore, the biolimus-eluting stent with bioabsorbable polymer has shown excellent results in comparison with first28-30 as well as second-generation DES31,32. The results of NOBLE and EXCEL may help to understand the clinical impact of EES and BES specifically for ULMCA.
Intravascular imaging to guide ULMCA PCI
IVUS guidance compared with angiography guidance has been associated with reduced one-year rates of definite/probable stent thrombosis, myocardial infarction and composite adjudicated major adverse cardiac events (i.e., cardiac death, myocardial infarction, or stent thrombosis)33,34. Specifically for ULMCA PCI, a propensity score matching of the MAIN-COMPARE registry (n=201) has associated IVUS-guided PCI to lower three-year mortality35. In both NOBLE and EXCEL, IVUS-guided PCI is strongly recommended pre-treatment and post-treatment to optimise lumen dimensions in the left main segment and for all non-left main lesions36. An exception is made for distal lesions or tortuous vessels. All left main lesions in which IVUS is used will undergo rigorous core laboratory evaluation (Cardiovascular Research Foundation, New York, USA in the EXCEL trial and Belfast Health & Social Care Trust, Belfast, Northern Ireland in the NOBLE trial).
In this regard, these trials will help to understand the PCI results according to the baseline IVUS criteria and IVUS post-PCI predictors of clinical events. Although a two by two randomised trial for IVUS guidance would be ideal, the EXCEL and NOBLE protocols are already sufficiently complex that adding another level of randomisation is not practical. Moreover, although IVUS assessment is relatively simple, not all sites are expert in the use of IVUS guidance for complex left main stenosis.
Primary endpoints
EXCEL and NOBLE had different sample size calculation due to the difference in their respective primary endpoints. In NOBLE, the sample size calculation is based on the combined primary endpoint of death, stroke (defined as ischaemic or haemorrhagic cerebrovascular event verified by brain CT), non-index treatment-related MI and new revascularisation (MACCE) after two years (Table 1). In EXCEL, the primary endpoint is defined as death, MI and stroke (modified Rankin scale [mRS] ≥1 and increase by ≥1 from baseline at three years). EXCEL has completed its enrolment with a total number of 1,905 patients (Figure 1).
Recommendations on bifurcation treatment
Bifurcation lesions may be present in about 70% of ULMCA cases and have been associated with the occurrence of ischaemic events after PCI37. In EXCEL and NOBLE protocols, a single-stent crossover provisional technique is recommended whenever possible for treatment of bifurcations. After implantation of the first stent, if there is uncertainty concerning the adequacy of side branch patency, an FFR determination is recommended, with a value of ≤0.80 indicating that side branch dilatation should be performed.
The decision to use a primary two-stent technique is left to the operator’s best judgement. However, a primary two-stent strategy rather than a single crossover stent technique should be considered when the side branch (usually the left circumflex) is large (>3 mm), with significant disease (by angiographic or IVUS assessment) and lesion length >5 mm, or when there are other special anatomic considerations (e.g., heavy calcification).
In both trials, the strategy of a two-stent technique (crossover or primary two stents) for bifurcation treatment may include any of the following: T-stent, TAP, mini-crush (reverse crush), or culotte bifurcation stent techniques. The final decision is made according to the lesion morphology and the experience of the operator. The use of kissing balloons after provisional second stents is strongly recommended in a two-stent strategy. However, based on the Nordic Bifurcation study38, in NOBLE the culotte technique is preferred in case a two-stent strategy is needed.
Long-term forecasting and comparison of mortality in the EXCEL trial using the SYNTAX score II (SSII)
The SSII was developed in the landmark, all-comers, randomised SYNTAX (Synergy between PCI with TAXUS and Cardiac Surgery) trial (n=1,800)24,25 where selection bias would have been minimal. The SSII is composed of the anatomical SYNTAX score, presence of ULMCA disease, and six clinical characteristics (age, creatinine clearance [CrCl], left ventricular ejection fraction [LVEF], sex, chronic obstructive pulmonary disease [COPD], and peripheral vascular disease [PVD]). SSII has been externally validated in the multinational DELTA (n=2,891) and CREDO-Kyoto (n=3,896) registries39,40. Moreover, international guidelines have implemented the SSII as a risk stratification tool for complex coronary artery disease (class IIa, level of evidence B)7.
Recently, a prospective validation of the SYNTAX score II has been proposed to forecast and compare the four-year mortality in the EXCEL trial41. After completion of patient recruitment in EXCEL, using the actual baseline clinical and angiographic data from each enrolled patient, the SYNTAX score II was calculated. Scores were assigned for the presence and magnitude of each predictor directly based on the Cox proportional hazards model coefficients generating different scores and four-year mortality predictions for PCI and CABG40. To determine the 95% prediction intervals (PI), the trial was simulated 10,000 times based on consecutive bootstrap samples42.
The SYNTAX score II indicated at least an equipoise for long-term mortality between CABG and PCI in subjects with ULMCA in the EXCEL trial. For the entire study cohort, the four-year predicted mortalities were 8.5% and 10.5% in the PCI and CABG arms, respectively (odds ratios [OR] 0.79; 95% PI: 0.43-1.50). Figure 2 demonstrates the first 1,000 trial simulations. It has been found that there is a 40.4% (n=4,040) chance that the mortality predictions will be significantly lower in favour of PCI, a 4.4% (n=440) chance that the mortality will significantly favour CABG, and a 55.2% chance of having neutral results. In subjects with low (≤22) anatomical SYNTAX scores, the predicted OR was 0.69 (95% PI: 0.34-1.45); in intermediate anatomical SYNTAX scores23-32, the predicted OR was 0.93 (95% PI: 0.53-1.62) (Figure 2).
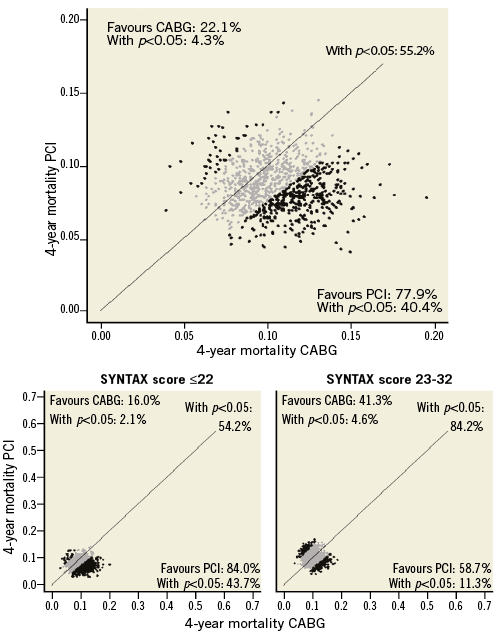
Figure 2. First 1,000 four-year mortality simulations of the EXCEL trial on the SYNTAX score II. Each dot represents one simulated trial mortality in both randomisation arms based on individual predictions. The diagonal line represents identical mortality for CABG and PCI. A dot plotted to the left of the diagonal line favours CABG (actual percentages shown in top left corner), and one to the right favours PCI (actual percentages shown in bottom right corner). Simulated trials with a significant (p<0.05) mortality difference between CABG and PCI are coloured black (actual percentage shown in parentheses in respective corners). Simulated trials with a non-significant (p≥0.05) mortality difference between CABG and PCI are coloured grey. (Modified from Campos et al41)
Based on four-year mortality predictions in EXCEL, clinical characteristics shifted long-term mortality predictions either in favour of PCI (older age, male gender, COPD) or CABG (younger age, lower creatinine clearance, female gender, reduced LVEF) (Table 2). The explanation for these predictions is that, as mentioned previously, ULMCA revascularisation, when limited to intermediate anatomical complexity, may have adequate results with PCI7.
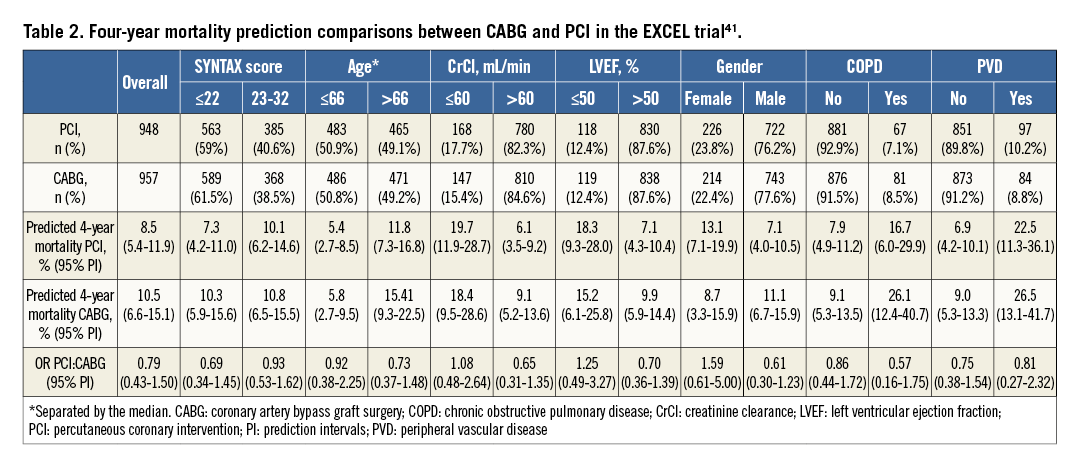
The major limitation of these predictions is also their greatest strength: the complete absence of the EXCEL trial outcomes. Therefore, at present, it not possible to assess whether these predictions are accurate. On the other hand, it enables unbiased validation of the SYNTAX score II, promoting understanding of the multiple risk factors involved in ULMCA disease and decision making on the most appropriate revascularisation modality.
Conclusion
The main results of both the EXCEL and the NOBLE trials are expected in 2016, which will therefore be a promising year for cardiology. The similarities and differences between these studies will, in the end, be complementary in the sense of throwing light on numerous aspects of revascularisation strategies and increasing our understanding of the role and mechanisms of their risk stratification and correlated therapeutic adjunctive tools.
Conflict of interest statement
The authors have no conflicts of interest to declare.