Abstract
Aims: To evaluate the angiographic and clinical outcome of patients undergoing paclitaxel-eluting stent (PES) implantation for unprotected left main coronary artery (ULMCA) stenosis in a “real-world” multicentre, prospective registry. Percutaneous coronary intervention (PCI) is an increasingly utilised method of revascularisation in patients with ULMCA.
Methods and results: A prospective registry including all patients with a significant (>50%) ULMCA stenosis. Of 151 such patients, the target lesion involved the distal bifurcation in 100 patients (66%), which was treated predominantly by a “provisional T-stenting” strategy. In the distal ULMCA disease group, 72% had only one stent implantation while 28% had multiple (either two or three) stents implanted. At a median follow-up of 1,123±80 days, cardiac death occurred in five patients (3.3%) and major adverse cardiac and cerebrovascular events (MACCE) in 32 patients (21.2%). The three-year survival rate was 93.3%.
Conclusions: In the drug-eluting stent era, paclitaxel-eluting stent implantation of ULMCA stenosis provided excellent immediate and long-term results in this selected population, suggesting that this approach may be considered as a safe and effective alternative to CABG for selected patients with ULMCA who are treated in experienced institutions performing large numbers of PCI procedures.
Abbreviations
BMS bare metal stent
CABG coronary artery bypass graft
CAD coronary artery disease
DES drug-eluting stent
DLMD distal left main disease
FRIEND French multicentre RegIstry for stEnting of uNprotecteD LMCA Stenosis
IVUS intravascular ultrasound
LAD left anterior descending artery
LCX left circumflex artery
LMCA left main coronary artery
MACE major adverse cardiac event
MACCE major adverse cardiac and cerebrovascular event
MI myocardial infarction
MLD minimal luminal diameter
MSCT multislice computer tomography
PES paclitaxel-eluting stent
PCI percutaneous coronary intervention
PTCA percutaneous transluminal coronary angioplasty
QCA quantitative coronary angiography
RCA right coronary artery
RVD reference vessel diameter
SES sirolimus eluting stent
STEMI ST-elevation myocardial infarction
TIMI Thrombolysis In Myocardial Infarction
TLR target lesion revascularisation
TVR target vessel revascularisation
ULMCA unprotected left main coronary artery
Introduction
Left main coronary artery (LMCA) disease has remained a challenge for percutaneous coronary intervention (PCI) throughout the last 25 years. Present guidelines consider this finding a major indication for coronary artery bypass grafting (CABG1) based mostly on the CASS (Coronary Artery Surgery Study2) and ECSS (European Coronary Surgery Study) trials3. These trials showed that in comparison with medical therapy, CABG improves survival in patients with ULMCA during a five-year follow-up period. However, these benefits diminish after 10- and 15-year follow-up with a mortality rate of over 50% with both treatment strategies4-6. Other recent studies suggest that percutaneous coronary intervention (PCI) with DES in this lesion subset is a feasible alternative offering similar results when compared with surgical revascularisation7,8.
The aim of the present study was to evaluate the early and long-term outcomes of ULMCA stenosis percutaneous intervention using the TAXUS® Express2™ PES stent system (Boston Scientific, Natick, MA, USA) in a “real-world” prospectively multicentre, registry.
Methods
Patient selection and study population
The French multicentre RegIstry for stEnting of. uNprotecteD LMCA Stenosis (FRIEND) trial included 23 clinical centres and a coordinating centre. The participating hospitals were selected on the basis of recognised experience with PCI for unprotected LMCA stenoses. To be eligible for the registry, patients had to have angiographic evidence of ≥50% diameter stenosis of the LMCA suitable for stent placement, with either anginal symptoms or documented myocardial ischaemia. Exclusion criteria consisted of contra-indications to antiplatelet or anticoagulation therapy, emergency situations such as acute ST-elevation myocardial infarction (STEMI) or cardiogenic shock, serum creatinine >1.8 mg/dl, reference vessel diameter <2.5 mm or >4.5 mm, in-lesion restenosis, severe obstructive pulmonary disease, pregnancy, and life expectancy less than three years due to underlying medical conditions. Between December 2005 and July 2006, a total of 600 patients with ULMCA disease were screened; 445 patients (74%) did not meet the clinical or angiographic requirements for inclusion or refused to give informed consent. Of these, 45.4% had CABG treatment and 36% had PCI treatment. In the other patients (18.7%), the coronary features were unsuitable for CABG or PCI and were treated by medical therapy. Therefore, 155 consecutive patients (26%) who underwent PES placement in an unprotected LMCA stenosis were enrolled in this prospective registry (Figure 1).
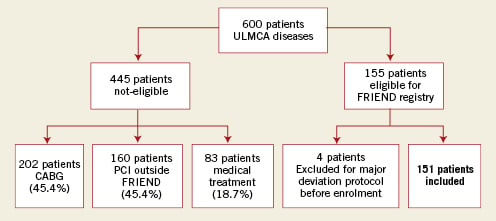
Figure 1. Selection of study population. CABG: coronary artery bypass graft; PCI: percutaneous coronary intervention; ULMCA: unprotected left main coronary artery
Angioplasty procedure
PCI technique, including the selection of arterial access or predilation devices, IIb/IIIa inhibitors, prophylactic intra-aortic balloon pump use, and intravascular ultrasound (IVUS) guidance was left to the discretion of the operator. However, the use of a “provisional T-stenting ” strategy was strongly recommended in the treatment of distal left main lesions in which a single PES was implanted from the left main preferentially through the left anterior descending artery (LAD). A second stent was implanted in the proximal circumflex only if there was residual moderate to severe dissection or significant residual stenosis after final kissing-balloon inflation. All elective patients were pre-treated with aspirin and clopidogrel beginning at least three days before the procedure. Unfractionated heparin 50-70 U/kg (with a goal activated clotting time of ≥250 sec) or bivalirudin was administered during the procedure. All patients were instructed to take aspirin 160 mg daily and indefinitely, and clopidogrel 75 mg for at least 12 months. Cardiac enzymes were determined routinely after percutaneous coronary procedures, at eight hour intervals during the first 24 hours or until discharge. At least one post-procedural 12-lead electrocardiogram was obtained and compared with pre-intervention tracings.
Angiographic methods
Quantitative coronary angiography was performed by an off-site, independent core laboratory (Corisis system) using a validated edge-detection program. The projection showing the maximal degree of stenosis was selected at baseline and was used for systematic angiographic control. For purposes of bifurcation analysis, the LMCA of every patient was divided into the parent vessel and side branch. By convention, the parent vessel was defined as the LMCA and proximal LAD, and the side branch as the LCX. The target lesion was defined as involving the distal LMCA if it was within 3 mm of the circumflex ostium. For the parent vessel and side branch, the reference vessel diameter (RVD), minimal luminal diameter (MLD), acute luminal gain (MLD immediately after the procedure minus the MLD before the procedure), binary restenosis (diameter stenosis of ≥50% relative to the angiographically uninvolved left main lumen), percent diameter stenosis, and late luminal loss (MLD immediately after the procedure minus the MLD at follow-up) were measured separately. In-stent restenosis was defined angiographic restenosis anywhere inside or within 5mm of the stented segment.
Endpoints and definitions
The primary endpoint was the occurrence of in-stent restenosis at nine-month follow-up. The secondary endpoints were the rate of major adverse cardiac and cerebrovascular events (MACCE) at 1, 12, 18 and 36 months. The LMCA was defined as “unprotected” if there was no history of CABG. Non-Q-wave myocardial infarction (MI) was defined as creatine kinase greater than two times the upper limit of normal with an abnormal CK-MB in the absence of pathological Q-waves. Stent thrombosis was defined as any of the following: angiographic demonstration of stent closure or intra-stent filling defect, unexplained sudden death, or MI without concomitant documentation of a patent stent. Acute stent thrombosis was defined as thrombosis occurring within 24 hours, subacute thrombosis between 24 hours and 30 days, and late thrombosis more than 30 days after the index procedure. Target lesion revascularisation (TLR) was defined as any repeat intervention (surgical or percutaneous) to treat a stenosis anywhere within the LMCA or within 10 mm distal to the LAD and LCX ostia. Target lesion revascularisation was further characterised as “ischaemia-driven” if signs or symptoms of ischaemia were present. Target vessel revascularisation (TVR) was defined as any repeat intervention (surgical or percutaneous) to treat a stenosis anywhere outside the LMCA to the LAD or LCX coronary arteries. MACCE were defined as any MI, any TLR, thrombosis, stroke, or death. Technical success was defined as a final diameter stenosis ≤30% and Thrombolysis in Myocardial Infarction (TIMI) flow grade 3.
Data collection and follow-up
The study protocol was approved by the FRIEND review board, the French Society of Cardiology, and was conducted in accordance with the Declaration of Helsinki. All patients signed informed consents before elective procedures, and data were reported using a standard case report form, and then forwarded to the coordinating centre. Clinical information about death, myocardial infarction, repeated revascularisation, bypass surgery and stroke was obtained during clinic visits or telephone interviews and was evaluated by a clinical event committee, which was independent of the operators. All the patients were followed up for at least 36 months.
Statistical analysis
The size of the cohort of patients was based on being able to show a 50% relative reduction in in-stent restenosis compared to the literature rate of 30% for BMS9-12, with alpha and beta errors of 5% (i.e., p<0.05) and 20% (i.e., 80% power), respectively. This required 131 patients. With the estimated attrition due to death or lost for angiographic follow-up, the total number of patients required was a maximum of 150. Categorical variables were expressed as counts and percentages, and continuous variables as mean ±SD. Angiographic characteristics and coronary angiography measurements were presented according to distal or non-distal ULMCA disease. Results were compared using a Student’s t-test for continuous variables, Chi-square test or Fisher’s exact test for categorical variables. Logistic regression analyses were used to determine risk factors influencing nine-month in-stent restenosis and MACCE rates. The considered covariates were: age, diabetes mellitus, EuroSCORE, vessel reference diameter, distal ULMCA disease, baseline angulation between LAD and LCX (< or >70°), the use of single vs. multiple stents and final post PCI MLD.
Cumulative probability of survival free from cardiac death, total death and MACCE were calculated for the whole population, and for distal versus non-distal ULMCA disease as sub-groups. Survival free from MACCE was also calculated in distal LMCA disease for patients with one stent versus 2-3 stents in distal left main lesion. All p-values were 2-tailed, with statistical significance set at a level of <0.05. Confidence intervals were calculated at the 95% level. All analyses were performed using SAS software version 8.2 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
Using an intention-to-treat analysis, 155 patients were enrolled in this trial. However, four patients were excluded at the beginning of enrolment for major protocol deviation (one death before coronary stenting, one acute coronary syndrome <12 hours, one patient with contra-indication to antiplatelet therapy and one PCI involving only balloon angioplasty). Thus, 151 patients were included in this prospective registry after percutaneous intervention of the ULMCA using one or more PES.
Clinical, procedural and angiographic characteristics are summarised respectively in Tables 1, 2 and 3. The procedure was elective for stable angina or silent ischaemia in 89 patients (59%), with no emergency revascularisation procedures for ST-elevation myocardial infarction or cardiogenic shock. Diabetes mellitus was only present in five patients (3.3%) and renal failure in 11 patients (7.3%), but 55 patients (36.4%) had additional disease in the RCA, 34 of which were treated during the index procedure. Twenty-seven percent of patients had an estimated EuroSCORE operative mortality >5%. Mean SYNTAX score was 17.45±7.1 with a very low percentage of high SYNTAX score >33 (4%).
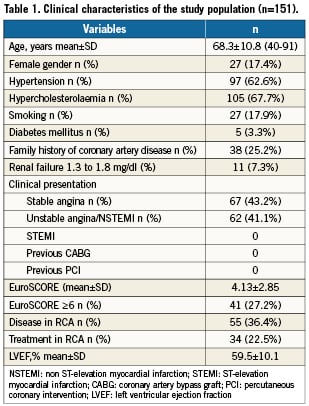
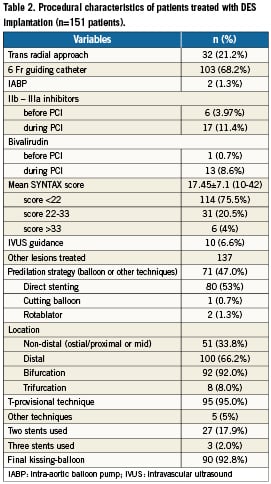
One hundred (66%) patients had distal LMCA stenosis: 92 were bifurcations and eight were trifurcations. For this group, the provisional T-technique was performed in 95% of cases, with 72% of patients receiving only one stent, and 28% receiving two or three stents. Final kissing-balloon dilation was performed in 93% of cases, and 20 patients (13%) received glycoprotein IIb/IIIa inhibitors before or during the procedure. Technical success was achieved in all patients.
Early outcomes
Three patients (2%) with high-risk EuroSCORE died of acute stent thrombosis during hospitalisation. One patient underwent TLR for residual dissection of LCX outside the previously stented segment. The combined endpoint of MACCE including death, stroke, any MI, TLR or thrombosis thus occurred in six patients (4%) before hospital discharge.
Angiographic follow-up
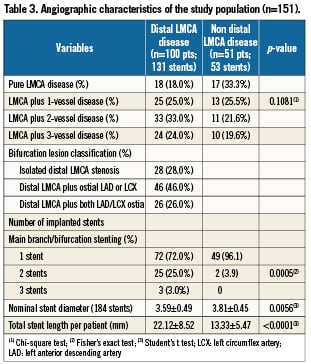
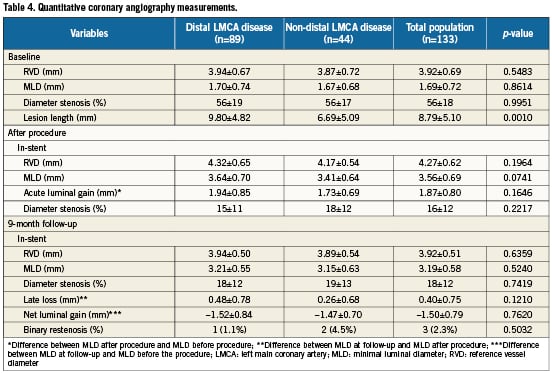
All patients were asked to return for angiographic follow-up. Overall, follow-up quantitative coronary angiography was performed in 133 patients (88%). Angiography was not performed in 18 patients because of death before the scheduled angiography (four patients), patient refusal (eight patients), age >80 or serious comorbidities (six patients), or the absence of clinical symptoms of cardiac ischaemia. Baseline and follow-up angiographic results are shown in Table 4. As shown, despite a similar LMCA reference vessel diameter, patients with distal lesion location tended to have a longer lesion length and a slightly greater MLD. In-stent acute luminal gain was similar in the two groups, but late loss in the distal ULMCA disease was almost double that of proximal or mid lesions (0.48±0.78 mm vs. 0.26±0.68 mm; p=0.12). Binary restenosis occurred in only three of the 133 patients studied (2.3%), including two (4.5%) of 44 patients with ostial or mid-shaft LMCA stenosis and in one (1.1%) of 89 patients with bifurcation involvement.
Mid-term and late outcomes
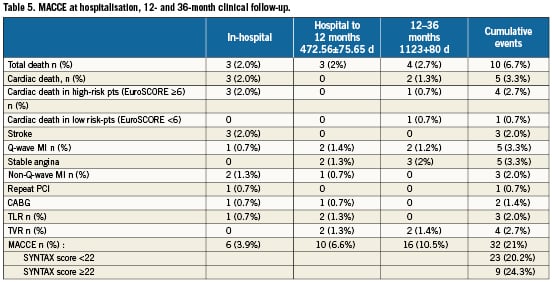
MACCE between hospital discharge and 12-month follow-up occurred in another 10 patients, so the cumulative incidence MACCE occurred in 16 patients (8%) by 12months. The mean long-term follow-up was 3.1 years (range 1.7 to 3.8 years; median 36.2 months). There were 10 deaths (6.7%), of which five (3.3%) were considered cardiac. MACCE occurred in 32 (21%) patients, which included eight (5.3%) myocardial infarcts, three (2%) strokes, and four (2.7%) TVRs. The three-year survival was 93.3%. Kaplan-Meier MACCE free of survival is shown in Figure 2. There was no significant difference in clinical outcome between patients with vs. without distal left main disease, although there was a non-significant trend towards lower MACCE in non-distal (i.e., proximal or mid) lesions as shown in Figure 3 (hazard ratio [HR] 1.04; 95% confidence interval [CI] 0.43 to 2.54; p=0.92). For distal lesions, the MACCE rate through 36-month follow-up remained slightly lower for patients receiving a single stent versus multiple (two or three) stents, but this difference did not reach statistically significant differences (HR 1.05; 95% CI 0.36 to 3.05; p=0.92 [Figure 4]). Finally, the MACCE rate was independent of whether the SYNTAX score was <22 or ≥22 (20.2% vs. 24.3%)( HR 2.86; 95% CI 0.38 to 1.05; p=0.92).
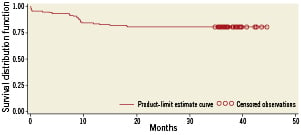
Figure 2. Actuarial rate of event-free survival.
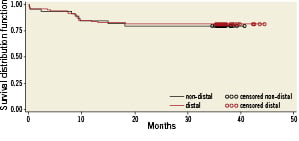
Figure 3. Actuarial rate of event-free survival between non-distal and distal left main disease.
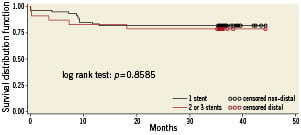
Figure 4. Actuarial rate of event-free survival between one and two or three stents implantation in distal left main disease.
According to the Academic Research Consortium definition, there were three episodes of acute or sub-acute in-stent thromboses and two probable very late in-stent thrombosis 2.5 years after the index procedure, while not on dual antiplatelet therapy.
Discussion
The FRIEND registry is one of the first prospective multicentre studies presenting long-term results of unprotected LM stenting. The early results of LM stenting with high procedural and clinical success (98% and 95%, respectively) and low periprocedural mortality (1.9%) and MACCE (3.9%) prove safety and feasibility of this procedure, despite the initial high risk profile of study population (EuroSCORE 4.2; ACS in 47% of patients). The present study is similar to these other DES series8-10 of ULMCA in showing a favourable (2%), 30-day mortality after stenting of unprotected LMCA stenosis in elective treatment, supporting consideration of this therapy as an alternative to bypass surgery in selected patients.
Long-term mortality of 6.7% and the three-year survival of 93.3% are very satisfactory and compare favourably with survival presented in long-term outcomes of coronary artery bypass surgery in the CASS (Coronary Artery Surgery Study) trial2,11 and the Duke University database11.
These extremely favourable short- and long-term outcomes in the current study, with no major events during the follow-up period have several potential explanations. Firstly, this population has a lower risk profile compared with other trials12,13; it had a low percentage (3.3%) of diabetic patients, left ventricular function was well preserved, renal function was good, mean age was 68.3±10.8 years, a large reference vessel diameter of 3.92±0.69 mm and a majority of patients (75.5%) with a low SYNTAX score (<22).
Secondly, this prospective multicentre registry of 23 participating centres is the first to systematically study the provisional T-stenting approach for distal left main disease (DLMD), rather than the double stent techniques used in many previous studies. The prior literature has consistently shown significantly worse long-term outcomes in patients undergoing percutaneous treatment of distal compared to proximal or mid-vessel LMCA lesions. Valgimigli et al12 showed that distal LMCA disease carried independent negative prognostic implications that should be considered when selecting the most appropriate patients for LMCA intervention. With our use of provisional T-stenting (and mostly [72%] single stent technique and final kissing-balloon in 93%), however, the procedural success and the short-term (30 days) outcome was remarkably similar between proximal-mid and distal lesions. Although there was still a trend towards a slightly higher rate of events at 12-month follow-up, driven mainly by a higher rate of TVR in the DLMD group, the avoidance of more complex multi-sent techniques such as crush, V, culotte or kissing stenting in the current series may well account for the excellent mid-term results. Angiographically confirmed restenosis occurred in 2.3% of patients. This result is lower than in studies involving only DES patients. These results could change future practice possibly using routine MSCT rather than QCA follow-up. We cannot, however, be certain of the benefit here given the small number of multiple stent patients in our series. At the same time, DES implantation in non-bifurcation left main coronary artery lesions showed an excellent (0.9%) long-term restenosis rate, with a favourable (7.4%) rate of any major adverse clinical event, compared to prior studies. Unlike the left main Taxus pilot study13, we did not find any impact of bifurcation angle between LAD and LCX (
Our results validate the recent large randomised trial (ISAR-LEFT MAIN [Intracoronary Stenting and Angiographic Results: Drug-Eluting Stents for Unprotected Coronary Left Main Lesions]), which found that SES and PES were equally effective and safe in patients undergoing unprotected LMCA stenting14. After 12 months, the incidences of death (6.6% vs. 5.0%), MI (4.6% vs.5.0%), stroke (1.0% vs. 1.7%), and major adverse cardiac event (death, MI, or revascularisation; 15.8% vs. 13.6%) were similar in the SES and PES groups, as were angiographic restenosis rates at six to nine months (19.4% vs.16.0%) and two-year revascularisation rates (7.8% vs. 6.5%).
Concerns have been raised recently regarding the long-term safety of DES, with particular regard to late stent thrombosis and late mortality15-17. Increasing concern over stent thrombosis, which might have more catastrophic consequences in patients undergoing unprotected LMCA stenting, and a lack of long-term clinical data have hampered the widespread use of PCI with DES as an alternative to CABG. A recent multicentre registry of 731 patients undergoing LMCA stenting with DES found that the rate of definite or probable thrombosis after 30 months was 0.95%18. Similar results were observed in another large registry (DELFT study [Sirolimus Versus Paclitaxel Drug-Eluting Stent for Left Main Registry]), with three-year rates of definite, probable, and possible stent thrombosis of 0.6%, 1.1%, and 4.4%, respectively19, and in a clinical study14 with a two-year rate of definite or probable stent thrombosis of 1.3%. We observed a similar incidence of definite or probable stent thrombosis (1.4%), providing further evidence that DES implantation in patients with unprotected LMCA disease results in lower or, at worst, similar rates of stent thrombosis and long-term mortality than are observed in patients with other coronary lesions20.
In addition, our study provides the longer-term follow-up results up to median three years in routine clinical practice. These findings support and extend the recent results of the SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) study, in which the patient subset with LM disease treated with DES had a similar overall MACCE at one-year follow-up to those treated with CABG.
In contrast with the FRIEND registry, the aim of the SYNTAX trial21 (which included planned complete revascularisation with multivessel PCI) was to investigate the non-inferiority of multivessel PCI with DES to CABG. Besides the exclusion of patients with post-PCI lesions in the SYNTAX study, there are other differences between the SYNTAX and FRIEND trials: patients included in the FRIEND registry always exhibited a left main lesion with bifurcation or trifurcation lesion and three-vessel disease (66% and 46%, respectively). The patients in the SYNTAX study PCI arm had more treated lesions (mean: 3.6), longer stented segments (mean: 86.1 mm) and more frequent total occlusion (24.2%) than the FRIEND study patients. Additionally, 88% of the FRIEND study patients underwent routine angiographic follow-up, even if this approach may increase the incidence of non clinically-driven revascularisation, especially in some patient groups (e.g., left main disease).
In spite of the differences between the patients in the SYNTAX and FRIEND trials, the two-year cumulative MACCE rates and the rates of TLR, death and stroke were slightly lower in the FRIEND registry, which suggests, as well as the fact that the mean SYNTAX score was low, the possible preventive role of systematic provisional T-stenting.
Study limitations
First, the data do not necessarily apply to interventional cardiologists with less prior experience with LMCA stenting. Second, the protocol did mandate the routine use of intravascular ultrasound to assess the result of stenting. Third, the results of our study are very encouraging but they cannot be conclusive: our findings in carefully selected patients may not be generally applicable to the entire range of unprotected LMCA stenosis.
Conclusions
The event rate after percutaneous treatment of ULMCA disease in a FRIEND multicentre registry was remarkably low, with an excellent short- and long-term prognosis in a low surgical risk population. Our findings extend the previous knowledge about risk stratification and technical approach, and may be helpful in identifying the most appropriate LMCA patients in whom catheter-based intervention may offer the best alternative to bypass surgery. Other long-term additional data from large scale randomised trials such as SYNTAX, will be forthcoming to shed further light on this important subgroup of patients.
Acknowledgements
The authors thank all investigators and research coordinators who contributed to the successful completion of this study. The promoter of the study was the Société Française de Cardiologie. We would like to thank the experts who reviewed the major adverse events: Professor Victor Legrand, Professor Jean Jacques Goy and Professor Michel Bertrand.
Conflict of interest
All authors have no conflict of interest to declare in relation to this paper.
Grant sponsor
Boston Scientific, Montigny Le Bretonneux, France.

