Abstract
Aims: To assess two-year outcomes following first vs. new-generation drug-eluting stent (DES) implantation in unprotected left main (ULMCA) percutaneous coronary intervention.
Methods and results: All eligible patients from our two-centre registry treated with first and new-generation DES from October 2006 to November 2010 were analysed. The study objective was major adverse cardiac events (MACE), defined as all-cause mortality, target vessel revascularisation (TVR) and myocardial infarction (MI) at two years. In total, 186 patients were included: 93 (50.0%) treated with first vs. 93 (50.0%) with new-generation DES. No differences were observed in baseline clinical characteristics except for higher EuroSCORE with new-generation DES (3.6±2.5 vs. 4.6±2.7; p=0.007). No significant difference was observed in stenting techniques; two stents were used respectively in 53.8% vs. 44.1% (p=0.187). Notably, intravascular ultrasound guidance was more frequent with new-generation DES (46.2% vs. 61.3%; p=0.040). At 730.0 (interquartile range 365.5-1,224.5) days, there was a trend towards improved MACE with new-generation DES (31.2% vs. 19.6%; p=0.070) and a significant reduction in TVR (23.7% vs. 12.0%; p=0.038) and MI (4.3% vs. 0%; p=0.044). Notably, there were four cases of definite stent thrombosis (ST) with first vs. none with new-generation DES (p=0.044).
Conclusions: In our study, new-generation DES had a trend for less MACE and improved results with regard to MI, TVR and definite ST at two-year follow-up.
Introduction
Unprotected left main coronary artery (ULMCA) disease remains a class I indication for coronary artery bypass grafting (CABG)1,2. However, with the advent of drug-eluting stents (DES), a significant reduction in restenosis and target lesion revascularisation (TLR) has been observed following the percutaneous coronary intervention (PCI) of ULMCA disease3-9.
The new-generation DES were developed with the aim of improving the efficacy and safety of PCI. These stents, however, have not been extensively studied in the ULMCA subgroup10-13. Recently, the LEMAX (Left main XIENCE V) study reported favourable results following everolimus-eluting stent (EES) implantation in ULMCA14 as did a prospective randomised trial of everolimus and zotarolimus-eluting stents for treatment of ULMCA disease (ISAR-LEFT MAIN 2). However, to our knowledge, no previous study has reported the outcomes of ULMCA PCI, comparing both first and all new-generation DES implanted over a contemporary time period.
Methods
PATIENTS AND PROCEDURES
Between October 2006 (when the new-generation DES became available) and November 2010, all eligible patients treated for ULMCA disease in the San Raffaele Scientific Institute, Milan, Italy, and the EMO-GVM Centro Cuore Columbus, Milan, Italy, with DES implantation (first or new-generation) were included in our registry. Patients with ST-elevation myocardial infarction (STEMI), cardiogenic shock and contraindications to dual antiplatelet therapy (DAPT) were excluded.
Full written informed consent was obtained for the procedure and for subsequent data collection.
The decision to perform PCI instead of surgery was considered when there was suitable anatomy for stenting and there was a preference by the patient and referring physician for PCI or when there was suitable anatomy for stenting and the patient was high risk for CABG as deemed by the cardiothoracic surgeon.
Each patient was preloaded with clopidogrel and continued on clopidogrel 75 mg daily in combination with aspirin 100 mg for 12 months. Aspirin 100 mg was continued indefinitely thereafter. At the start of the procedure, weight-adjusted unfractionated heparin or bivalirudin was administered. Coronary angioplasty and stent implantation, including bifurcation strategy in the case of distal disease, were performed according to operator preference with the aim of complete coverage of the diseased segment.
The choice of DES used was at the discretion of the operator and according to stock availability and the required size and length. The first-generation DES used were: sirolimus-eluting stents (SES) (CYPHER SELECT™; Cordis, Johnson and Johnson, Warren, NJ, USA) and paclitaxel-eluting stents (PES) (TAXUS® Liberté®; Boston Scientific, Natick, MA, USA). The new-generation stents were: EES (XIENCE V®/XIENCE PRIME™; Abbott Vascular, Santa Clara, CA, USA, and PROMUS®/PROMUS Element™; Boston Scientific), zotarolimus-eluting stents (ZES) (Endeavor® Resolute; Medtronic Inc., Minneapolis, MI, USA), and biolimus A9-eluting stents (BES) (BioMatrix™; Biosensors Inc., Newport Beach, CA, USA, and Nobori®; Terumo Medical Corp., Tokyo, Japan).
Clinical follow-up was performed at one, six, 12 and 24 months via telephone calls or clinic visit. Angiographic follow-up was not mandatory unless there were clinical symptoms or subjective evidence of ischaemia on functional testing.
DEFINITIONS
The events analysed during hospital stay and at two-year clinical follow-up were major adverse cardiac events (MACE), defined as a composite of death, myocardial infarction (MI) and target vessel revascularisation (TVR).
Deaths were classified as either cardiac or non-cardiac. Death from unknown cause was adjudicated as cardiac. Periprocedural non-Q-wave MI was defined as the elevation of the serum creatinine kinase (CK) isoenzyme MB three times the upper limit of normal, in the absence of new pathological Q-waves. Q-wave MI was defined as the development of new pathological Q-waves in two or more contiguous leads with or without CK-MB levels elevated above normal. Spontaneous MI was defined as the occurrence after hospital discharge of any value of troponin and/or CK-MB greater than the upper limit of normal if associated with clinical and/or electrocardiographic changes15.
TLR was defined as any revascularisation performed on the treated segment (stent and 5 mm proximal and distal). TVR was defined as any reintervention performed on the treated vessel and also included treatment of any segment in the left anterior descending and circumflex arteries. Stent thrombosis (ST) was adjudicated as per the Academic Research Consortium (ARC) definitions15.
STATISTICAL ANALYSIS
Continuous variables are expressed as mean±SD and analysed with the Student’s t-test or Wilcoxon rank-sum test depending on the variable distribution. Categorical variables were compared with the chi-squared test with Yates correction for continuity or the Fisher’s exact test as appropriate. A regression model analysis was performed to determine the independent predictors of study endpoints using purposeful selection of covariates; variables identified at univariate analysis and of clinical importance from previous literature were eligible for inclusion into the multivariable model and included age, sex, diabetes mellitus, hypertension, hypercholesterolaemia, smoking, family history of coronary artery disease, unstable angina, chronic kidney disease, left ventricular ejection fraction, European System for Cardiac Operative Risk Evaluation (EuroSCORE) and Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) score ≥33. These results are reported as odds ratios (OR) with 95% confidence intervals (CI). Survival was recorded by Kaplan-Meier analysis and the log-rank method was used for comparison. Statistical analysis was performed with Statistical Package for Social Sciences Version 18.0 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered statistically significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agreed to the manuscript as written.
Results
In total, 186 patients underwent PCI for ULMCA: 93 (50.0%) of these were treated with first-generation DES (49 SES and 44 PES) and 93 (50.0%) with new-generation DES (73 EES, 11 ZES and 9 BES).
BASELINE AND PROCEDURAL CHARACTERISTICS
The baseline characteristics of the study population are illustrated in Table 1. A significantly higher EuroSCORE was found in those treated with new-generation DES (3.6±2.5 vs. 4.6±2.7; p=0.007).
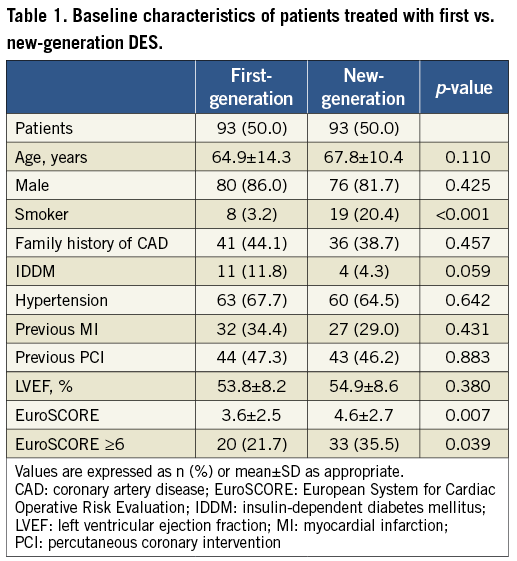
With regard to procedural and lesion characteristics (Table 2), there were no differences between first and new-generation DES in the frequency of distal ULMCA disease (respectively 81.7% vs. 74.2%; p=0.216), the presence of Medina true bifurcations (72.0% vs. 61.3%; p=0.120) or in the use of a two-stent strategy (53.8% vs. 44.1%; p=0.187). When a two-stent strategy was employed, culotte was the most frequent technique used both in first (n=29; 58.0%) and new-generation (n=19; 46.3%) DES, followed by the mini-crush (n=10; 20.0% in first, and n=8; 19.5% in new-generation DES). However, IVUS was used more frequently to guide the procedure in the new-generation group (46.2% vs. 61.3%; p=0.040). In addition, in this group of patients the stent diameter was greater (3.5±0.3 mm vs. 3.6±0.3 mm; p=0.027) and the stent length shorter (20.8±7.8 mm vs. 18.6±7.4 mm; p=0.048).
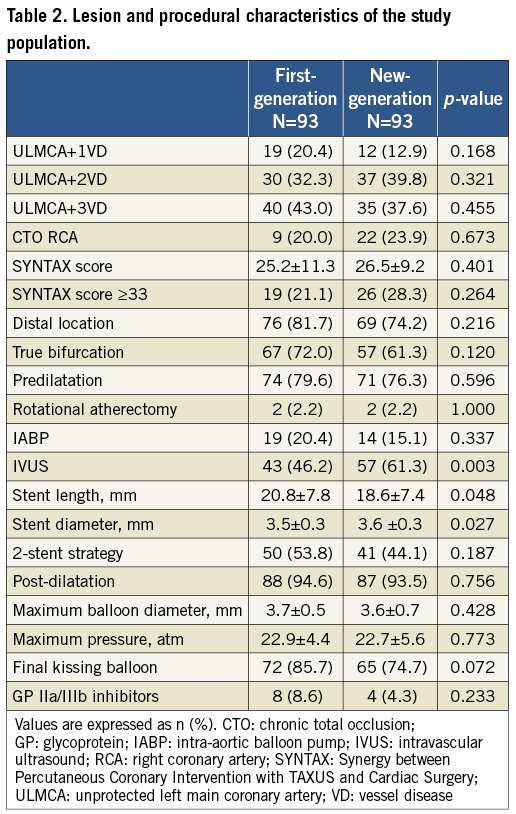
With regard to DAPT, in total 96.8% of the first and 100% of the new-generation group were compliant with our recommendation. In the first-generation group, two had clopidogrel stopped due to restenosis and the decision to proceed to CABG. A further patient treated with a PES stopped clopidogrel after one month with no adverse consequences.
IN-HOSPITAL OUTCOMES
There were no differences observed according to grouping in the in-hospital outcomes. MACE occurred in three (3.2%) of the first-generation vs. four (4.3%) of the new-generation group (p=0.700). This was mostly driven by periprocedural MI, with two (2.2%) in the first vs. four (4.3%) in the new-generation group (p=0.424). Only one death (cardiac death at day 17) was observed in a patient who underwent a hybrid procedure (PCI with first-generation DES followed by surgical aortic valve replacement).
TWO-YEAR OUTCOMES
The outcomes at two years are shown in Table 3. There was no difference between groups in the clinical follow-up duration (first-generation 887.9±513.5 days vs. new-generation 748.4±497.4 days). With new-generation DES there was a statistical trend for a reduction in the study objective of MACE (31.2% vs. 19.6%; p=0.070). In addition, a trend in improved cardiac mortality (7.5% vs. 2.2%; p=0.091), but with no differences in all-cause mortality (10.8% vs. 7.6%; p=0.459), was observed in the new-generation DES group.
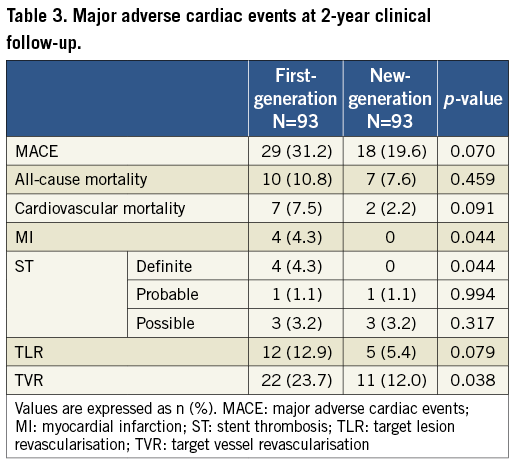
An advantage in the occurrence of MI (4.3% vs. 0%; 0.044) was also shown in this group. Notably, patients who had MI at follow-up were the patients who experienced definite ST, which consequently was significantly less frequent with new-generation DES (4.3% vs. 0%; p=0.044). Regarding the four cases of definite ST in first-generation DES, three of them occurred following PES, the characteristics of which are illustrated in Table 4. All patients survived the repeat intervention.
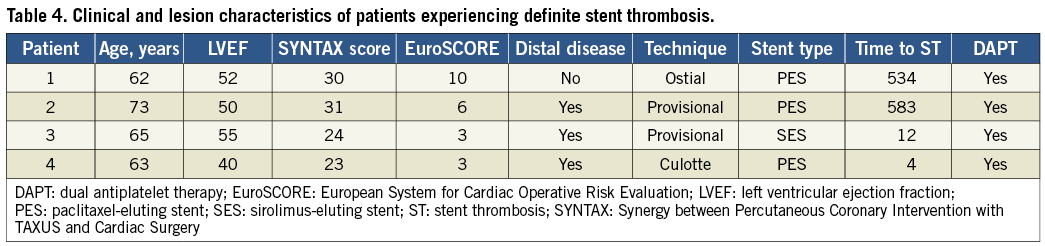
Only one (1.1%) probable ST was reported in first-generation DES and one (1.1%) probable ST in new-generation DES (p=0.994). Regarding the only patient who experienced a probable ST with new-generation DES, it was a sudden death at day 20 in a patient following ZES implantation who was taking DAPT. The probable ST with first-generation DES occurred 1,226 days following SES in a patient who presented with an acute MI and died prior to undergoing repeat intervention. This patient was taking only aspirin monotherapy.
Overall, 73.1% of those undergoing first vs. 56.5% of those with new-generation DES implantation underwent angiographic follow-up (p=0.018). A benefit in favour of new-generation DES was additionally observed in TVR (23.7% vs. 12.0%; p=0.038) as well as a trend in TLR (12.9% vs. 5.4%; p=0.079).
Interestingly, there were no differences in patients treated with IVUS vs. those treated with only angiography guidance in TVR (15.2% vs. 22.1%; p=0.224), MI (3.0% vs. 1.2%; p=0.384) or MACE (IVUS 21.2% vs. angiography 31.4%; p=0.115).
Furthermore, when specifically PES vs. new-generation DES were analysed, there was a significant improvement in MACE (38.6% vs. 20.7%; p=0.026), MI (6.8% vs. 0%; p=0.011) and TVR (27.3% vs. 13.0%; p=0.042) with all new-generation DES, with a trend for improved cardiovascular mortality (9.1% vs. 2.2%; p=0.066). Additionally, when the SES group was compared with all new-generation DES, there was a trend for improved TLR (14.3% vs. 5.4%; p=0.073), Figure 1.
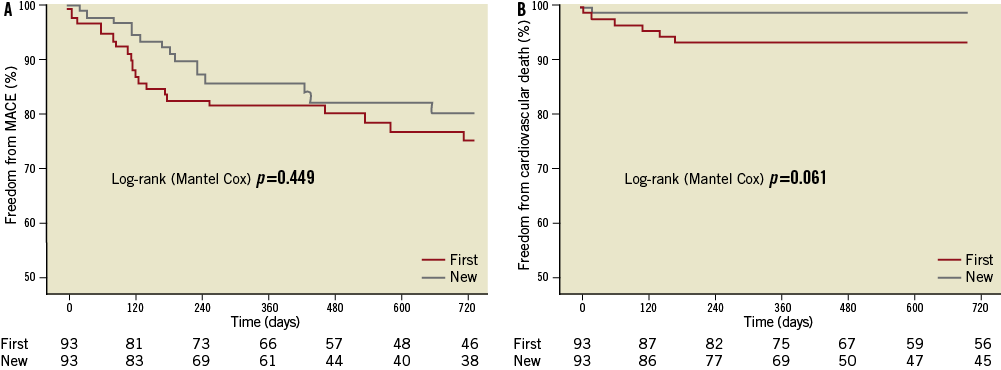
Figure 1. Kaplan-Meier curves demonstrating freedom from study endpoints. Freedom from events: A) major adverse cardiac events; B) cardiac mortality and myocardial infarction in first- ( ) vs. new- ( ) generation drug-eluting stents.
MULTIVARIATE ANALYSIS
Predictors of MACE on multivariable analysis were EuroSCORE (OR 1.134; 95% CI: 1.000-1.286; p=0.0509) and the use of PES (OR 2.119; 95% CI: 0.978-4.591; p=0.0568).
Discussion
The main findings of our study are: 1) new-generation DES led to a trend towards a reduction of MACE at two years as compared with first-generation DES; 2) benefits in MI and TVR were observed, as was a trend towards lower cardiac mortality with new-generation DES as compared with first-generation DES; 3) definite ST occurred less frequently with new-generation DES; 4) the use of PES was associated with MACE.
With the advent of DES, outcomes of ULMCA PCI have improved significantly, with encouraging results reported at midterm clinical follow-up3-5,9,16-21. The new-generation DES were subsequently developed and have shown superior results compared to first-generation DES10,11. However, these studies typically did not include the ULMCA, and consequently there is a paucity of data in this subgroup10-13. One study (LEMAX) reported favourable results in 173 patients following EES implantation in the ULMCA14. Recently, the results of the Prospective Randomized Trial of Everolimus- and Zotarolimus-eluting Stents for Treatment of Unprotected Left Main Coronary Artery Disease (ISAR-LEFT MAIN 2) were published22 comparing outcomes with EES vs. ZES. The primary study endpoint at one year of MACE was 14.3% in the EES group vs. 17.5% in the ZES group (p=0.25). However, to our knowledge, no previous study has reported outcomes of ULMCA PCI, comparing both first and all new-generation DES implanted over a contemporary time period.
In our study, we compared the results of patients undergoing ULMCA PCI with first and new-generation DES over a similar time period. The choice of DES was at the discretion of the operator and depended on availability.
At two-year clinical follow-up, there was a trend for reduced MACE favouring the new-generation DES group (31.2% vs. 19.6%; p=0.070). These stents have different platforms, polymer and drug combinations with the aim of improving PCI safety and efficacy by providing more rapid endothelialisation and effective suppression of neointimal hyperplasia. Due to these factors, rates of restenosis and ST have been demonstrated to be lower10,11,23. In the Trial of Everolimus-Eluting Stents and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice (COMPARE) which evaluated 1,800 unselected patients (only 2.0% ULMCA), there was a significant reduction in the composite endpoint of all-cause mortality, MI and TVR at two years in those patients randomised to EES (9.0% vs. 13.7%; relative risk [RR] 0.66; 95% CI: 0.50-0.86)24. It was also demonstrated to be significant between one-year and two-year follow-up (p=0.02). Similar results were demonstrated in the Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System (SPIRIT) IV study, where 3,687 patients were randomised to EES (n=2,458) or PES (n=1,229). At two-year follow-up, the incidence of TLR was significantly reduced with the EES (4.5% vs. 6.5%; p=0.004) in addition to the rates of MI (2.5% vs. 3.9%; p=0.02) and ST (0.4% vs. 1.2%; p=0.008)25. Indeed, the use of PES in our study actually correlated with MACE at two years. It must be noted, however, that no clear superiority has previously been demonstrated with new-generation stents compared with the first-generation SES26-28, which we have included in this group, but only with PES. However, in the only randomised controlled trial of PES vs. SES performed in the setting of ULMCA, there was no difference between the two stent types in the composite endpoint of death, MI or TLR21.
From a safety perspective, in our study there was a tendency for a reduction in cardiac mortality amongst the new-generation group (7.5% vs. 2.2%; p=0.091), despite a higher EuroSCORE in this cohort. This is comparable to the 1.2% cardiac mortality reported in the LEMAX study at one-year clinical follow-up14.
Furthermore, with the new-generation DES there were no MI at two-year clinical follow-up compared with four of the first-generation group (p=0.044). Notably, all reported MI were secondary to the occurrence of definite ST. It is reassuring that there were no such events in the new-generation group, as concerns have been raised regarding the risk of late and very late ST following DES implantation. This is particularly important in the ULMCA cohort where such an event could prove fatal. Only one probable subacute ST occurred in a patient treated with the new-generation DES. The more rapid endothelialisation with the new-generation DES is the most plausible explanation for this low rate. Interestingly, three out of four of the definite ST occurred following PES, with previous reports showing a similar higher rate of ST with PES as compared with EES10,11. It must also be noted that, in our series, definite ST occurred only in patients with intermediate SYNTAX score, not in the high-score group. Clearly, because of the small sample size and the rarity of such events in the population, we cannot exclude that such a finding was a consequence of the small number of patients.
Regarding efficacy, there also appears to be a benefit regarding revascularisation with the new-generation DES. In our study, there was a reduction in TVR (23.7% vs. 12.0%; p=0.038) and a trend for reduced TLR (12.9% vs. 5.4%; p=0.079) with the new-generation stents at two-year clinical follow-up, Figure 2. In addition to the use of the newer technology causing neointimal suppression, a higher use of IVUS guidance in the new-generation group (46.2% vs. 61.3% p=0.003) may have contributed, by ensuring adequate stent expansion and complete lesion coverage. This latter tool has been demonstrated to improve mortality in ULMCA PCI with DES, however not the risk of MI or TVR29. Indeed, in our study there were no differences in TVR, MI or MACE between those treated with IVUS vs. angiography guidance. We cannot exclude that the large experience of our centre in IVUS-guided procedures in the last 20 years with the development by the operators of an “IVUS eye” could be the explanation of such a finding. A further contributing factor may be the reduced stent length and greater stent diameter in the new-generation group. It might also be fair to point out that routine angiographic follow-up was performed more frequently in the first-generation group, which may have led to more angiographically driven rather than clinically driven TVR.
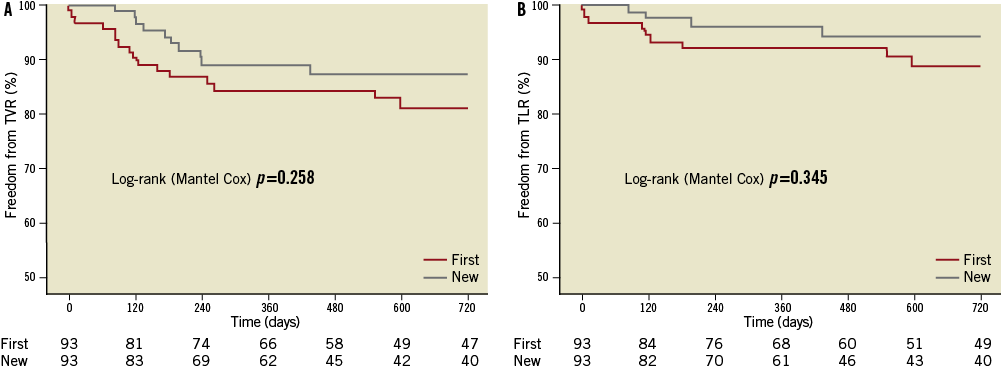
Figure 2. Kaplan-Meier curves demonstrating freedom from revascularisation. Freedom from events: A) target vessel revascularisation; and B) target lesion revascularisation in first- ( ) vs. new- ( ) generation drug-eluting stents.
Comparing our TVR rate with other data, in the LEMAX study, the rate of TVR at one-year follow-up was 7.0%14. However, our follow-up period was longer than in LEMAX. Also, in our study there was more use of a two-stent strategy (44.1% vs. 22.0%). This was despite a similar number of cases affecting the distal bifurcation (in our study 74.2%; in LEMAX 80.9%). The distal location of ULMCA disease has been shown to be an important predictor of MACE30.
Our study suggests improvement in safety and efficacy outcomes with new-generation DES for ULMCA PCI; however, this needs to be investigated further in a larger population, with a longer follow-up period and in a randomised fashion. Recently, the results of ISAR-LEFT MAIN 2 were reported showing very encouraging results. This and other future studies should help provide more insight into the possible advantages of new-generation DES in this complex lesion subset.
Limitations
The major limitation was that this was a non-randomised study, with all the inherent issues. The choice of DES was at the discretion of the operator and depended on availability of the required size and length. No sample size has been calculated a priori. In addition, due to the small study population it was not feasible to perform a propensity analysis to adjust for baseline characteristics. Furthermore, we cannot exclude that the more frequent use of larger and shorter stents observed in the new-generation group might have impacted on the study outcomes. In addition, the number of patients included in our registry may not have been adequate to detect differences in safety endpoints with such a low event rate. Finally, the length of clinical follow-up was limited.
Conclusions
In our registry study, the use of new-generation DES for ULMCA disease demonstrated a statistical trend for improved MACE and cardiac mortality at two-year follow-up. In addition, there was reduced MI, TVR and definite ST with the use of the new-generation DES. These results are hypothesis-generating and need to be confirmed at longer-term follow-up with larger patient numbers.
Conflict of interest statement
The authors have no conflicts of interest to declare.

