
Abstract
Aims: The aim of the study was to determine whether patients treated with drug-eluting stents in the proximal left anterior descending artery (LAD) carried a different long-term prognosis from patients treated in other coronary artery segments.
Methods and results: Ten-year clinical outcome expressed as all-cause mortality and major adverse cardiac events (MACE: cardiac death, acute myocardial infarction, or target vessel revascularisation) was determined for 1,479 patients with a single non-left main coronary stenosis treated with a first-generation drug-eluting stent in the SORT OUT II trial. The outcome of patients treated with stents in the proximal LAD (n=365) was compared with that of patients treated in a non-proximal LAD segment (n=1,114). Follow-up was 99.3% complete. All-cause mortality was 24.9% in the proximal LAD group vs. 26.3% in the non-proximal LAD group (p=0.60). MACE occurred less frequently in the proximal LAD group, 24.6% vs. 31.0% with a hazard ratio of 0.77 (95% confidence interval [CI]: 0.61-0.97, p=0.024). After multivariate analysis which included baseline characteristics that were unevenly distributed between the groups, the hazard ratio for MACE was 0.82 (95% CI: 0.65-1.03, p=0.09).
Conclusions: Patients treated with a drug-eluting stent in the proximal LAD have similar, if not better, long-term clinical outcome compared with patients stented in other coronary artery segments.
Abbreviations
CABG: coronary artery bypass grafting
CI: confidence interval
LAD: left anterior descending artery
MACE: major adverse cardiac events
PCI: percutaneous coronary intervention
SCAI: Society for Cardiovascular Angiography and Interventions
SORT OUT II: The Danish Organization on Randomized Trials With Clinical Outcome II
Introduction
The left anterior descending coronary artery (LAD) supplies a large portion, often >50%, of the left ventricular myocardium. Coronary artery disease jeopardising this artery can have dire consequences for the left ventricular function and thereby for the clinical outcome. Thus, patients with proximal LAD lesions are thought to have a higher risk of future cardiac events compared with patients with lesions in other coronary segments. The optimal treatment of proximal LAD stenoses is a matter of debate1 and is given special consideration in coronary revascularisation guidelines with changing recommendations2,3. Randomised controlled trials have shown similar mortality and major adverse cardiac events (MACE) after treatment of single proximal LAD lesions with a drug-eluting stent (DES) and coronary artery bypass grafting (CABG)4,5. Recently, it was demonstrated that four-year mortality and MACE rates were similar for patients treated with a DES in the proximal LAD compared with those stented in other coronary artery segments6. However, in that study the proximal LAD group had a higher rate of myocardial infarction during follow-up, but interestingly not a higher rate of target lesion failure. A possible explanation is more severe coronary disease at baseline with, for example, more lesions treated in the proximal LAD group1,6. As also noted in the NOBLE left main trial7, a difference in outcome may be driven not by the lesion of interest (e.g., left main or proximal LAD), but by other target or de novo lesions. To determine the outcome after DES treatment of the proximal LAD per se, it may be more appropriate to study patients treated for just one lesion. Because MACE continue to occur in the long term after coronary stenting8, a follow-up longer than four years after stenting of the proximal LAD is interesting1.
We hypothesised that treatment of the proximal LAD segment with a DES did not infer a different 10-year clinical outcome with respect to MACE (cardiac death, myocardial infarction, or target vessel revascularisation) and survival from stenting of other non-left main stem coronary artery segments using data from the SORT OUT II database8,9.
Methods
SORT OUT II encompassed 2,098 patients and 33 subsequently included patients with diabetes in order to reach the pre-specified number needed to complete a substudy8-10. All five Danish PCI centres randomised unselected all-comer patients to PCI with either a sirolimus-eluting CYPHER® stent (Cordis, Cardinal Health, Milpitas, CA, USA) or a paclitaxel-eluting TAXUS™ stent (Boston Scientific, Marlborough, MA, USA). A daily dose of 75 mg clopidogrel was prescribed for 12 months and aspirin 75 mg indefinitely. The design, methods and main outcomes of the SORT OUT II trial (ClinicalTrials.gov: NCT00388934) have been published previously8,9. The study was approved by the local ethics committees, and all patients gave written informed consent.
For the present analyses we included patients who were treated for single non-left main coronary artery lesions. Of 2,131 patients included in the main study, 606 were excluded because of treatment of more than one lesion, 23 due to left main stem lesion and 23 because of graft disease. Thus, the study population comprised 365 patients who were treated with a stent in the proximal LAD (the proximal LAD group) and 1,114 patients who were stented in another coronary artery segment not located in the left main stem (the non-proximal LAD group). In SORT OUT II the proximal LAD was defined as the coronary segment located between the distal end of the left main stem and the take-off of the first diagonal branch9. Stenting that extended from the proximal LAD into the mid LAD was classified as proximal LAD stenting. Stent treatment of a side branch counted as a separate lesion. As previously described, all coronary angiograms were reviewed by two authors (A.M. Galløe, N. Bligaard) in order to classify the lesion characteristics8,9.
The primary MACE endpoint was a composite of cardiac death, myocardial infarction, or target vessel revascularisation. Secondary endpoints were components of the primary endpoint, all-cause death, target lesion revascularisation, definite stent thrombosis, and definite or possible stent thrombosis. Cardiac death was defined as any death not clearly attributed to non-cardiac causes as previously described8. Target vessel revascularisation, target lesion revascularisation, and stent thrombosis were defined according to the Academic Research Consortium11. In SORT OUT II a treatment failure was defined as definite or probable stent thrombosis, target lesion revascularisation, angiographic restenosis even if left untreated, or cardiac death definitely connected to the initial PCI12. Complete follow-up was performed in all patients who had not emigrated. All endpoints were adjudicated by the events committee as previously described8,9.
STATISTICS
The cumulative proportion of patients experiencing an event was analysed according to the appearance of the first event for each patient. All-cause death was censored for emigration and the other endpoints were censored for emigration and death. The outcome of the proximal LAD vs. the non-proximal LAD group was compared using the log-rank test and reported as hazard ratios with 95% confidence intervals. Between-group differences in baseline characteristics were assessed using the Student’s t-test, the Mann-Whitney test or the chi-square test. Important patient characteristics found to be unevenly distributed between the two groups underwent: 1) testing for interaction with proximal LAD vs. non-proximal LAD grouping with regard to outcomes, and 2) testing of whether these characteristics were predictors of clinical outcomes and, if so, they were, together with proximal LAD vs. non-proximal LAD grouping, forced into a multivariate Cox regression with calculation of hazard ratios for clinical outcome.
Results
The baseline demographics of the two groups are presented in Table 1. Follow-up was complete, except for 10 patients who had emigrated, resulting in a 99.3% follow-up at 10 years. In 14 of the 384 patients who died (3.6%), the cause of death could not be classified. The proximal LAD group had better outcome for MACE and myocardial infarction (Table 2) with hazard ratios vs. the non-proximal LAD group <1.00 for all examined individual endpoints. Survival curves for MACE, cardiac death, myocardial infarction and target vessel revascularisation are presented in Figure 1A-Figure 1D, and survival curves for all-cause death, target lesion revascularisation, definite, and definite and probable stent thrombosis, respectively, are depicted in Figure 2A-Figure 2D.
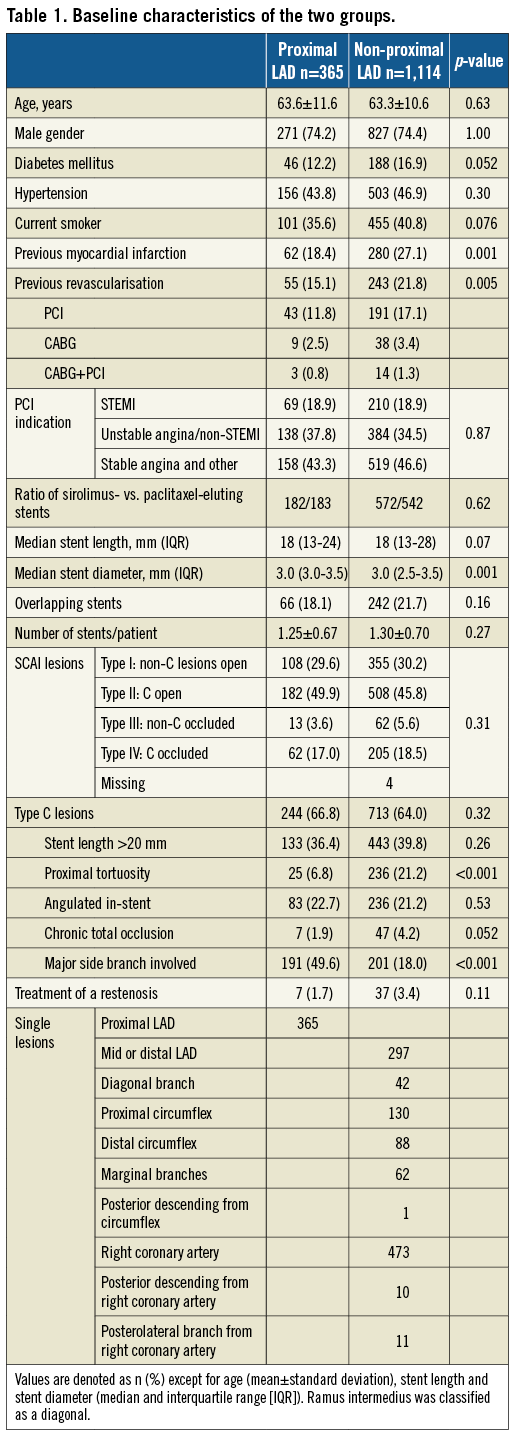
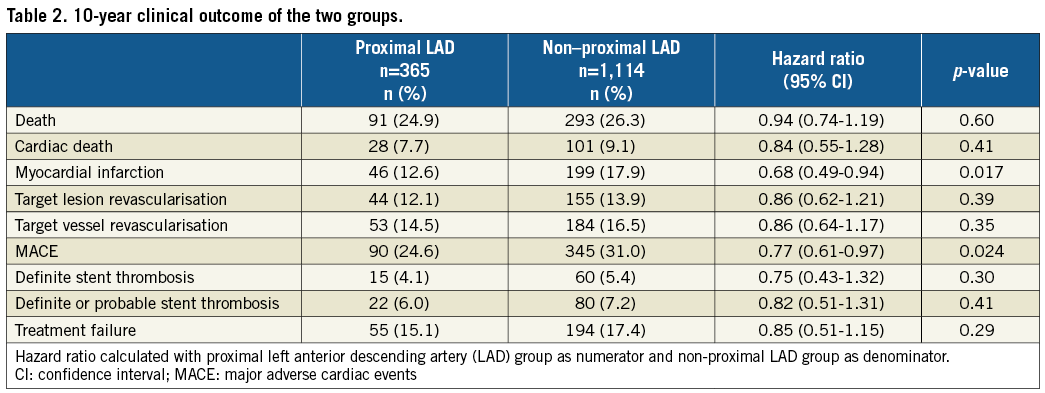
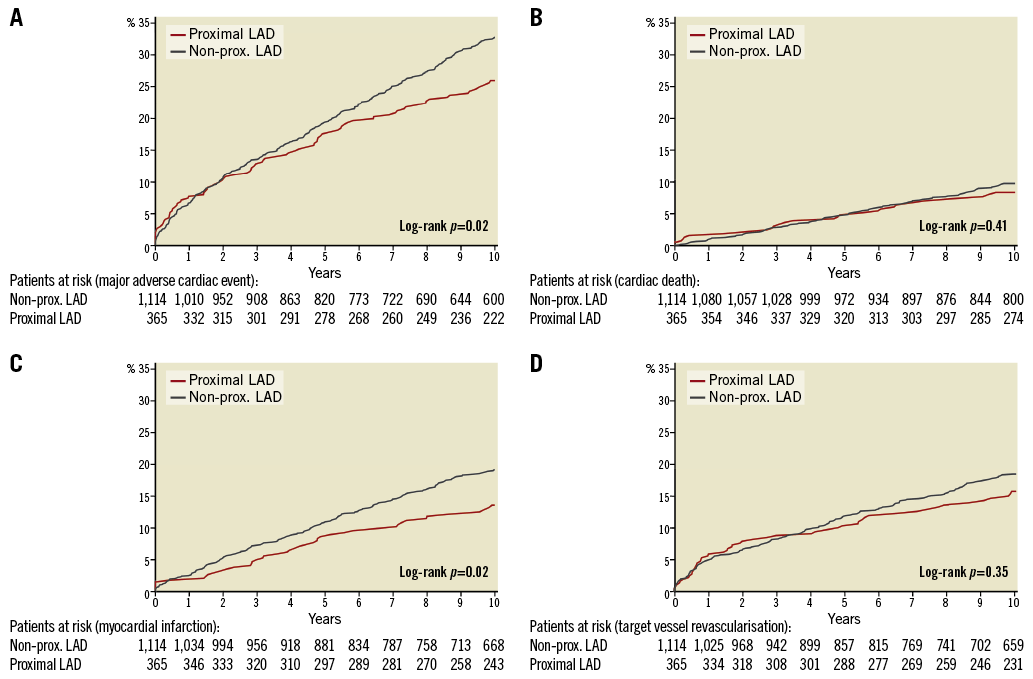
Figure 1. Major adverse cardiac events and their individual components. The cumulative incidence (%) of MACE (A), cardiac death (B), myocardial infarction (C), target vessel revascularisation (D) by time (years). The number of patients at risk each year in the two groups is noted below the abscissa. LAD: left anterior descending artery; MACE: major adverse cardiac events; prox.: proximal
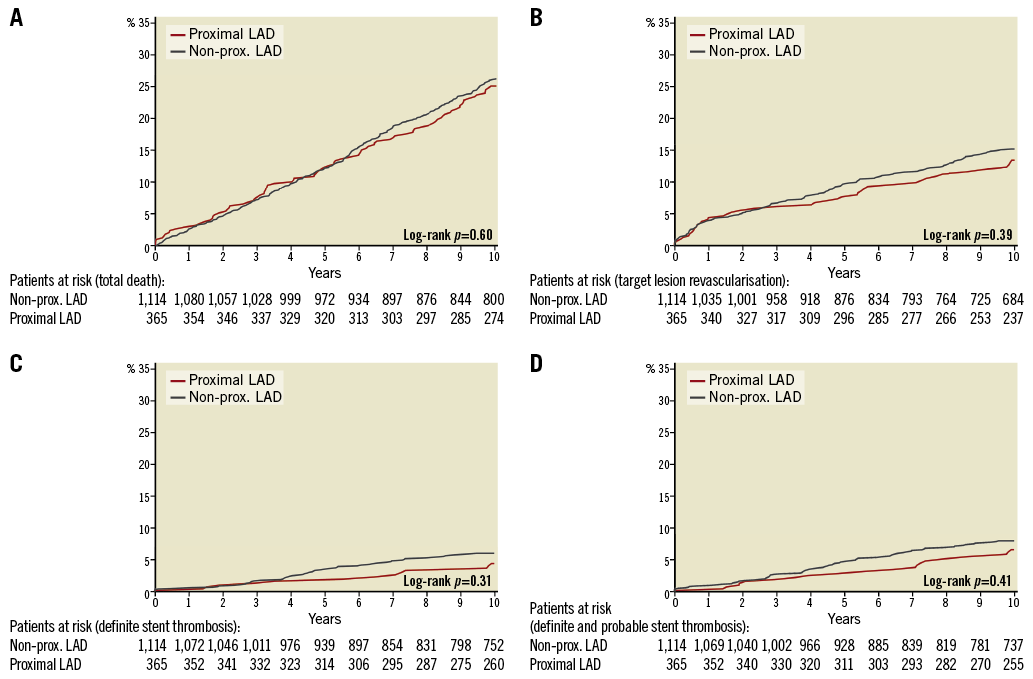
Figure 2. Ten-year clinical outcome for secondary endpoints. The cumulative incidence (%) of all-cause death (A), target lesion revascularisation (B), definite stent thrombosis (C), and definite or probable stent thrombosis (D) by time (years). The number of patients at risk each year in the two groups is noted below the abscissa. LAD: left anterior descending artery; prox.: proximal
The absolute difference in MACE was 6.5%. A little more than half of this difference is well explained by the differences in cardiac death and treatment failure, that includes definite and probable stent thrombosis, target lesion revascularisation and target vessel-related myocardial infarctions (Table 2). The number of myocardial infarctions that can be ascribed as treatment failure was 28 of 46 (60.9%) in the proximal LAD group and 109 of 199 (54.5%) in the non-proximal LAD group, p=0.51.
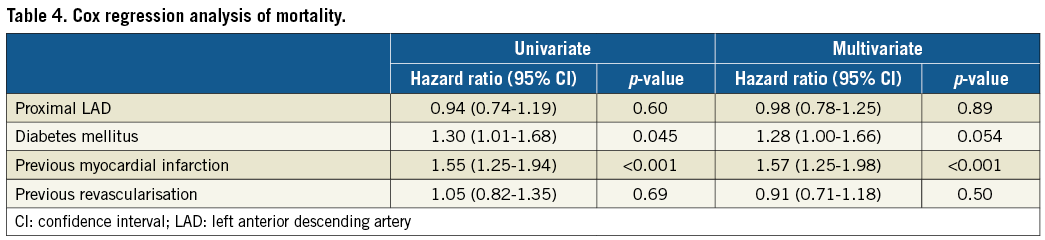
The results of multivariate Cox regression analyses are presented in Table 3 and Table 4. The hazard ratio for MACE occurring in the proximal LAD vs. non-proximal LAD group increased slightly from 0.77 to 0.82 (7% relative change) when the covariates diabetes, previous myocardial infarction, and previous revascularisation were included in the multivariate analysis. The hazard ratio of all-cause death was <1.00 for the proximal LAD group also after multivariate analysis.
There was no interaction in terms of MACE or mortality between the proximal LAD group vs. the non-proximal LAD group and the use of sirolimus- or paclitaxel-eluting stents (p=0.49 and p=0.48), diabetes mellitus (p=0.22 and p=0.62), previous myocardial infarction (p=0.73 and p=0.75), or previous revascularisation (p=0.79 and p=0.47). If patients with a previous revascularisation were excluded from the analysis, the hazard ratios for the proximal LAD group (n=310) compared with the non-proximal LAD group (n=871) were 0.81 (95% CI: 0.62-1.05, p=0.12) for MACE and 0.91 (95% CI: 0.70-1.18, p=0.46) for all-cause mortality.
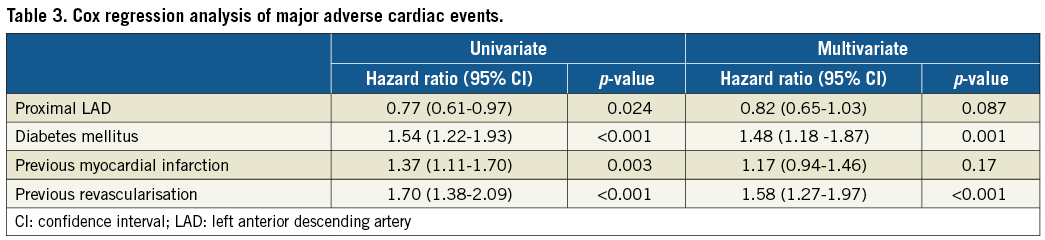
The higher risk of death with a previous myocardial infarction was largely driven by a higher risk of cardiac death. The hazard ratio for cardiac death with a previous myocardial infarction was 2.01 (95% CI: 1.40-2.88, p<0.001). A significant interaction on MACE and mortality (p=0.001 for both) between proximal LAD vs. non-proximal LAD grouping was observed. Patients with chronic occlusion in the proximal LAD group (n=7) fared worse. If chronic occlusion was forced into the multivariate analysis, the hazard ratios and p-values were similar for the remaining covariates, as presented in Table 3 and Table 4.
OUTCOME IN PROXIMAL LAD AND NON-PROXIMAL LAD GROUPS IF PATIENTS TREATED FOR MULTIPLE LESIONS ARE INCLUDED
Patients treated for graft or left main lesions were excluded. Treatment of multiple lesions was significantly more prevalent in the proximal LAD group (37.3% vs. 23.5%, p=0.0001). Nevertheless, outcome was neutral between the groups. Among those treated for a proximal LAD lesion, 174 of the 582 patients (29.9%) had a MACE compared with 478 of 1,456 patients (32.8%) in the non-proximal LAD group (hazard ratio for proximal LAD group 0.89 [95% CI: 0.75-1.05, p=0.22]). Also, all-cause mortality was similar in the proximal LAD and non-proximal LAD groups (hazard ratio 1.03 [95% CI: 0.85-1.24, p=0.96]).
REPRESENTATIVENESS
The 30-day and one-year mortality in the SORT OUT II study population was 0.9% and 3.0%, respectively10. In comparison, the 30-day and one-year mortality in unselected nationwide data from a population with a similar distribution of patients with ST-elevation myocardial infarction, non-ST-elevation myocardial infarction/unstable angina and stable angina pectoris reported in the Danish Heart Registry was 2.1% and 4.7%, respectively13. Accordingly, patients with the highest 30-day mortality were not included in SORT OUT II. However, the SORT OUT II study population had a 30-day to one-year mortality comparable to the unselected population, i.e., 2.1% vs. 2.6%. The percentage of patients treated for one lesion only in SORT OUT II (69.4%) was similar to the percentage reported for all patients who underwent PCI in our country (also 69.4%)13.
Discussion
The present study with 10-year follow-up of patients randomised to implantation of one of the two first-generation drug-eluting stents for a single coronary artery lesion demonstrates that patients stented in the proximal LAD had a similar survival rate yet a lower MACE rate compared to those stented in another non-left main stem coronary artery segment. A number of demographic characteristics that might have impacted on the clinical outcome were not evenly distributed between the two groups. On the other hand, inclusion of these demographic characteristics in a multivariate Cox regression model changed the hazard ratio for MACE by only 7%, i.e., from 0.77 at univariate analysis to 0.82 after multivariate analysis. It is worth noting that the hazard ratio for the proximal LAD group was <1.00 for any examined endpoint and, concerning the lesion-specific outcomes, such as stent thrombosis, target lesion revascularisation, and treatment failure, the hazard ratios for the proximal LAD group ranged from 0.75 to 0.86. Interestingly, the cumulative incidence curves for MACE, target vessel and target lesion revascularisation and stent thrombosis, respectively, in our study seemed to diverge in favour of the proximal LAD group after three to four years, i.e., later than the observation period in the PROTECT trial6. We found no difference in all-cause mortality between the proximal LAD and non-proximal LAD groups in univariate or multivariate analyses. Non-cardiac deaths accounted for approximately two thirds of all deaths in our study, corresponding to the findings of a long-term study of Danish patients with ST-elevation myocardial infarction14. Accordingly, the long-term impact of revascularisation and coronary artery disease on survival is diluted by non-cardiac deaths.
If patients treated for multiple lesions were included in the analysis, the 10-year outcome for MACE and survival was still similar for patients stented in the proximal LAD compared with patients stented in non-proximal LAD segments. In two large studies with two and four years of follow-up, Kerner et al15 and Roguin et al6 found that stenting of the proximal LAD did not confer a different prognosis, nor should it be regarded differently from stenting of other coronary artery lesions.
Our findings indicate that patients treated with a DES in the proximal LAD have a 10-year clinical outcome at least as good as patients treated with a DES in another non-left main coronary segment. Thus, our results, in accordance with the studies by Kerner et al and Roguin et al6,15, do not support the contention of a higher risk with stenting of the proximal LAD, now up to 10 years, and a special status of the proximal LAD in coronary revascularisation guidelines.
Stent and lesion characteristics could partly explain the relatively lower MACE rate in our proximal LAD group. A reference diameter ≥3 mm, less proximal tortuosity, and higher blood flow are inherent characteristics of the proximal LAD. Stent length, use of overlapping stents, frequency of type C lesions and SCAI lesion types were similarly distributed between the groups. That the hazard ratios for proximal LAD vs. non-proximal LAD groups would be comparable also with newer-generation drug-eluting stents is suggested by the lack of interaction between proximal LAD vs. non-proximal LAD groups and use of sirolimus- or zotarolimus-coated stents on outcomes in the PROTECT trial6. In addition, it is unlikely that treatment of the proximal LAD with later-generation DES would result in a worse outcome than that observed in our study.
We found a 12% target lesion revascularisation rate 10 years after treatment of a proximal LAD lesion with first-generation drug-eluting stents, a result fairly similar to the one which may be expected after CABG using the left internal mammary artery to the LAD, where the reported long-term patency rates for this bypass graft vary slightly according to whether patients with multivessel disease are also included (85% to 90% patency rates)16,17 or if patients have only single proximal LAD lesions (89% to 100% patency rates)5,18,19.
Limitations
Left ventricular ejection function was not recorded systematically as part of the SORT OUT II trial. However, previous myocardial infarction may be a surrogate of left ventricular ejection fraction and was a predictor of mortality (especially cardiac death); it was included in our multivariate models. The two groups may have been different with respect to unknown confounders that might have had an impact on their clinical outcome. Although we performed a secondary analysis including patients treated for multiple lesions, our results may not necessarily apply to patients treated for multiple lesions since this cohort only constituted less than one third of our study population. However, we do feel that our results apply to the outcome of stenting of the proximal LAD segment per se. Ischaemia burden, as determined by stress testing or fractional flow reserve, was not recorded in SORT OUT II. Therefore, some treated lesions may not have been flow-limiting, whereas some flow-limiting lesions may have been left untreated. However, the indication for treatment represents the practice for high-volume operators in high-volume centres during the inclusion period.
Conclusions
At 10-year follow-up, patients treated with a first-generation drug-eluting stent in the proximal LAD had similar or better clinical outcome compared to patients stented in another non-left main stem coronary lesion. Consequently, the contention that stenting of the proximal LAD carries a higher risk compared with other coronary segments is not supported by our results.
| Impact on daily practice Our results do not support the contention that stenting of the proximal LAD segment is associated with a higher risk than stenting of other non-left main coronary segments, including in the very long term and with the use of first-generation stents. Location is not everything. Future studies and treatment recommendations regarding coronary drug-eluting stents should focus on plaque burden, composition and vulnerability in addition to stent characteristics and implantation technique. |
Acknowledgements
The authors acknowledge the work of the clinical events committee: Jørgen L. Jeppesen, MD, Amager Hvidovre Hospital Glostrup, University of Copenhagen; Søren Boesgaard, MD, Department of Cardiology, Copenhagen University Hospital, Rigshospitalet; and John Godtfredsen, MD, Department of Cardiology, Copenhagen University Hospital, Herlev. The Danish Heart Registry (DHR) has contributed with collection of data. The SORT OUT II investigators are: Niels Bligaard, MD; Leif Thuesen, MD; Henning Kelbæk, MD; Per Thayssen, MD; Jens Aarøe, MD; Peter R. Hansen, MD; Jens F. Lassen, MD; Kari Saunamäki, MD; Anders Junker, MD; Jan Ravkilde, MD; Ulrik Abildgaard, MD; Hans H. Tilsted, MD; Thomas Engstrøm, MD.
Funding
Boston Scientific, Denmark, and Cordis, Denmark, have both donated unrestricted grants to conduct the study at the departments involved. They had no role in the design and conduct of the study, the collection, management, analysis, or interpretation of data, or the preparation, review, and approval of the manuscript.
Conflict of interest statement
The authors have no conflicts of interest to declare.

