
Abstract
Aims: Our aim was to compare, in a large unprotected left main coronary artery (ULMCA) all-comer registry, the long-term clinical outcome after percutaneous coronary intervention (PCI) with first-generation drug-eluting stents (DES) versus coronary artery bypass grafting (CABG) in patients with acute coronary syndrome (ACS).
Methods and results: Of a total of 2,775 patients enrolled in the Drug Eluting Stents for Left Main Coronary Artery Disease (DELTA) multicentre registry, 379 (13.7%) patients with ACS treated with PCI (n=272) or CABG (n=107) were analysed. Baseline demographics were considerably different in the two groups before propensity matching. No significant differences emerged for the composite endpoint of all-cause death, myocardial infarction (MI), and cerebrovascular accident (HR 1.11, 95% CI: 0.63-1.94; p=0.727), all-cause death (HR 1.26, 95% CI: 0.68-2.32; p=0.462), the composite endpoint of all-cause death and MI (HR 1.02, 95% CI: 0.56-1.84; p=0.956), and major adverse cardiac and cerebrovascular events (HR 0.82, 95% CI: 0.50-1.36; p=0.821). However, a higher incidence of target vessel revascularisation (HR 4.67, 95% CI: 1.33-16.47; p=0.008) was observed in the PCI compared with the CABG group, which was confirmed in the propensity score-matched analysis.
Conclusions: In the DELTA all-comer, multinational registry, PCI for ACS in ULMCA is associated with comparable clinical outcomes to those observed with CABG at long-term follow-up, despite the use of first-generation DES.
Introduction
The optimal revascularisation option for unprotected left main coronary artery (ULMCA) in the setting of acute coronary syndrome (ACS) remains the subject of debate. Current guidelines are not yet conclusive in favour of PCI or CABG1,2. While CABG remains the standard-of-care vs. PCI in most cases, ACS remains an underinvestigated clinical subset. Accordingly, all available data regarding this particular subset are derived from small retrospective registries and are inconclusive3.
The advent of drug-eluting stents (DES) was associated with a drastic reduction in in-stent restenosis in comparison to bare metal stents (BMS)4,5. However, large randomised trials and registries which considered DES vs. CABG comparisons in the setting of ULMCA have systematically excluded patients with ACS6,7. Therefore, the aim of this study was to assess the long-term clinical outcome of patients with ULMCA and ACS, comparing PCI vs. CABG. To do this, we interrogated the Drug Eluting Stents for Left Main Coronary Artery Disease (DELTA) registry8.
Methods
PATIENT POPULATION
The methods of the DELTA registry were published previously8. In brief, the DELTA registry included all comers with ULMCA stenosis treated with PCI and “first-generation” DES (sirolimus- and paclitaxel-eluting stents) or CABG between April 2002 and April 2006 in 14 international centres. Acute coronary syndrome was defined as non-ST-segment myocardial infarction (NSTEMI) or ST-segment myocardial infarction (STEMI) at clinical presentation. Of the 2,775 patients initially enrolled, 379 (13.7%) patients presented with ACS and were divided into patients treated with PCI (n=272) and patients treated with CABG (n=107) (Figure 1).
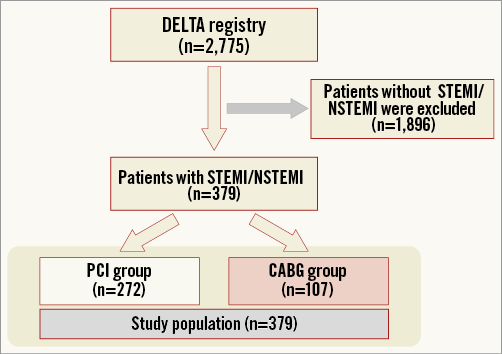
Figure 1. Study population flow chart. CABG: coronary artery bypass graft; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction
All patients were evaluated by both interventional cardiologists and cardiac surgeons, and the decision to perform PCI or CABG was made based on the following: 1) haemodynamic conditions; 2) lesion characteristics; 3) vessel size; 4) the presence of comorbidities; 5) quality of arterial and/or venous conduits for grafting; and 6) patient and/or referring physician preferences. All data relating to hospital admissions, procedures, and outcomes were collected in each centre via the hospital recording network. Information with regard to the clinical status at the latest clinical follow-up available was collected by clinical visits, telephone interviews, and referring physicians.
Whether to use an intra-aortic balloon pump (IABP) was left to the discretion of the operator. When IABP was used, in most cases it was in the context of haemodynamically unstable patients and complex bifurcation lesions9. Dual antiplatelet therapy (i.e., aspirin 100 mg daily and clopidogrel 75 mg daily or ticlopidine 250 mg twice daily) was administered for at least 12 months in the PCI group. In the South Korean centre, cilostazol was also prescribed. Detailed information on adherence as well as reasons and date for discontinuation of dual antiplatelet therapy were obtained in all patients.
Data analysis was performed with the approval of the institutional ethics committees of the hospitals and/or universities involved.
DEFINITIONS
The following events were analysed cumulatively up to the last clinical follow-up available: cardiac and overall death, myocardial infarction (MI), cerebrovascular accident (CVA), target lesion revascularisation (TLR), and target vessel revascularisation (TVR). TLR was defined as any repeat intervention of the target lesion or other complication of the target lesion. TVR was defined as any repeat intervention of any segment of the target vessel, defined as the entire major coronary vessel proximal and distal to the target lesion, including upstream and downstream branches and the target lesion itself. The occurrence of stent thrombosis (ST) was defined on the basis of the Academic Research Consortium definitions10 in the PCI group. Cardiac death was defined as any death due to a cardiac cause (e.g., MI, low-output failure, fatal arrhythmia), procedure-related deaths, and death of unknown cause. CVA was defined as stroke, transient ischaemic attacks, and reversible ischaemic neurological deficits adjudicated by a neurologist and confirmed by computed tomography scanning. In-hospital non-Q-wave MI was defined as the elevation of the serum creatine kinase (CK) isoenzyme myocardial band that was three times the upper limit of normal in the PCI group and five times the upper limit of normal in the CABG group, in the absence of new pathological Q-waves. In this analysis we included as cumulative MIs all Q-wave MIs that occurred during hospital stay and follow-up and all spontaneous MIs occurring after hospital discharge. Q-wave MI was defined as the development of new pathological Q-waves in ≥2 contiguous leads with or without CK or CK-myocardial band levels elevated above normal. Spontaneous MI was defined as the occurrence after hospital discharge of any value of troponin and/or CK-myocardial band greater than the upper limit of normal if associated with clinical and/or electrocardiogram change. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) was calculated. Diagnostic angiograms were scored according to the SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) score algorithm at the site laboratory11. Major adverse cardiac and cerebrovascular events (MACCE) were defined as the composite endpoint of death, CVA, MI, and TVR.
STUDY ENDPOINTS
The primary study endpoint was the occurrence of the composite of all-cause death, MI, and CVA at long-term follow-up. Secondary endpoints were occurrence of all-cause death and the composite of all-cause death and MI, MACCE, TVR, and TLR at long-term follow-up.
STATISTICAL ANALYSIS
Data are presented as percentages or mean±standard deviation. Differences in proportions were tested with the chi-square test or Fisher’s exact test, and differences in continuous variables were tested with a Student’s t-test. Cumulative event curves were generated with the Kaplan-Meier method and compared by the log-rank test. A propensity score analysis was performed to minimise selection bias between the two treatment groups (CABG vs. PCI). For each patient, a propensity score indicating the likelihood of having PCI was calculated by using a non-parsimonious multivariable logistic regression. A propensity score, indicating the predicted probability of receiving a specific treatment conditional on the observed covariates, was then calculated from the logistic equation for each patient. Variables included in the logistic regression model to calculate the propensity score were age, sex, hypertension, smoking habit, family history of coronary artery disease (CAD), diabetes mellitus, chronic kidney disease, left ventricular ejection fraction (LVEF), EuroSCORE, type of ACS (NSTEMI or STEMI), multivessel disease, intra-aortic balloon pump (IABP) and concomitant right coronary artery disease. The C-statistic was 0.71 and the Hosmer-Lemeshow test p-value was 0.45, confirming good discrimination and goodness-of-fit of the propensity score model, respectively. The individual propensity score was incorporated into Cox proportional hazards regression models as a covariate as well as the treatment group as the variable of interest to calculate the adjusted hazard ratio (HR). Clinical outcomes in the matched population were analysed with Cox proportional hazards regression stratified on matched pairs. Multivariable Cox proportional hazards regression modelling was performed to determine the independent predictors of the primary endpoint (death, CVA or MI) with purposeful selection of covariates. Variables associated on univariate analysis (all with a p-value <0.1) and those judged to be of clinical importance from previous published reports were eligible for inclusion in the multivariable model-building process. The goodness-of-fit of the Cox multivariable model was assessed with the Grønnesby-Borgan-May test. Results are reported as HR with associated 95% confidence interval (CI) and p-value. Analyses were carried out using SPSS for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
PATIENT POPULATION
The study population flow chart is shown in Figure 1. From the initial patient population of 2,775 patients of the DELTA registry, a total of 379 patients with NSTEMI (318, 83.9%) and STEMI (61, 16.1%) were included. Among them, 272 (71.8%) were treated with PCI and 107 (28.2%) with CABG. The mean follow-up duration was 1,020.3 vs. 1,060.4 days, respectively (p=0.592).
Baseline clinical characteristics are summarised in Table 1. Patients in the PCI group were less frequently diabetics (26.1% vs. 40.0%, p=0.005) and had higher LVEF. Clinical presentation also differed, since the percentage of STEMIs was higher in the PCI subgroup (19.9% vs. 6.5%, p=0.001).
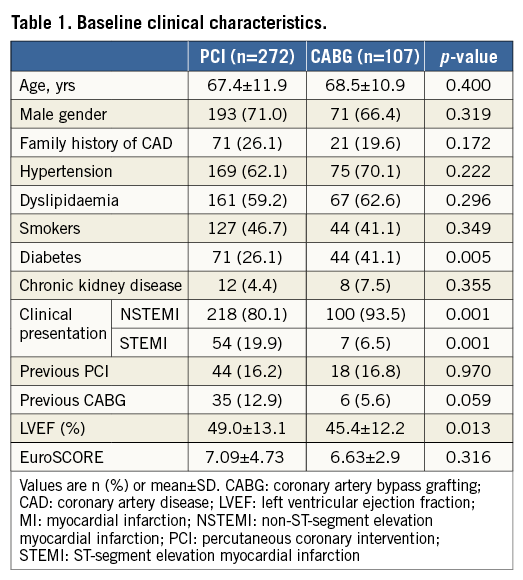
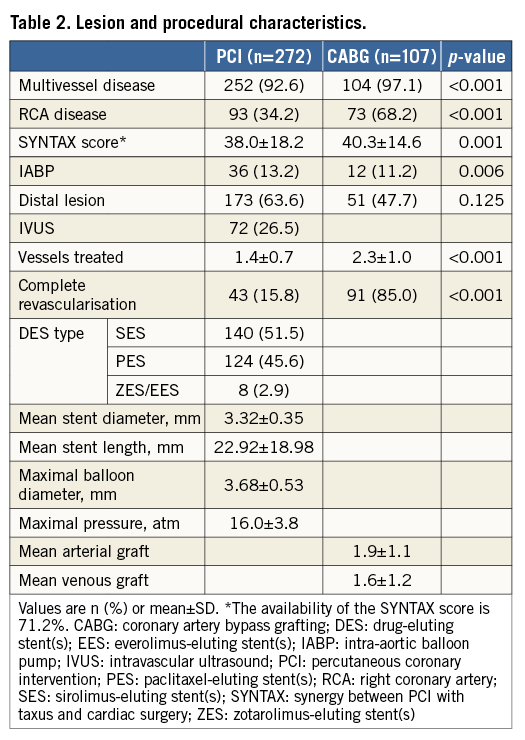
Lesion and procedural characteristics are shown in Table 2. As expected, in the PCI group, the SYNTAX score was lower (38.0±18.2, p=0.001), with lower occurrence of multivessel disease (92.6% vs. 97.1%, p<0.001), concomitant right coronary artery disease (34.2% vs. 68.2%, p<0.001) and number of treated vessels (1.4±0.7 vs. 2.3±1.0, p<0.001), as well as rate of complete revascularisation (15.8% vs. 85.0%, p<0.001). However, the use of IABP was more frequent in patients treated with PCI (13.2% vs. 11.2%, p=0.006).
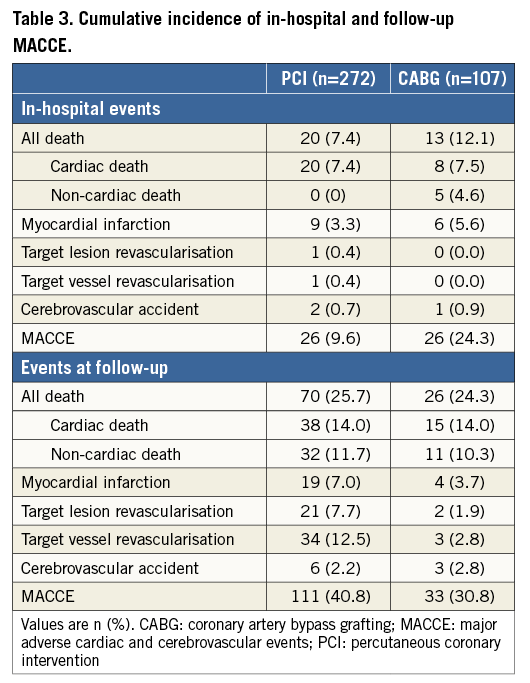
IN-HOSPITAL AND FOLLOW-UP MACCE
The median follow-up period was 1,120 days (interquartile range [IQR]: 441 to 1,521). In-hospital and follow-up MACCE are illustrated in Table 3. Definite ST occurred in five patients (1.8%), whereas probable ST occurred in four patients (1.5%). Of note, angiographic follow-up rates in the PCI and CABG groups were 38.6% and 5.6%, respectively (p<0.001). Five in-hospital deaths were observed in the CABG patient subgroup: two patients died because of multi-organ failure, one patient died of sepsis, one of postoperative respiratory insufficiency, and one of disseminated intravascular coagulation.
STUDY ENDPOINTS
No significant differences in the composite endpoint of all-cause death, MI, and CVA (unadjusted HR 0.96, 95% CI: 0.63-1.47; p=0.862; propensity score-adjusted HR 1.11, 95% CI: 0.63-1.94; p=0.727), all-cause death (unadjusted HR 1.00, 95% CI: 0.64-1.57; p=0.988; propensity score-adjusted HR 1.26, 95% CI: 0.68-2.32; p=0.462), the composite endpoint of death and MI (unadjusted HR 0.85, 95% CI: 0.55-1.33; p=0.481; propensity score-adjusted HR 1.02, 95% CI: 0.56-1.84; p=0.956), and MACCE (unadjusted HR 0.82, 95% CI: 0.55-1.21; p=0.320; propensity score-adjusted HR 0.82, 95% CI: 0.50-1.36; p=0.821) were seen between the two groups. A higher TVR rate (unadjusted HR 6.90, 95% CI: 2.00-23.76; p<0.001; propensity score-adjusted HR 4.67, 95% CI: 1.33-16.47; p=0.008) was observed in the PCI group. A higher TLR rate was also observed in the PCI group (unadjusted HR 6.5, 95% CI: 1.40-30.23; p=0.017); however, the propensity score-adjusted TLR rate confirmed only a trend (HR 3.77, 95% CI: 0.79-18.07; p=0.097).
Kaplan-Meier survival curves for all-cause death, the composite endpoint of all-cause death and MI, MACCE, and the composite endpoint of all-cause death, MI, and CVA are illustrated in Figure 2.
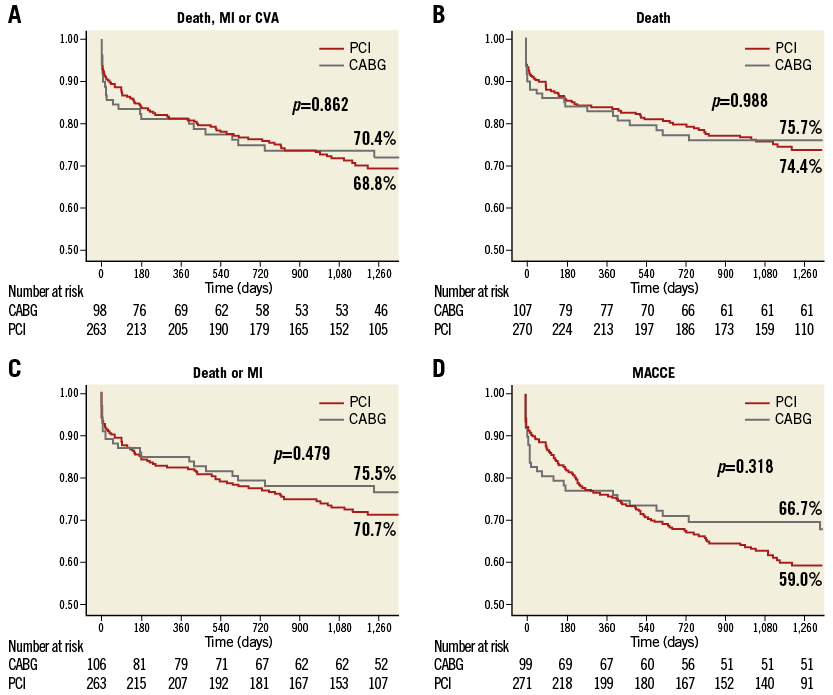
Figure 2. Freedom from cardiac and cerebrovascular events in PCI versus CABG in the overall population. Freedom from cardiac death, myocardial infarction (MI), and cerebrovascular accidents (CVA) (A), from death (B), from death and MI (C), and from major adverse cardiac and cerebrovascular events (MACCE) (D) after percutaneous coronary intervention (PCI) (red line) versus coronary artery bypass grafting (CABG) (grey line) in the overall population. Patients at risk at different times are reported below each graph.
MULTIVARIABLE ANALYSIS FOR PREDICTORS OF THE PRIMARY ENDPOINT
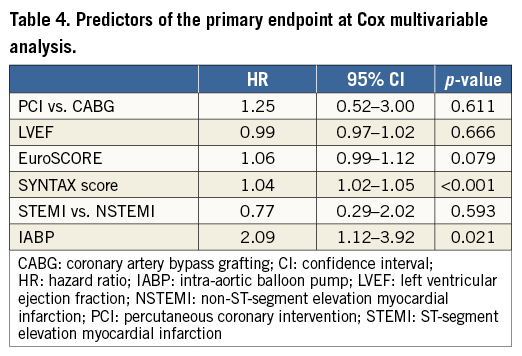
At Cox regression multivariable analysis, SYNTAX score (HR 1.04, 95% CI: 1.02-1.05; p<0.001), and IABP (HR 2.09, 95% CI: 1.12-3.92; p=0.021) were predictors of the primary endpoint (Table 4). The clinical presentation (STEMI vs. NSTEMI) did not affect the primary endpoint (HR 0.77, 95% CI: 0.29-2.02; p=0.593).
PROPENSITY SCORE-MATCHED ANALYSIS
After propensity score matching was performed, 62 pairs were matched. Baseline clinical and lesion characteristics of the matched groups are shown in Table 5. After propensity score matching, there were no significant differences in the composite endpoint of all-cause death, MI, and CVA (HR 1.43, 95% CI: 0.70 to 2.88; p=0.326), all-cause death (HR 1.10, 95% CI: 0.51 to 2.38; p=0.805), or the composite endpoint of death and MI (HR 1.56, 95% CI: 0.75 to 3.21; p=0.232).
An advantage of CABG over PCI was observed in the composite secondary endpoint of MACCE (HR 2.17, 95% CI: 1.17 to 4.03; p=0.015), driven by the benefit of CABG in terms of TVR (HR 23.42, 95% CI: 3.00 to 184.02; p=0.003). Differences in terms of TLR showed a trend favouring CABG (HR 108.47, 95% CI: 0.44 to 26,577.04; p=0.095).
Kaplan-Meier survival curves for all-cause death, the composite endpoint of all-cause death and MI, MACCE, and the composite endpoint of all-cause death, MI, and CVA are illustrated in Figure 3.
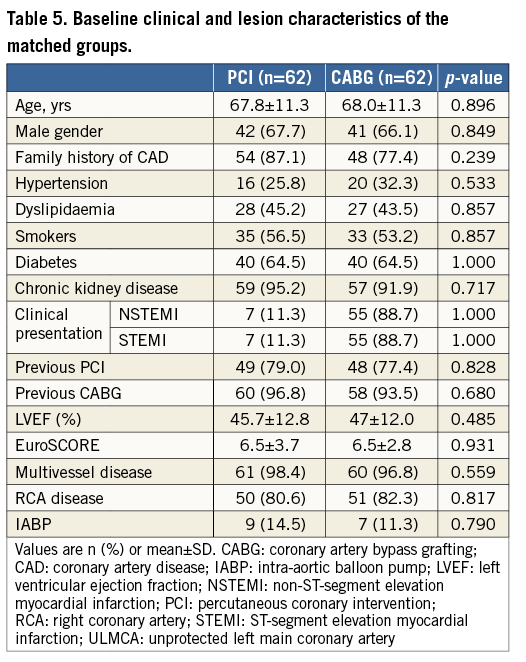
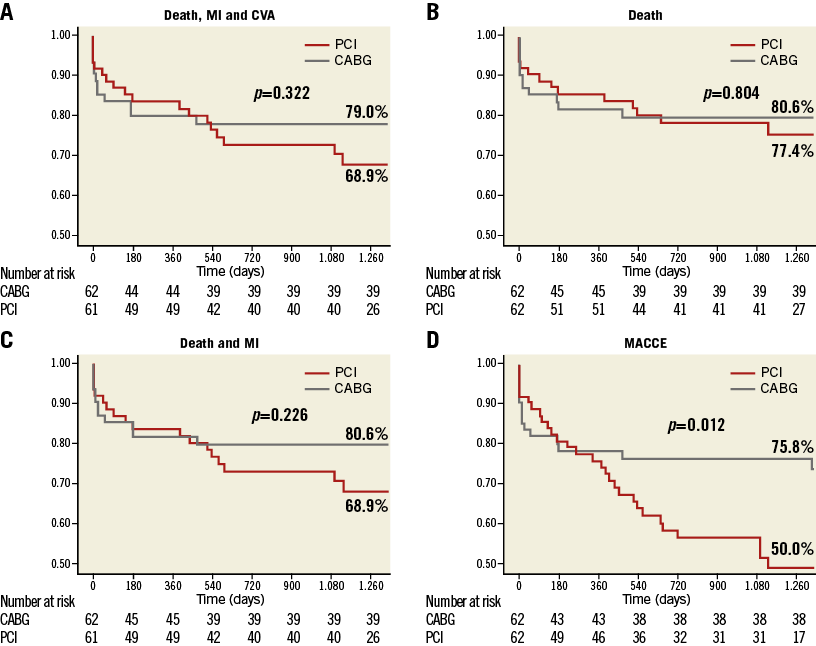
Figure 3. Freedom from cardiac and cerebrovascular events in PCI versus CABG in the propensity score-matched groups. Freedom from cardiac death, myocardial infarction (MI), and cerebrovascular accidents (CVA) (A), from death (B), from death and MI (C), and from major adverse cardiac and cerebrovascular events (MACCE) (D) after percutaneous coronary intervention (PCI) (red line) versus coronary artery bypass grafting (CABG) (grey line) in the propensity score-matched groups. Patients at risk at different times are reported below each graph.
Discussion
The main findings of this large, multicentre, multinational, all-comer registry are the following: 1) no difference was found at a median follow-up of 1,120 days (IQR: 441 to 1,521) in the occurrence of the primary endpoint (death, MI, and CVA) between PCI with DES implantation and CABG for ULMCA disease both in the propensity-adjusted analysis as well as in the propensity-matched analysis; 2) in the propensity score-matched population an advantage of CABG over PCI in terms of MACCE was observed, exclusively driven by a lower incidence of repeat revascularisation in the CABG group; 3) SYNTAX score and the need for haemodynamic support were found to be correlated to the occurrence of death, MI, and CVA; and 4) PCI in patients with ACS and ULMCA has proven to be a safe procedure, considering the low mortality and ST rates, despite the all-comer design of the study and the very high SYNTAX score of the population.
The present study confirms the findings of the main DELTA study, this time in the setting of ACS. Accordingly, in patients with ULMCA disease presenting with STEMI or NSTEMI, characterised per se by high thrombotic milieu and potential haemodynamic instability, no differences in the primary endpoint of death, MI, and CVA were observed. Our findings are in line with previous findings from the MAIN-COMPARE study12; however, the latter excluded patients with STEMI and/or haemodynamic instability. In contrast, 12.7% of our patient population was treated with IABP. In addition, the rate of patients treated with IABP was significantly higher for patients undergoing PCI vs. CABG (respectively, 13.2 vs. 11.1%, p=0.006). Furthermore, a considerable proportion of patients enrolled in MAIN-COMPARE were treated with BMS, in contrast to our cohort of patients who were exclusively treated with DES.
We observed a higher MACCE rate in PCI patients vs. CABG only in the limited, propensity score-matched population, exclusively driven by higher TVR occurrence. The SYNTAX trial showed similar findings in patients with stable clinical presentation6.
Interestingly, the Kaplan-Meier curves begin to “open” after one to two years of follow-up in favour of CABG. Previous reports confirm this finding, particularly regarding first-generation DES13. Intuitively, different findings would have been possible if new-generation DES had been used in our study population. Furthermore, the highly significant difference in angiographic follow-up between the two groups (PCI vs. CABG, respectively: 38.6% vs. 5.6%, p<0.001) may partially explain the differences noted between the two groups in TVR, since mere angiographic findings may have triggered further intervention in downstream vessels.
In the present study, the SYNTAX score emerged as an independent predictor of the predefined primary endpoint. The prognostic value of the SYNTAX score in patients with ACS has been previously shown in the setting of both STEMI14 and NSTEMI15. Our findings are in line with these studies and suggest that anatomically and technically complicated revascularisation is, per se, associated with poor outcome in the ACS setting, for both surgical and percutaneous revascularisation modalities. Moreover, the SYNTAX score in the PCI subgroup was 38.0±18.2, which is higher with respect to the current recommendation1, reflecting the fact that the involved institutions were high-volume, experienced, tertiary referral PCI centres. This fact is also reflected by the lack of significant difference in distal LM lesions between PCI and CABG subgroups (63.6% vs. 47.7%, respectively; p=0125), confirming that technically challenging LM bifurcations were not addressed selectively for CABG.
The SYNTAX left main trial showed a significantly higher rate of stroke in CABG vs. PCI16, in contrast to our findings, where no significant difference was observed. This result may have several explanations: 1) the SYNTAX trial had a randomised design, with an LM cohort being predefined and powered, in contrast to our observational data; 2) our patient population included only ACS patients, where the risk of stroke following PCI was higher; and 3) similar to our findings, the SYNTAX LM trial did not show significant differences for stroke in patients with SYNTAX score >33.
Of note, the complete revascularisation rate was significantly higher in patients undergoing CABG as compared to PCI (respectively, 85% vs. 16%, p<0.001). This finding may be important in terms of long-term prognostic stratification, considering that the benefit in terms of outcome of CABG vs. PCI increases steadily past five years of clinical follow-up. Accordingly, our findings are limited because of the relatively short duration of the follow-up (three years).
Study limitations
This is a retrospective analysis of a large multicentre registry, where no randomisation has been systematically performed. Accordingly, inherent selection biases related to the observational nature of the present study cannot be excluded, despite the fact that propensity score-matched analysis bore similar results to the overall population analysis. Second, our results reflect a multinational experience, where PCI was performed with first-generation DES. In comparison, current use of second-generation DES resulted in lower rates of TLR17-19, suggesting that our findings may have differed if newer-generation DES had been used. Third, current advances in dual antiplatelet treatment (DAPT) with new thienopyridinic agents have shown improved outcome after PCI in comparison to clopidogrel, particularly in the setting of ACS20,21. In the present study, clopidogrel was used for all patients. Therefore, we cannot exclude potential differences if one of the new thienopyridinic agents had been used instead. We acknowledge that, in our registry, the proportion of patients undergoing PCI is double that of those undergoing CABG. This phenomenon might reflect the practice of selected high-volume tertiary centres, such as the ones included in our study.
Conclusions
In the clinical setting of ACS in a large multicentre registry assessing CABG versus PCI with first-generation DES, no differences were observed in the primary endpoint of death, CVA, and MI, at a median follow-up of 1,120 days. In the propensity-matched analysis, the rate of TVR was significantly higher for patients treated with PCI vs. CABG, driving a significant difference in MACCE in favour of CABG. These findings need to be confirmed by larger, randomised trials.
| Impact on daily practice Optimal revascularisation strategy (CABG vs. PCI) remains a matter of debate in the ACS setting. The present subgroup analysis of the large multicentre DELTA registry shows no differences between CABG and PCI (using first-generation DES) for the composite of death, cerebrovascular accident and myocardial infarction at long-term follow-up, although target vessel revascularisation was more frequent in the PCI patient subgroup, when propensity score matching was performed. |
Guest Editor
This paper has been guest edited by Henning Kelbaek, MD, DMSc; Department of Cardiology, Zealand University Hospital, Roskilde, Denmark.
Conflict of interest statement
D. Capodanno has received speaker’s honoraria from Eli Lilly and AstraZeneca. R. Makkar is a consultant to Medtronic and Abiomed, has received speaker’s fees from Lilly and Medtronic, and has received equity from Entourage Medical Technologies. R. Mehran is a consultant to Abbott Vascular, The Medicines Company, Janssen, and Regado, and has received research grant support from Bristol-Myers Squibb/Sanofi, The Medicines Company, and Lilly/Daiichi Sankyo. C. Naber is a speaker for Abbott Vascular, Cordis, Biotronik, Biosensors, Cirdus, Medtronic, Stentys, Daiichi Sankyo, and The Medicines Company, has received research support from Abbott Vascular, Biotronik, Sadra Medical, Stentys, and Icon, and is on the advisory board of Biotronik and Abbott Vascular. J. Moses is a consultant to Cordis and Boston Scientific.The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

