Abstract
Aims: We sought to assess the efficacy and safety of everolimus-eluting stents for unprotected left main disease.
Methods and results: A total of 173 consecutive patients with de novo significant unprotected left main stenosis received an everolimus-eluting stent in four French centres. Among them, 140 (81 %) had involvement of the distal portion of left main, and 129/140 (92%) were treated with provisional side branch T-stenting, with a side branch stenting rate of 20%. Angiographic success was achieved in all cases. At 12 months, the cumulative rate of major adverse cardiac or cerebrovascular events (MACCE) was 26/173 (15%) including death from any cause (N=5, 2.9%), stroke (N=4, 2.3%), Q-wave myocardial infarction (MI) (N=2, 1.2%), non-Q-wave MI (N=6, 3.5%) and any repeat revascularisation (N=16, 9.3%). At one year, the rate of target-lesion revascularisation (TLR) was 5/173 (2.9%), target-vessel revascularisation was 12/173 (7 %) and the rate of definite or probable left main stent thrombosis 1/173 (0.6 %).
Conclusions: Unprotected left main stenting using everolimus-eluting stents and a strategy of provisional side branch T-stenting for distal lesions, is safe and effective in the midterm, with a relatively low rate of events and reintervention at one year.
Introduction
Coronary bypass grafting (CABG) has long been considered the most appropriate strategy for patients with unprotected left main (LM) disease1, and previous guidelines used to restrict percutaneous coronary intervention (PCI) to patients not eligible for CABG. Several reports have proved the feasibility of PCI and shown favourable results of first generation paclitaxel- and sirolimus-eluting stents2-9 in this lesion subset. Over the past years, evidence from non-randomised registries3,6, a subanalysis of the Synergy Between Percutaneous Coronary Intervention and Cardiac Surgery (SYNTAX) trial7 and one small randomised study8 led to the recently updated recommendations that PCI may be considered an alternative to surgery in certain patients10,11. Data from two recent large randomised studies4,9, showing that patients with LM disease revascularised with PCI have safety outcomes comparable to CABG, despite a higher need for revascularisation, support this recommendation.
Despite these promising results, certain issues remain unresolved. Firstly, though a rare occurrence, stent thrombosis has serious clinical consequences. Secondly, the need for re-intervention is still a problem in this complex subset of lesions. Thirdly, the optimal strategy for stenting of distal left main is still debated.
Second-generation DES were designed with the goal of improving safety, efficacy and device performance. Although some trials have reported the superiority of second-generation DES compared to first generation DES in unselected populations12,13, no data are available on the use of second-generation DES in unprotected LM disease.
Consequently, we sought to assess the efficacy and safety of the XIENCE V® (Abbott Vascular, Santa Clara, CA, USA) everolimus-eluting stent (EES) using a uniform approach of provisional side branch (SB) T-stenting for distal lesions.
Methods
Study population
Between December 2007 and May 2009, 173 consecutive patients undergoing unprotected LM stenting with XIENCE V EES in four French centres were entered into a prospective registry, the LEMAX (Left Main XIENCE V) pilot study. EES was the default stent used during the study period for patients with LM disease that matched the inclusion criteria. The inclusion criteria were the presence of stable or unstable angina and/or documented ischaemia and evidence of de novo >50% LM lesion by visual estimate, considered amenable to PCI. Patients presenting with ST elevation myocardial infarction (MI) or cardiogenic shock were excluded. The study was approved by each institutional review committee. Patients provided written informed consent.
Procedure
Patients not previously treated with clopidogrel received a loading dose of 300 mg or 600 mg. The antithrombotic regimen was given at the discretion of the operator. Patients were instructed to take aspirin ≥75 mg daily indefinitely and clopidogrel 75 mg daily for 12 months.
In distal LM lesions, the strategy was provisional SB T-stenting, and T-stenting was the recommended technique for SB stenting. It was recommended that Finet’s adaptation of Murray’s law14 be applied in order to select the optimal stent diameter in distal lesions treated with a single stent. The proximal optimisation technique (POT) described by O.Darremont15 (expansion of the LM stent at the carina using a short oversized balloon) was recommended for optimising apposition and expansion of the stent at the bifurcation. Coverage of the ostium of the left main was encouraged in all cases, regardless of the lesion location. Intravascular ultrasound and intra-aortic balloon pump were used at the discretion of the operator.
Treatment of other lesions during the same procedure was allowed. Bare metal stents were allowed in lesions other than the LM, to comply with French policy for stents reimbursement. Staged procedures were permitted.
Quantitative coronary analysis
Coronary angiograms were analysed with a semiautomated edge-contour-detection computer analysis system (QAngio XA version 7.1; Medis, Leiden, The Netherlands) by an independent core laboratory (Centre Européen de Recherche Cardiovasculaire [CERC] Massy, France). The baseline diagnostic angiograms were reviewed by two experienced interventional cardiologists who scored all angiograms according to the SYNTAX score algorithm16.
Endpoint definitions and follow-up
The LEMAX study was monitored by the CERC (www.cerc-europe.org) and events were adjudicated by an independent clinical events committee (CEC).
Angiographic success was defined as <30% residual stenosis in the stented lesion and <50% in unstented SB, in the presence of Throm-bolysis In Myocardial Infarction (TIMI) flow grade 3 in both branches.
Major adverse cardiac or cerebrovascular events (MACCE) were defined as death from any cause, any cerebrovascular event, any MI or any repeat revascularisation. MACCE were assigned in a non-hierarchical way. Non-Q wave MI was defined as creatine phosphokinase elevation ≥3 times the upper limit of normal in the absence of pathological Q-waves. A cerebrovascular event was defined as stroke, transient ischaemic attack (TIA) or reversible ischaemic neurological deficit adjudicated by the CEC. Repeat revascularisation was defined as any PCI or CABG during follow-up. Target lesion revascularisation (TLR) was defined as any repeat revascularisation to treat a >50% stenosis anywhere within the LM or 5 mm distal within the LAD and LCX ostia. Target vessel revascularisation (TVR) was defined as any repeat revascularisation to treat a >50% stenosis of the left coronary artery. Stent thrombosis (ST) was recorded according to the Academic Research Consortium definitions17. Deaths were classified as either cardiac or non-cardiac. Death due to unknown causes was adjudicated as cardiac.
All patients were followed by clinical visit or telephone call at one month and one year. A non-invasive ischaemia test was recommended six to nine months postprocedure. One of the study centres performed routine angiographic follow up at 12 months.
Statistical analysis
Statistical analysis was performed by an independent academic institution (INSERM unit 970, Paris Centre de Recherche Cardiovasculaire, Paris, France). Continuous variables are shown as mean ±1 SD and categorical variables as counts and percentages. Survival curves were estimated by the Kaplan-Meier method. The log-rank test was used to compare survival among groups. Univariable analysis was performed with all the baseline, procedural and angiographic characteristics reported in Tables 1 and 2. Cox regression analysis was used to determine independent predictors of MACCE, with those variables with a p-value <0.10 in the univariable analysis being included in a backward stepwise mutivariable model. All statistical tests were 2-tailed and a p-value <0.05 was considered statistically significant. Data were analysed with SAS version 9.2 (SAS Institute Inc. Cary, NC, USA).
Results
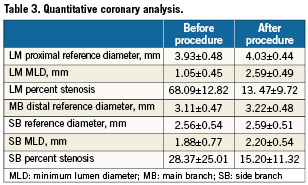
Baseline and procedural characteristics are summarised in Tables 1 and 2. Quantitative coronary angiography measurements are reported in Table 3.
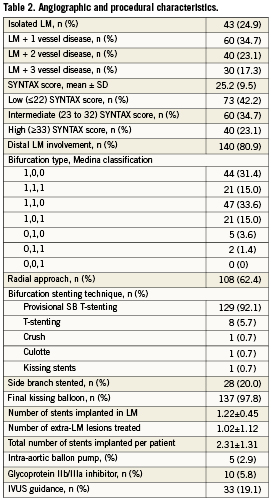
In summary, patients were aged 69±11 years; 27% were diabetic and 29% had undergone previous PCI. A total of 75% had significant stenosis in segments other than the LM and 59% had extra-LM lesions treated during the same procedure and/or during staged PCI. Mean SYNTAX score was 25±9.5. The distal portion of LM was involved in 81%, provisional SB T-stenting was performed in 92%, with a SB stenting rate of 20%, and final kissing balloon was peformed in 98%. In total, 400 stents were implanted (378 EES and 22 bare metal stents).
In-hospital outcomes
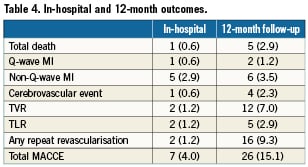
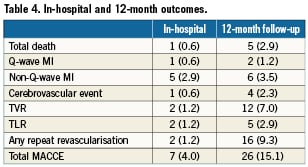
In-hospital outcomes are reported in Table 4. The procedure was angiographically successful in all cases. One patient developed subacute stent thrombosis resulting in cardiogenic shock, underwent repeat PCI and died at day 14. A second patient developed a subocclusion of the LAD distal to the LM stent two hours after the procedure, underwent emergency repeat PCI, and sustained non-Q-wave MI. Other non-Q-wave MIs were revealed by asymptomatic creatine phosphokinase elevation. The overall in-hospital MACCE was 4.0%: death in one patient (0.6%), Q-wave MI in one (0.6%), non-Q-wave MI in five (2.9%) , TIA in one (0.6%) and repeat revascularisation in two patients (1.2%).
One-year outcomes
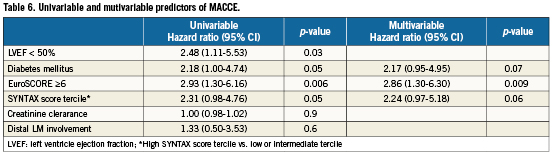
Clinical follow-up at one year was completed in all but one patient (99.4%). Outcomes are described in Table 4. At one-year follow-up, the cumulative rate of MACCE was 15.1%. Death occurred in five patients, causes of death are listed in Table 5. Target vessel revascularisation was performed in 12 patients (7.0%), five were due to TLR (2.9%). Of the five TLR procedures carried out, two were in-hospital and were by PCI. A third case was due to LAD dissection caused by the LM stent and presenting as non-Q-wave MI which was treated by re-PCI seven days after the index procedure. The fourth case of TLR was due to focal restenosis and was also by PCI. A fifth patient developed restenosis of the distal LM and underwent CABG. The total rate of definite or probable LM stent thrombosis at one year was 0.6% (one case). This case was a definite subacute LM stent thrombosis which occurred during hospitalisation.
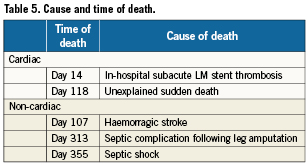
MACCE rates were stratified according to the three SYNTAX score subgroups described in the SYNTAX trial7. Patients with low (≤22) SYNTAX score had similar outcomes to the subgroup of intermediate (22 to 32) SYNTAX score (12.7% vs. 11.3%, respectively, p=ns). Conversely, as shown in Figure 1, the high SYNTAX score group (≥33) demonstrated higher MACCE rates than in patients with SYNTAX score <33 (25.0 % vs. 12.0%, respectively, p= 0.05). Patients at high risk classified by EuroSCORE ≥6 had a significantly higher MACCE rate at 12 months than patients at low risk (24.3% vs. 9.7%, respectively, p=0.006, Figure 2). Table 6 shows the univariable and multivariable analysis for predictors of MACCE. The presence of a EuroSCORE ≥6 was the only independent predictor of MACCE (hazard ratio 2.86, 95% CI 1.3 to 6.3, p=0.009).
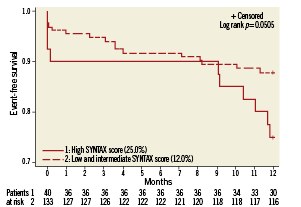
Figure 1. Kaplan-Meier curves for freedom from MACCE in patients with high SYNTAX score compared with patients with low or intermediate SYNTAX score (Log rank p=0.05)
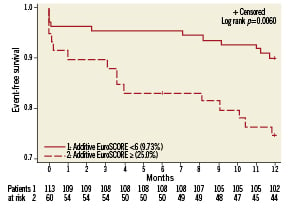
Figure 2. Kaplan-Meier curves for freedom from MACCE in patients with high (≥6) EuroSCORE compared with patients with EuroSCORE <6 (Log rank p=0.006)
Angiographic follow-up and restenosis
Angiographic follow-up was obtained in 81 patients (47%) at a median of 336 days. Patients were asymptomatic in 67% of cases, and none of them had LM restenosis. Among patients with symptoms or ischaemia, LM restenosis was present in two, one was in-stent in the distal left main, the second was at the stent edge in the ostium of the intermediate branch.
Discussion
The main finding of our study is that the use of XIENCE V EES is feasible, safe and effective in the mid term in a real-life population with unprotected left main disease. In addition, in the presence of distal LM involvement, a strategy of provisional SB T-stenting is applicable to more than 90% of patients, with a low rate of SB stenting (20%).
Use of a second-generation drug-eluting stent for unprotected LM disease
PCI with paclitaxel and sirolimus-eluting stents in unprotected LM stenosis has been associated with encouraging mid- to long-term results2-7. Several observational studies comparing these two types of first-generation DES for LM stenting showed that both DES had comparable outcomes18,19. A recent large randomised study5 showed that sirolimus- (SES) and paclitaxel-eluting stents (PES) were equally effective and safe in this setting. Despite these promising results with first-generation DES, the need for re-intervention as well as stent thrombosis remain unresolved. Efforts have therefore focused on designing safer and more effective DES. XIENCE V is a second-generation DES which releases everolimus from a thin coating of a biocompatible fluoropolymer. The platform is a cobalt-chromium stent frame with thin struts. Two recent randomised trials12,13 have shown superiority of EES over PES in unselected patients in terms of safety and efficacy. Data regarding the use of EES for unprotected LM disease are not yet available. Our study shows that elective LM stenting with EES is feasible, with a 100 % angiographic success. This approach is safe, with a 1-year cardiac death rate of 1.2%, and effective, with a TLR rate of 2.9% and a TVR rate of 7% at one year. The main LM stenting trials using PES and SES2,4-7,9,18,23,24 reported TLR rates ranging from 3.15 to 15.8%, TVR rates from 6 to 12%, MACCE rates varying from 8.7 to 41%. The TLR, TVR and MACCE rates of 2.9, 7.0 and 15.1%, respectively, found in our population, compare favourably with these trials using previous generation DES. There is a considerable heterogeneity in the risk profile, inclusion criteria and technical approach among the different studies, therefore this comparison if of limited value. Moreover, it cannot be determined whether these encouraging results are attributable to the use of EES alone, or also reflect the strategy employed, especially in case of distal LM involvement.
Stent thrombosis
Over the past few years, concerns have been raised regarding the risk of stent thrombosis (ST) following DES implantation. However, recent data alleviate concerns about this issue. A multicentre registry evaluated the occurrence of ST in 731 patients undergoing elective LM stenting with PES and SES20. The rate of definite or probable ST at 30 months was 0.9. Our rate of 0.6% of definite or probable ST at one year is consistent with these findings.
PCI of distal LM lesions
Distal left main is involved in 60% to 90% of patients. Stent implantation in this subset remains challenging and provides less optimal outcomes than those achieved after non-distal LM stenting21. Current available data suggest that results are less favourable when distal LM lesions are treated by two stents compared to a single-stent approach21,22. A 1-stent approach is regarded as the best option when the side branch is small and/or mildly diseased. However, when the LCX is not a small branch and is significantly diseased, there is little consensus, and few data, regarding the optimal dual stent technique. Our preferred approach was the provisional SB T-stenting technique, followed by kissing balloon. When the result at the ostium of the SB was not satisfactory, the SB was stented, and final kissing balloon was performed. This approach yielded a SB stenting rate of 20%. Given that in our previous study with PES2, SB stenting was shown to substantially increase the risk of stent thrombosis, efforts were made to obtain the best possible result at the ostium of the SB with the provisional SB T-stenting technique in order to avoid SB stenting whenever possible. We believe that optimising the expansion of the stent at the carina with the POT technique15 produces curved expansion of the stent into the bifurcation and facilitates recrossing with the wire through the most distal strut; it also facilitates balloon crossing and kissing inflation with properly sized balloons.
There is a lack of evidence as to whether the ostium of the left main needs to be covered with stent in shaft and distal lesions. However, in our series with PES2 as well as in other reported series23, there were cases of restenosis at the stent edge in the LM ostium. This could have been caused by geographical miss when performing kissing balloon with the balloons being positioned too proximally, or could be genuine proximal edge restenosis. To avoid this phenomenon, we covered the ostium of the left main regardless of lesion location. This approach seems to be safe and effective in the midterm.
Post-revascularisation surveillance
Restenosis may still occur after LM stenting with DES, and no surveillance strategy to detect this problem has yet been established. In the absence of symptoms or ischaemia, routine angiographic follow-up after LM stenting is no longer recommended by the practice guidelines10. Previous studies have shown that an “oculostenotic reflex” by routine angiographic follow-up may inflate the need for repeat revascularisation, especially at the LCX ostium23,24. One study showed that two thirds of out-of-hospital TLR for DES restenosis were not ischaemia-driven24. In our study, a non-invasive ischaemia test was recommended six to nine months postprocedure. One centre carried out routine angiographic follow-up. Of the patients who underwent routine angiographic follow-up without evidence of ischaemia, none had LM restenosis. Conversely, all patients who underwent TLR had evidence of ischaemia. This non-invasive follow-up modality appears safe in the midterm. In addition, the role of multi-slice computed tomography (MSCT) for detection of LM restenosis needs to be evaluated. A single-center study reported a high accuracy of MSCT for the detection of LM stent restenosis25, compared to conventional angiography, suggesting that this novel technology could be a first-line alternative after LM stenting. However, these results require further evaluation.
Risk stratification after PCI for LM disease
In patients with unprotected LM disease, prediction of individual outcomes can assist physicians in assessing the risks and benefits of different therapeutic options. EuroSCORE, a prognostic scoring system developed for patients undergoing cardiac surgery26, has also been used to stratify the risk of patients undergoing PCI. However, it does not incorporate any information regarding the anatomy and extent of coronary artery disease. The SYNTAX score is an emerging tool that was developed in order to characterise the extent and complexity of coronary lesions16 as a determinant of outcomes after PCI. It has been proposed to predict outcomes and assist in selecting an optimal treatment strategy, whether PCI or CABG. In the SYNTAX trial7, as well as in study by Capodanno27, SYNTAX score was found to be an independent predictor of MACCE in patients underdoing LM stenting. However, in a large study with 3-year follow-up28, SYNTAX score was weakly predictive of MACCE in this setting. In our study we found an increase in MACCE at one year in the subgroup of high (≥33) SYNTAX score compared to the subgroup of patients with SYNTAX score <33 (25% vs. 12%, p=0.05) which is consistent with previously reported data7,27,28. However, the SYNTAX score alone did not remain an independent predictor of MACCE. This suggests that generalisation of the results of some studies7,27 assessing the role of SYNTAX score to predict outcomes after LM stenting, is limited. The association between EuroSCORE and outcomes after LM stenting has also been addressed in different studies7,28,29 and was found to be predictive of MACCE. In our series, a high EuroSCORE (≥6) was a strong independent predictor of MACCE in our population. All these findings suggest that a combination of clinical and angiographic information would be more suitable for prediction of outcomes. This hypothesis was developed in a recent study by Capodanno et al30 that created a combined risk model and tested its performance on a population undergoing LM PCI. A Global Risk Classification was created by combination of SYNTAX score and EuroSCORE strata, and three new classes of risk were defined. The authors found a significant improvement in the prediction of outcomes with the inclusion of EuroSCORE in a SYNTAX score-based model. In our population, as shown in Figure 3, when MACCE was stratified according to the three risk categories of the Global Risk Classification, the distribution of MACCE in the lowest risk class (7.6%), was well separated by that observed both in the intermediate (17.7%) and the highest class (28.1%) (Log rank p=0.0181). Therefore, our findings support those of Capodanno et al, suggesting that clinical and angiographic information are both important for assessing individual risk of patients undergoing left main PCI.
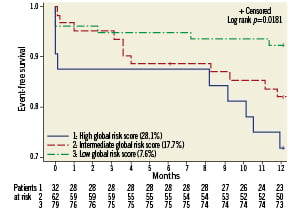
Figure 3. Kaplan-Meier curves for freedom from MACCE according to Global Risk Classificaion (Log rank p= 0.0181)
Limitations
This is a non-randomised study with a relatively small number of patients and, as only 47% of patients underwent angiographic follow-up, the restenosis rate may have been underestimated. Furthermore, because EES was the only type of stent implanted in the left main, these results may not be applicable to all types of DES.
Conclusions
We conclude that unprotected LM stenting with EES is feasible, safe and effective in the midterm, with a MACCE rate of 15.1%, a TLR rate of 2.9 % and a 0.6% rate of LM stent thrombosis at one year. Randomised clinical trials with prolonged follow-up comparing PCI with new generation DES vs. CABG are warranted to establish the role of these new devices in patients with unprotected LM disease. The upcoming EXCEL trial (Evaluation of Xience Prime versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularisation), which will randomise patients with unprotected LM disease and a SYNTAX score <33 to either PCI with EES or CABG, will shed more light on this subject.
Acknowledgments
The authors thank Catherine Dupic for revising style and grammar.
Conflict of interest statement
N. Dumonteil and N. Boudou have received speaker honorarium from Abbott. T.Lefèvre has received minor fees from Abbott, Cordis, Boston, Biosensor and Terumo. B. Chevalier is a consultant for Abbott Vascular. The other authors have no conflict of interest to declare.

