Abstract
Background: Improvements in drug-eluting stent design have led to a reduced frequency of repeat revascularisation and new biodegradable polymer coatings may allow a shorter duration of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI).
Aims: The Improved Drug-Eluting stent for All-comers Left Main (IDEAL-LM) study aims to investigate long-term clinical outcomes after implantation of a biodegradable polymer platinum-chromium everolimus-eluting stent (BP-PtCr-EES) followed by 4 months DAPT compared to a durable polymer cobalt-chromium everolimus-eluting stent (DP-CoCr-EES) followed by 12 months DAPT in patients undergoing PCI of unprotected left main coronary artery (LMCA) disease.
Methods: This is a multicentre randomised clinical trial study in patients with an indication for coronary artery revascularisation who have been accepted for PCI for LMCA disease after Heart Team consultation. Patients were randomly assigned in a 1:1 ratio to receive either the BP-PtCr-EES or the DP-CoCr-EES. The primary endpoint was a non-inferiority comparison of the rate of major adverse cardiovascular events (MACE), defined as all-cause death, myocardial infarction, or ischaemia-driven target vessel revascularisation at 2 years.
Results: Between December 2014 and October 2016, 818 patients (410 BP-PtCr-EES and 408 DP-CoCr-EES) were enrolled at 29 centres in Europe. At 2 years, the primary endpoint of MACE occurred in 59 patients (14.6%) in the BP-PtCr-EES group and 45 patients (11.4%) in the DP-CoCr-EES group; 1-sided upper 95% confidence interval (CI) 7.18%; p=0.04 for non-inferiority; p=0.17 for superiority. The secondary endpoint event of BARC 3 or 5 bleeding occurred in 11 patients (2.7%) in the BP-PtCr-EES group and 2 patients (0.5%) in the DP-CoCr-EES group (p=0.02).
Conclusions: In patients undergoing PCI of LMCA disease, after two years of follow-up, the use of a BP-PtCr-EES with 4 months of DAPT was non-inferior to a DP-CoCr-EES with 12 months of DAPT with respect to the composite endpoint of all-cause death, myocardial infarction or ischaemia-driven target vessel revascularisation.
Introduction
Randomised clinical trials of surgical and percutaneous revascularisation in patients with significant stenosis of an unprotected left main coronary artery (LMCA) have shown that percutaneous coronary intervention (PCI) with drug-eluting stents (DES) has comparable clinical outcomes to bypass surgery in terms of death, all myocardial infarction and stroke, but a higher rate of repeat revascularisation and non-periprocedural myocardial infarction123. As such, PCI is an acceptable alternative therapy for patients with LMCA disease, especially for those considered unsuitable for surgery due to advanced age or multiple comorbidities. Given the potentially catastrophic consequences of stent thrombosis in the LMCA, 12 months of dual antiplatelet therapy (DAPT) is reasonable. Biodegradable polymer (BP)-coated DES were developed to decrease the risk of durable polymer (DP)-related delayed vascular healing, potentially reducing the risk of stent thrombosis and allowing a shorter duration of DAPT45. Additionally, during PCI of LMCA disease, a large size discrepancy exists between the proximal and distal segments of the left main bifurcation and represents a potential substrate for strut malapposition. Therefore, the ability to over-expand a stent whilst maintaining radial strength is also an important consideration. A novel BP-coated platinum-chromium everolimus-eluting stent (BP-PtCr-EES) combines these features and may offer advantages for PCI in the LMCA67. To test this hypothesis, the Improved Drug-Eluting stent for All-comers Left Main (IDEAL-LM) study investigated clinical outcomes after BP-PtCr-EES implantation followed by only 4 months of DAPT compared to durable polymer cobalt-chromium everolimus-eluting stent (DP-CoCr-EES) implantation followed by 12 months of DAPT for treatment of unprotected LMCA disease.
Methods
Study design and patient population
The IDEAL-LM study is an investigator-initiated, international, multicentre, open-label randomised clinical trial comparing two stent designs coupled with two different durations of DAPT for PCI in LMCA disease. Patients had an indication for revascularisation according to current guidelines (LMCA >90% diameter stenosis or >50% diameter stenosis with documented ischaemia)8. PCI should be the revascularisation method of choice after discussion with the Heart Team. Patients with chronic and acute coronary syndromes were enrolled, excluding those with ST-elevation myocardial infarction within the prior 5 days. The complete inclusion and exclusion criteria are listed in Supplementary Table 1. Patients were randomly assigned in a 1:1 ratio to undergo PCI with either a BP-PtCr-EES followed by 4 months of DAPT or a DP-CoCr-EES followed by 12 months of DAPT. The use of intravascular ultrasound to optimise stent deployment was strongly recommended. Details of the main study design have been published9. All patients provided written consent before any study-specific procedures. Randomisation was performed immediately before the index procedure using web-based software (e-DREAM system; Diagram B.V.) with random blocks according to centre. Clinical follow-up was performed at discharge, 3 months, 6 months, 1 year and then annually through to 5 years. There was a prespecified substudy in 100 patients at 5 sites to assess vascular healing after 3 months using optical coherence tomography (OCT).
The study was initiated by the principal investigators and the protocol developed in consultation with the statistical committee. Boston Scientific provided funding, reviewed the protocol and participated in site selection but were not involved in any other aspect of the conduct of the study. Site monitoring, database management and statistical analyses were performed by the independent organisations listed in Supplementary Table 2. The study was approved by local institutional review boards, it adheres to the principles of the Declaration of Helsinki and Good Clinical Practice, and was registered at ClinicalTrials.gov (NCT02303717) before inclusion of the first patient.
Study devices
The BP-PtCr-EES (Synergy; Boston Scientific) has a platinum-chromium alloy platform with a strut thickness of 74-81 μm and a 4 μm thick coating on the abluminal side only of a biodegradable polymer (DL-lactide-coglycolide) which is hydrolysed to carbon dioxide and water over a period of 120 days. The polymer is mixed with an everolimus to give a dose equivalent of 1 μg/mm2 and is released over a period of 90 days. The DP-CoCr-EES (XIENCE; Abbot Vascular) has a cobalt–chromium alloy platform with a strut thickness of 81 μm and an 8 μm thick durable polymer coating. The polymer is polyvinylidene fluoride hexafluoropropylene and is loaded with everolimus10.
Procedural characteristics
PCI for LMCA was performed according to the standard procedures. For LMCA bifurcation lesions, provisional stenting with side branch opening was the preferred approach, but two-stent strategies, such as T and protrusion, culotte and crush were all acceptable. Any additional non-left main lesions undergoing PCI were to be treated with the same stent to which the patient had been assigned at randomisation. If a staged procedure was planned, treatment of the non–target vessel should have been performed within 30 days, again using the same stent type as the study stent. All procedural complications and adverse events were recorded throughout the PCI procedure and the follow-up period. Cardiac biomarkers (CK-MB or troponin) were measured before and at least 4 hours post-procedure.
Objectives and endpoints
The primary objective of the trial is to establish the non-inferiority of the BP-PtCr-EES group relative to the DP-CoCr-EES group for the composite primary endpoint of major adverse cardiac events (MACE), defined as all-cause death, myocardial infarction, and ischaemia-driven target vessel revascularisation (TVR) at 2 years. Secondary endpoints included individual components of the primary endpoint, a device-oriented composite endpoint (DOCE), defined as cardiac death, myocardial infarction not clearly attributable to a non-treated vessel and clinically-indicated target lesion revascularisation (TLR), stent thrombosis as per Academic Research Consortium criteria and bleeding as per Bleeding Academic Research Consortium criteria (BARC 1 to 5 and combined BARC 3 or 5)11. Procedural success was defined as attainment of a <30% diameter stenosis of the target lesion with no in-hospital DOCE. An independent Clinical Endpoint Committee adjudicated all study endpoints including bleeding events.
Statistical analysis
The trial was powered to assess non-inferiority for the primary endpoint at 2 years post-procedure. Reviewing event rates from published data, the primary endpoint rates at 2 years for both treatment groups were predicted to be 20%1213. Based on a prespecified absolute difference of 7.5% for the non-inferiority margin and a two-sided type I error of 0.05, a total of 818 patients provided 85% power. Non-inferiority would be shown if the upper limit of the 1-sided 95% confidence interval (CI) of the absolute risk difference was less than the non-inferiority margin of 7.5%. If non-inferiority was established, superiority testing would be performed, as well as calculation of 2-sided 95% CIs, both applied to the intention-to-treat population. Continuous variables are reported using descriptive statistics and compared using Wilcoxon’s rank sum or Student's t-tests. Categorical variables are expressed as frequency (%) and compared using the likelihood-ratio chi-square or Fisher’s exact tests. All statistical tests were interpreted at a 2-sided significance level of 0.05 and all CIs at a 2-sided level of 95% unless otherwise stated. Statistical analyses were performed by using SAS (version 9.4, SAS Institute Inc.).
Results
Baseline patient characteristics and follow-up
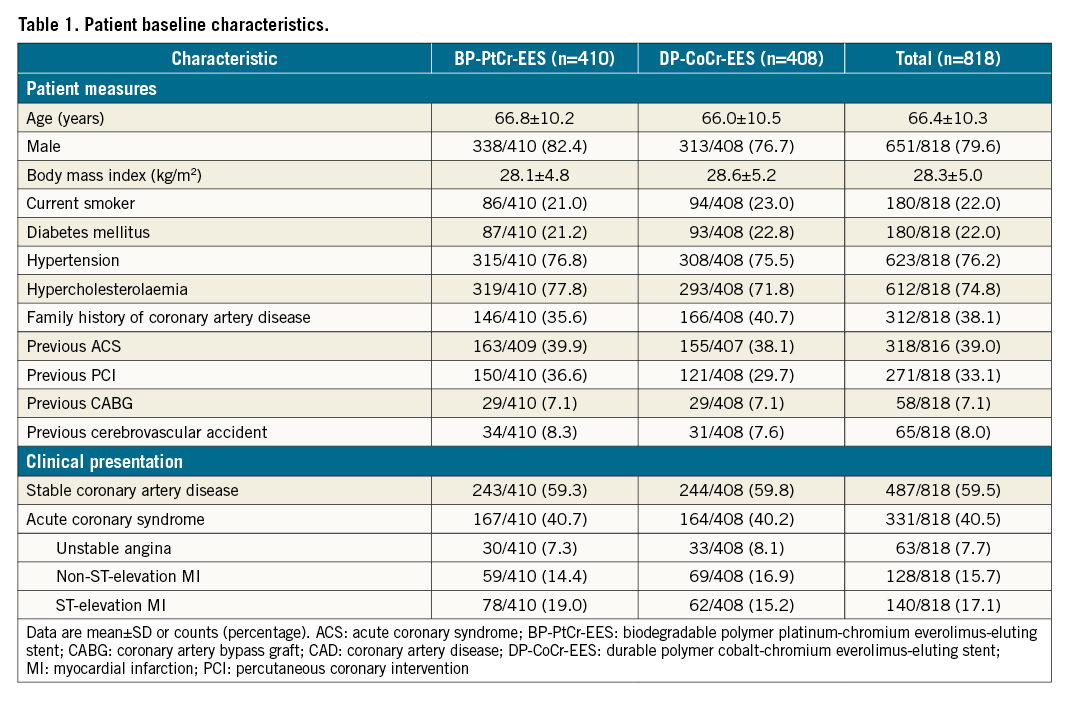
Between December 2014 and October 2016, 818 patients undergoing PCI for LMCA were randomly assigned to receive either BP-PtCr-EES (410 patients) or DP-CoCr-EES (408 patients). The study flow chart is presented in the Central illustration. Baseline clinical characteristics were well balanced between the groups and are shown in Table 1. Participants were mainly male (79.6%), with a mean age of 66.4±10.3 years, 22.0% had diabetes, 33.1% had previous PCI and 40.5% presented with an acute coronary syndrome. All patients received at least a short term of DAPT post-procedure and, according to the protocol, a major shift in DAPT usage was in the period from 4 months to 12 months (Supplementary Figure 1).
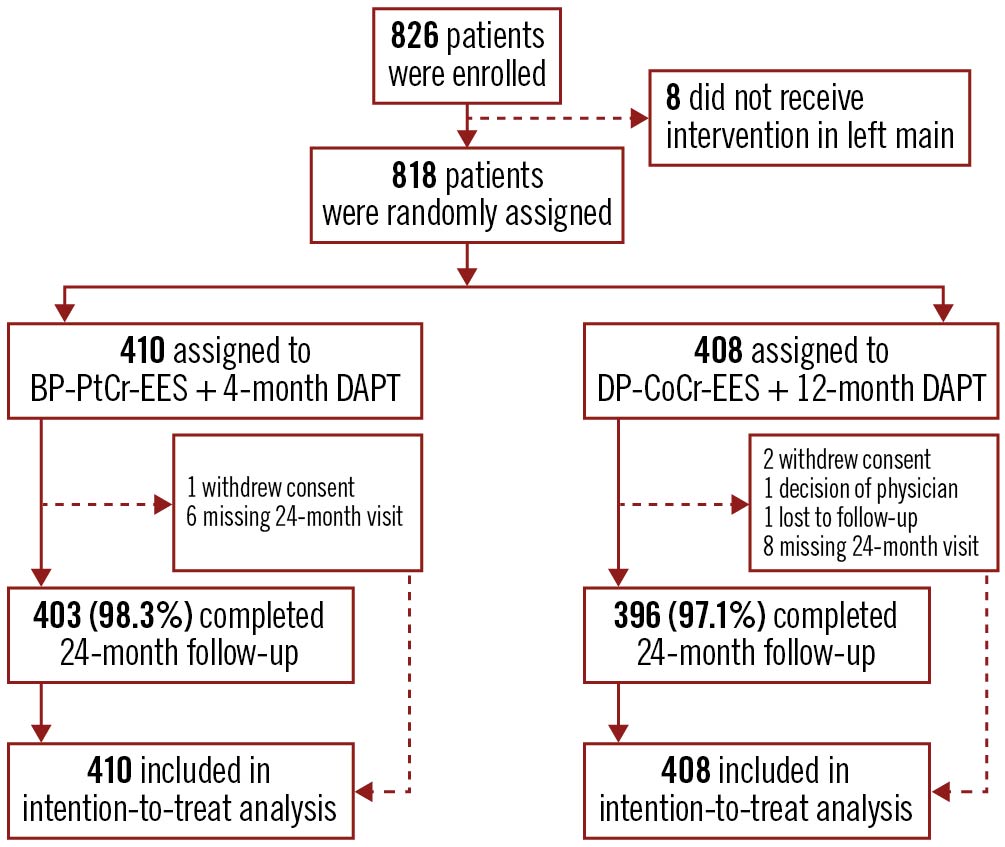
Central illustration. Study profile. BP-PtCr-EES: biodegradable polymer platinum-chromium everolimus-eluting stent; DAPT: dual antiplatelet therapy; DP-CoCr-EES: durable polymer cobalt-chromium everolimus-eluting stent
Lesion and procedural characteristics
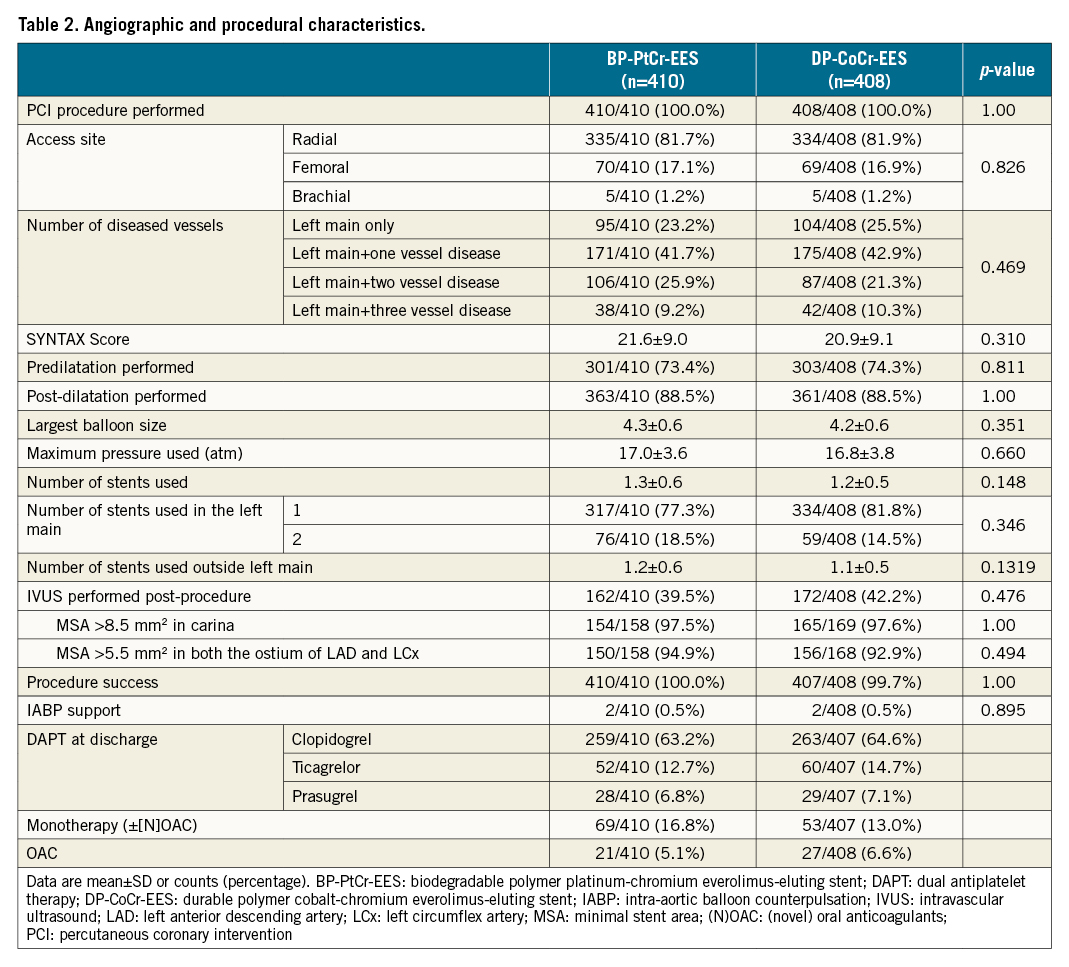
Lesion and procedural characteristics are summarised in Table 2. PCI for LMCA disease was performed in all patients. Isolated LMCA disease was present in 23.2% (95/410) of patients in the BP-PtCr-EES group and 25.5% (104/408) in the DP-CoCr-EES group. The SYNTAX score was 21.6±9.0 in the BP-PtCr-EES group and 20.9±9.1 in the DP-CoCr-EES group. Intravascular ultrasound was used in 39.5% and 42.2% of patients, respectively. Single-stent implantation was performed in the LMCA in 77.3% (317/410) and 81.8% (334/408) of patients in the BP-PtCr-EES and DP-CoCr-EES groups, respectively. Almost all patients (99.5%) received the assigned stent in the culprit lesion.
Clinical outcomes
At 2 years, the primary endpoint of MACE occurred in 59 patients (14.6%) in the BP-PtCr-EES group and 45 patients (11.4%) in the DP-CoCr-EES group (Table 3, Figure 1); 1-sided upper 95% CI 7.18%, p=0.04 for non-inferiority; p=0.17 for superiority. Rates of all-cause death (BP-PtCr-EES vs DP-CoCr-EES: 5.2% vs 5.3%; log-rank p=0.96), myocardial infarction (BP-PtCr-EES vs DP-CoCr-EES: 6.0% vs 3.5%; log-rank p=0.08) and ischaemia-driven TVR (BP-PtCr-EES vs DP-CoCr-EES: 7.4% vs 4.8%; log-rank p=0.15) did not significantly differ between groups through 2 years of follow-up. Rates of definite or probable stent thrombosis were not significantly different between groups (BP-PtCr-EES vs DP-CoCr-EES: 2.7% vs 1.3%; log-rank p=0.14). BARC 3 or 5 bleeding occurred in 11 (2.7%) patients assigned to the BP-PtCr-EES and 2 (0.5%) patients assigned to the DP-CoCr-EES (log-rank p=0.02) (Table 3, Figure 2). Of the 11 events in the BP-PtCr-EES group, 7 occurred when the patients were off DAPT and 4 occurred in patients also taking oral anticoagulant therapy (2 on-DAPT and 2 off-DAPT). There was no evidence for any treatment-by-subgroup interaction with respect to MACE across all prespecified subgroups (Figure 3). Landmark analyses up to 4 months, from 4 months to 1 year, and from 1 to 2 years showed no significant differences between groups with respect to MACE (Supplementary Figure 2).
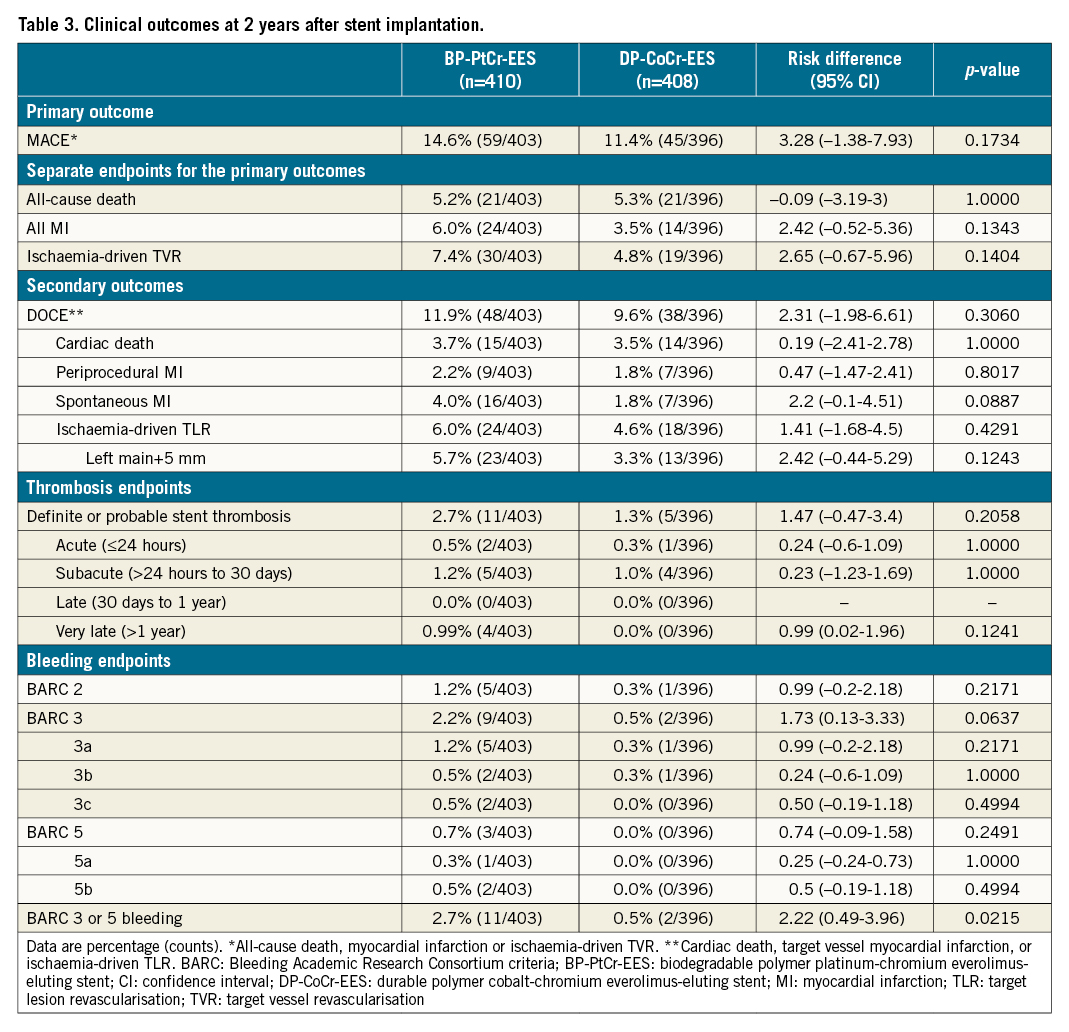
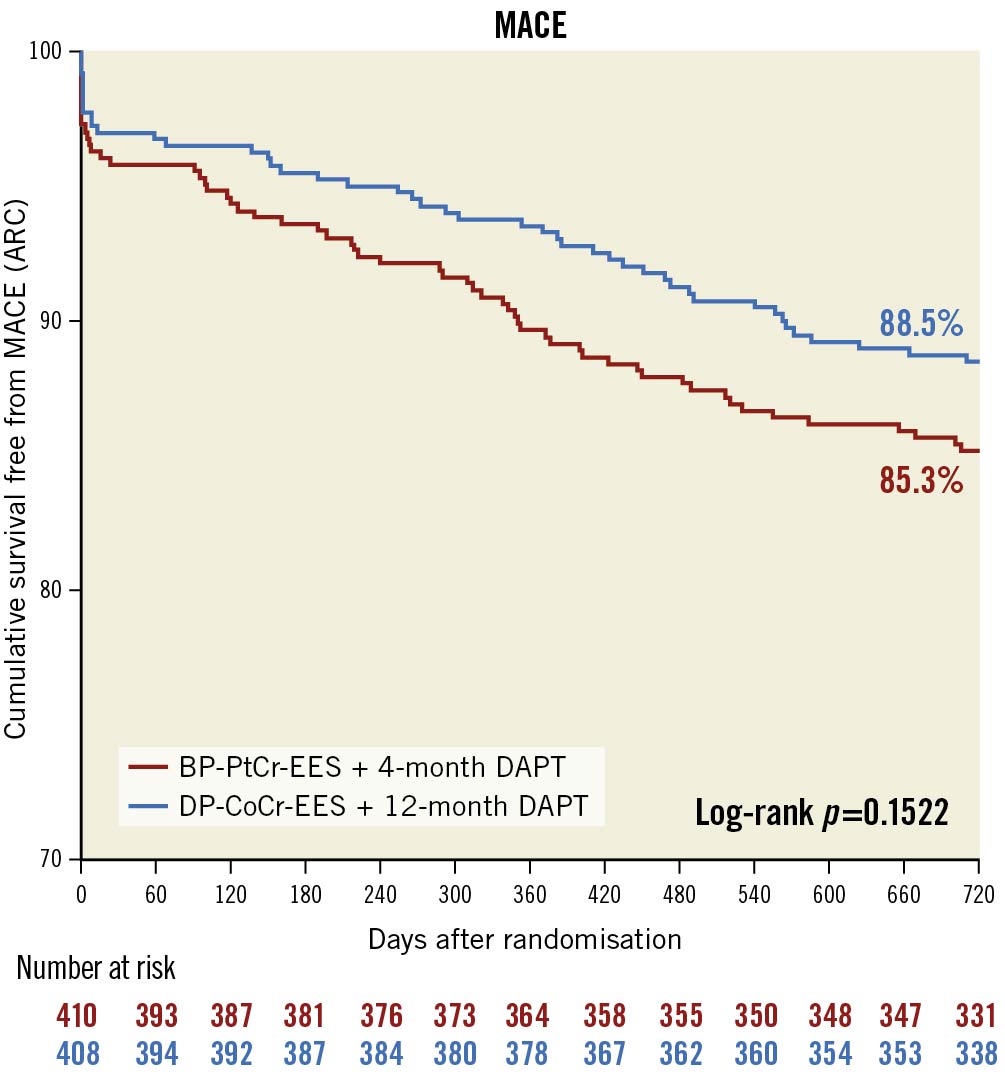
Figure 1. Cumulative event-free survival plot for primary endpoint over 2 years of follow-up. ARC: Academic Research Consortium; BP-PtCr-EES: biodegradable polymer platinum-chromium everolimus-eluting stent; DAPT: dual antiplatelet therapy; DP-CoCr-EES: durable polymer cobalt-chromium everolimus-eluting stent; MACE: major adverse cardiovascular events
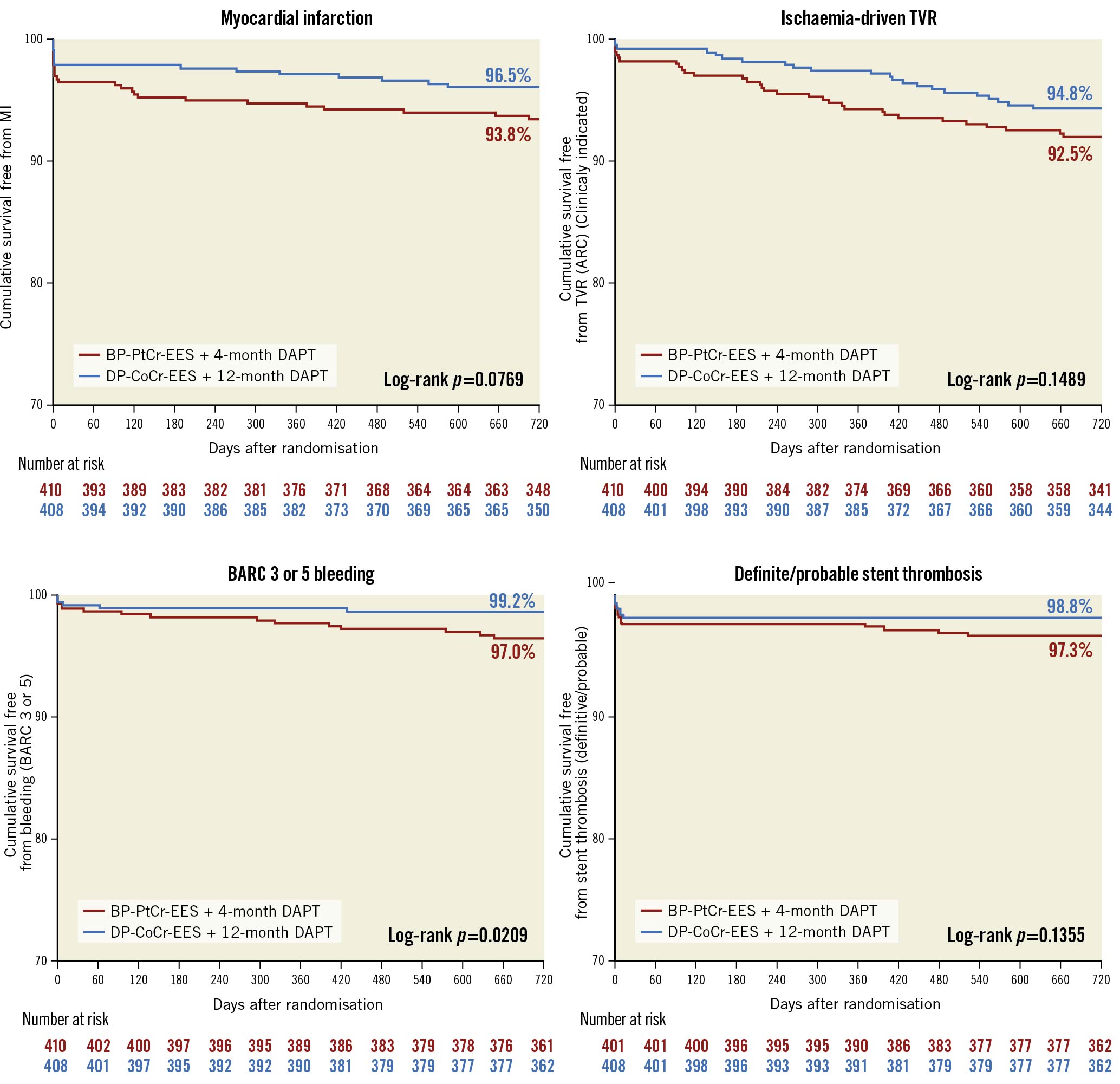
Figure 2. Cumulative event-free survival plot for secondary endpoint over 2 years of follow-up. ARC: Academic Research Consortium; BARC: Bleeding Academic Research Consortium; BP-PtCr-EES: biodegradable polymer platinum-chromium everolimus-eluting stent; DAPT: dual antiplatelet therapy; DP-CoCr-EES: durable polymer cobalt-chromium everolimus-eluting stent; MACE: major adverse cardiovascular events; MI: myocardial infarction; TVR: target vessel revascularisation
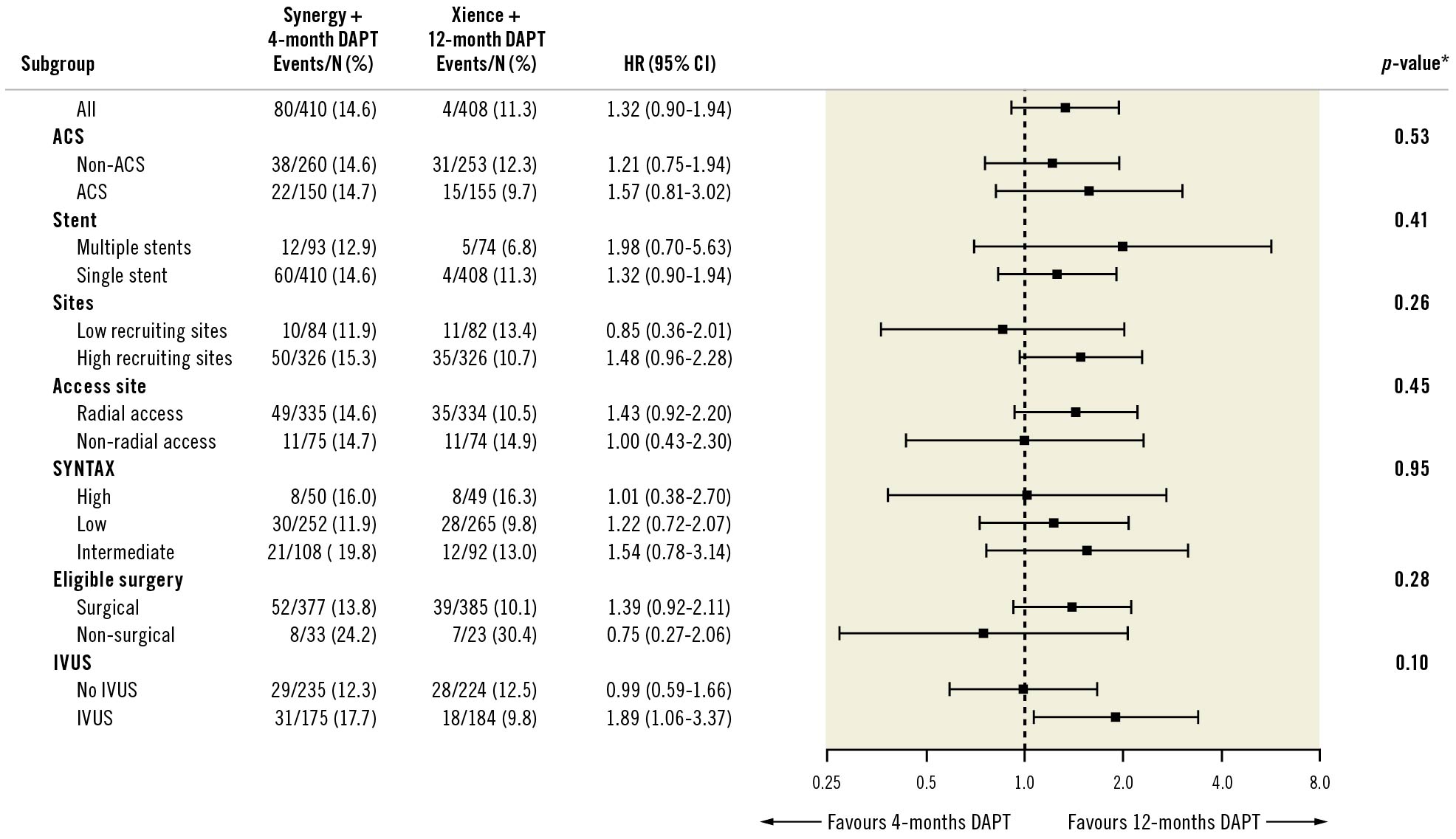
Figure 3. Stratified analyses of the primary endpoint at 2 years across subgroups. * p-value is the test of interaction between treatment and subgroup, unadjusted for multiplicity. ACS: acute coronary syndrome; CI: confidence interval; DAPT: dual antiplatelet therapy; HR: hazard ratio; IVUS: intravascular ultrasound
Discussion
In this all-comers, multicentre, single-blind, randomised clinical trial, LMCA PCI with BP-PtCr-EES followed by 4 months of DAPT was non-inferior to DP-CoCr-EES followed by 12 months of DAPT in terms of MACE at 2 years. No significant differences were documented in the individual components of the primary endpoint, but they all trended numerically higher in the BP-PtCr-EES with 4-month DAPT group than in the DP-CoCr-EES with 12-month DAPT group. The rates of definite and probable stent thrombosis were low and did not differ between groups. Counterintuitively, BARC 3 or 5 bleeding occurred more commonly in the short-duration DAPT group but the study was not powered to show a difference in bleeding events and so this could be the play of chance. Moreover, all 4 patients who experienced BARC 3-5 bleeding whilst taking an oral anticoagulant were in the BP-PtCr-EES group.
The specific design of the BP-PtCr-EES platform, as well as the polymer degradation kinetics, has been reported to provide favourable vascular healing in non-LMCA lesions, with rates of strut coverage and apposition at 3 months of 94.5% and 93.8%, respectively14. In the TRANSFORM-OCT (TRiple Assessment of Neointima Stent FOrmation to Reabsorbable polyMer With Optical Coherence Tomography) study, BP-PtCr-EES and DP-zotarolimus-eluting stents showed a similar healing response at 3 months15. The OCT substudy of the IDEAL-LM study showed similar and near complete vascular healing with both stents 3 months after implantation, with a strut coverage >20 μm for over 96% of the individual struts16. These findings support the view that the biodegradable and durable polymers utilised in contemporary DES are associated with favourable vascular healing, even in LMCA disease. Nevertheless, little is known about the efficacy and safety of a short duration of DAPT after PCI with contemporary DES in LMCA. Current guidelines from the ACC/AHA recommend at least 6-12 months of DAPT in patients undergoing PCI with DES17. The European Society of Cardiology Guidelines recommend a 3-month period of DAPT in patients at high bleeding risk (e.g., PRECISE-DAPT score ≥25) undergoing PCI for stable coronary artery disease18. The European Bifurcation Club recently consulted worldwide opinion leaders for a detailed DAPT strategy proposal involving bleeding risk, patient characteristics and procedural characteristics, such as imaging guidance and bifurcation approach19.
Recently, several large-scale randomised trials investigated the efficacy and safety of a short duration of DAPT (1 to 3 months) after PCI in specific study populations (e.g., all-comers, elderly, or high risk for bleeding or an ischaemic event)202122. Overall, a short duration of DAPT after PCI did not increase the incidence of ischaemic events and may reduce the risk of bleeding events. Nevertheless, it is noteworthy that only a small proportion of patients (from 1.8% to 8.2%) with LMCA disease were included in the aforementioned studies. Although no significant differences were observed in the ischaemic endpoints in IDEAL-LM, they all trended numerically higher in the BP-PtCr-EES/short DAPT group and bleeding was not reduced. Accordingly, the risks and benefits of a short duration of DAPT after PCI for LMCA disease need to be further investigated, taking into account the personalised risk of bleeding and ischaemic events.
In the EXCEL (Evaluation of XIENCE Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) trial, PCI with the same DP-CoCr-EES used in IDEAL-LM, coupled with a minimum of 12 months of DAPT, was non-inferior to CABG at 5 years with respect to a composite endpoint of all-cause death, stroke, or myocardial infarction2. The SYNTAX scores and the incidence of diabetes were similar in EXCEL to the patient population in IDEAL-LM. Continuation of DAPT beyond 1 year in EXCEL did not reduce the rate of death, myocardial infarction or stroke23.
In a patient-level pooled analysis of five multicentre registries including 700 patients treated for LMCA disease, the rate of target lesion failure in patients treated with two-stent techniques was significantly higher than in the one-stent group only when DAPT was interrupted before 1 year24. In IDEAL-LM, approximately 80% of patients underwent LMCA-PCI using a single-stent technique and we did not see an interaction between treatment with one or more stents and randomised treatment allocation.
Study limitations
Firstly, the observed rates of the primary endpoint were lower than predicted, with the difference being due to lower rates of all-cause death and ischaemia-driven TVR than in previous studies1213252627. As a specific example, all-cause death at two years was 5.0% in IDEAL-LM, compared to 10.0% in the ISAR-LEFT-MAIN study28. Generally, of course, this is good news for patients undergoing LMCA-PCI, but it means that the result of IDEAL-LM is not particularly robust. The predicted event rate of 20% in both groups coupled with an absolute non-inferiority margin of 7.5% yield a relative non-inferiority margin of 7.5/20=0.375. This is similar to the EXCEL trial in which the predicted event rates were 11% in both groups (excluding repeat revascularisation) with a non-inferiority margin of 4.2%, yielding a relative non-inferiority margin of 4.2/11=0.38. If we apply this to the observed event rates in IDEAL-LM, the equivalent absolute non-inferiority margin would be 4.3% and non-inferiority would not be confirmed. Alternatively, and retrospectively, one can state that due to the lower-than-predicted event rates, the trial is now underpowered, though potentially the original power of 85% could be recovered during ongoing follow-up. Given the methodological limitations of the study, and the numerically higher number of events in the BP-PtCr-EES followed by 4-month DAPT group, a larger dataset is required to evaluate this potentially concerning signal.
Secondly, the IDEAL-LM study was designed to compare two therapeutic strategies for LMCA-PCI. The duration of DAPT was coupled to and determined by the randomly assigned stent type but we acknowledge that this may hinder the interpretation of any between-group differences in outcomes. Finally, the majority of patients were treated using one-stent techniques and therefore more data are required for a more robust recommendation on DAPT duration for patients treated with two-stent strategies.
Conclusions
PCI with the BP-PtCr-EES followed by 4 months of DAPT was non-inferior to the DP-CoCr-EES followed by 12 months of DAPT with respect to MACE at 2 years in an all-comers population with LMCA disease. However, due to event rates that were lower than predicted, the trial is underpowered and the individual components of MACE all trend numerically higher in the BP-PtCr-EES with 4-month DAPT group. The findings of this trial should be interpreted only as hypothesis generating. The efficacy and safety of a short duration of DAPT after LMCA PCI requires further investigation and future studies should focus on the individual patient risk for bleeding and ischaemic events.
Data sharing
All data, including study participant data, data dictionary, statistical analysis plan, and informed consent, will not be shared.
Impact on daily practice
PCI for left main disease is frequently a primary strategy due to relative contraindications for bypass surgery. Stent design iterations intend to improve outcomes and minimise antiplatelet therapy for these patients where comorbidity is frequently present. This study demonstrated that a strategy of using a biodegradable polymer-coated platinum-chromium everolimus-eluting stent followed by four months of dual antiplatelet therapy was non-inferior to a strategy with a durable polymer-coated cobalt-chromium everolimus-eluting stent followed by 12 months dual antiplatelet therapy. Superiority in bleeding events was not achieved. As the overall event rate was low, this study cannot make definite conclusions, yet both strategies provided state-of-the-art outcomes.
Acknowledgements
All authors are indebted to Mrs. Colette Donaghy (Venn Life Sciences, Belfast, United Kingdom) and Mrs. Evelien Kolkman (Diagram, Zwolle, the Netherlands) for their assistance in coordinating the trial.
Conflict of interest statement
R. van Geuns reports receiving grants and personal fees from Boston Scientific, during the conduct of the study; grants and personal fees from Abbott Vascular, AstraZeneca, and Amgen; and personal fees from Sanofi, outside the submitted work. M. Lesiak has received speaker’s honoraria from Abbott Vascular, and Boston Scientific. Y. Onuma was an advisory board member of Abbott Vascular. M.B. McEntegart has a proctoring agreement with Boston Scientific and Vascular Perspectives. K.G. Oldroyd reports receiving grant support and lecture fees from AstraZeneca and lecture fees from Biosensors, Abbott Vascular, and GE. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

