
Abstract
Aims: The aim of this study is to demonstrate the non-inferiority of the BiOSS LIM C sirolimus-eluting cobalt-chromium bifurcation dedicated stent against the XIENCE stent regarding the patient-oriented composite endpoint (POCE) at 12 months among patients with left main coronary artery disease (LMCA).
Methods and results: The POLBOS LM study is a single-arm, prospective, multicentre study enrolling 260 patients (SYNTAX score ≤32) with a pre-specified performance goal based on the results of the EXCEL trial with contemporary percutaneous coronary intervention (PCI) for LMCA disease. Patient enrolment will comply with objective inclusion criteria of diameter stenosis ≥50% in the LMCA based on off-line quantitative coronary angiography (QCA) analysed by an independent core laboratory using dedicated bifurcation QCA software. The BiOSS LIM C is used for the treatment of LMCA disease with the same specific technical classification as for the BiOSS LIM (modified MADS classification) and the stent implantation is optimised by using pre-specified intravascular ultrasound criteria. The primary endpoint is POCE (a composite of all-cause death, stroke, any myocardial infarction, and any revascularisation) at 12 months.
Conclusions: The POLBOS LM study will indicate the efficacy of the BiOSS LIM C stent with contemporary PCI for distal left main bifurcation lesions in comparison with the XIENCE stent from the recent EXCEL trial, as a performance index.
Introduction
BACKGROUND
Left main coronary artery (LMCA) disease is associated with a relatively large amount of myocardium at risk; therefore, percutaneous coronary intervention (PCI) for LMCA disease has been considered one of the challenging subsets. Coronary artery bypass grafting (CABG) has shown good long-term clinical outcome for LMCA disease, whereas, in historical studies of PCI for LMCA, a higher incidence of repeat revascularisation has been reported1. The lesion frequently involves bifurcation segments, especially when a lesion is located in the distal part of the LMCA2. In that case, stenting techniques tend to be complex, being associated with a high incidence of adverse events1. However, stent technology and implantation techniques have evolved with concomitant medical therapy such as P2Y12 inhibitors, statins and PCSK9 inhibitors.
As a consequence, the clinical outcomes after LMCA stenting have become comparable to CABG for low- and intermediate-risk patients3,4,5. In the SYNTAX trial, which was the comparison trial of major adverse cardiac and cerebrovascular events (MACCE) between PCI using a first-generation paclitaxel-eluting stent and CABG, 705 patients had LMCA disease (LMCA subgroup). The five-year MACCE rate of the patients with low and intermediate SYNTAX scores (0-22 and 23-32) treated with PCI was comparable to that of the patients in the CABG group (low: 30.4% for PCI versus 31.5% for CABG, p=0.74; intermediate: 32.7% versus 32.3%, p=0.88)3,6. In the PRECOMBAT trial, comparing clinical outcomes after PCI using a first-generation sirolimus-eluting stent versus CABG in patients with LMCA disease (average SYNTAX score: 25), the five-year MACCE rate was comparable (17.5% for PCI versus 14.3% for CABG, p=0.26)5. The recent EXCEL trial was a prospective, international, open-label, multicentre trial that randomised 2,900 patients to compare PCI using a best-in-class stent (XIENCE; Abbott Vascular, Santa Clara, CA, USA) versus CABG in patients with LMCA disease with a SYNTAX score ≤32. In that trial, the three-year MACCE (all-cause mortality, stroke, or MI) rate in the PCI arm was non-inferior to the one in the CABG arm (15.4% versus 14.7%, p for non-inferiority=0.02)4. However, simultaneously with the publication of the EXCEL trial, the NOBLE trial (1,201 patients) reported that PCI treatment for LMCA disease failed to achieve non-inferiority against CABG in terms of the MACCE (all-cause mortality, non-procedural MI, any repeat coronary revascularisation and stroke) rate at five years7. In a recent meta-analysis including four studies as described previously, PCI and CABG showed comparable safety in patients with LMCA disease and low to intermediate SYNTAX scores, whereas repeat revascularisation was more common after PCI8. This suggests that the indication of PCI for LMCA disease should be discussed on a case-by-case basis by a local Heart Team.
THE BiOSS LIM C BIFURCATION-DEDICATED STENT AND THE RATIONALE OF THE STUDY
The difficulty of PCI treatment for LMCA lesions is presumably ascribed to the morphological complexity of the bifurcation such as vessel-size mismatch between the proximal main trunk and the distal branch, which can cause malapposition of the struts in the main trunk. Additionally, a complex multiple stent strategy is often required, which may result in considerable overlap and malapposition of the struts. It was reported that a two-stent strategy was associated with a stiffening process of the systolic and diastolic change in bifurcation angle and that this issue was an independent predictor of adverse events9.
Dedicated bifurcation stents have been developed to reduce these potential issues. The BiOSS LIM C sirolimus-eluting cobalt-chromium stent (Balton, Warsaw, Poland) is a dedicated coronary bifurcation stent for provisional side branch stenting with a strut thickness of 70 µm. The device is designed for implantation from the proximal main vessel to the distal main vessel (Figure 1), consisting of two main parts with different diameters with a 2.0-2.4 mm middle zone with two connecting struts. The ratio of the proximal part diameter to the distal part diameter varies between 1.15 and 1.3 mm, ensuring physiological compatibility and optimal flow conditions10. The bottle-shaped device balloon ensures the proximal optimisation technique (POT)-like effect after BiOSS LIM C implantation11. The maximum expansion capacity of the BiOSS LIM C is 6.15 mm, which is comparable to that of the XIENCE (5.6 mm).
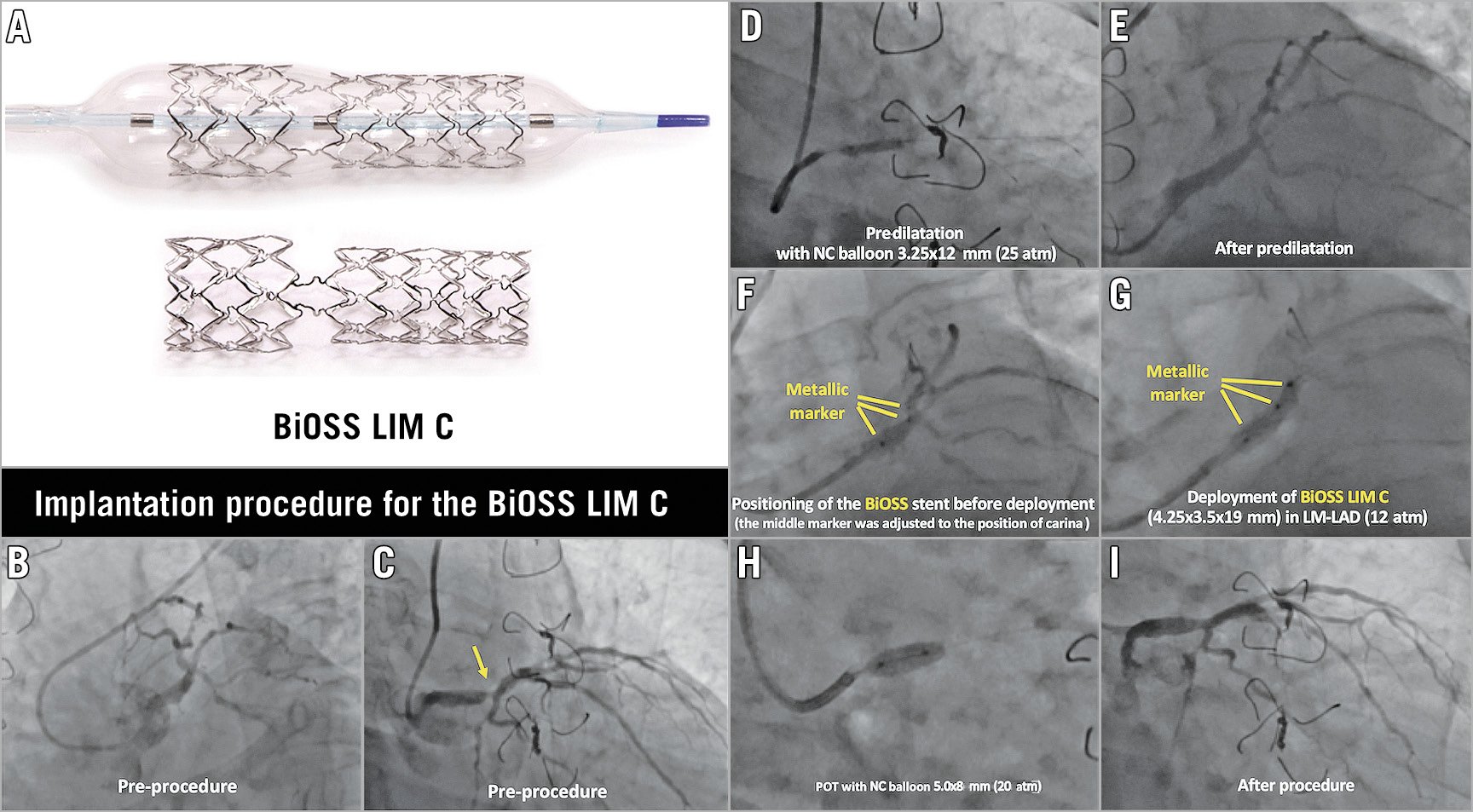
Figure 1. BiOSS® Stent/Bottle® Balloon structures and a case example of BiOSS® implantation in the LMCA. A) The macroscopic appearance of the BiOSS LIM C stent and its delivery balloon. The diameter of the balloon in the proximal and distal parts is different, reflecting the natural tapering of bifurcation anatomy. The transitional zone (corresponding to the bifurcation) is free from ring, but connects the proximal and distal parts with the two links. This enables easy access to the side branch after stenting in the main branch. The balloon has three metallic markers which are placed at the distal edge, transitional zone, and proximal edge. During implantation, the mid marker should be located at the point of the bifurcation carina, so that the transitional zone is precisely located in the polygon of confluence. B) – I) Example of an implantation procedure of the BiOSS LIM C stent. The patient had a Medina 1,0,0 left main bifurcation lesion (B & C). After predilatation with a non-compliant balloon 3.25×12 mm at 25 atm (D & E), the BiOSS stent was advanced in the LM towards the LAD. The BiOSS stent 4.25×3.5 ×19 mm was positioned precisely at the position of the carina using a metallic marker (F). After the deployment of the BiOSS stent 4.25×3.5 ×19 mm (G), the proximal optimisation technique was performed with a non-compliant balloon 5.0x8 mm at 20 atm (H). Final angiography demonstrated an excellent result (I). LAD: left anterior descending; LM: left main; POT: proximal optimisation technique
In the POLBOS II trial, the previous iteration of the BiOSS LIM sirolimus-eluting stent with 316L stainless steel demonstrated comparable one-year clinical outcomes to conventional drug-eluting stents in patients with bifurcation lesions12. The BiOSS stent was further upgraded using a cobalt-chromium platform (BiOSS LIM C stent). In a porcine model, the BiOSS LIM C stent showed comparable histological vascular healing to the Orsiro stent (Biotronik, Bülach, Switzerland) 28 days after implantation11. The first-in-man trial of the BiOSS LIM C stent, which investigated the clinical outcomes of 48 patients with bifurcation lesions 12 months after the implantation, demonstrated a high device success rate (100%) and low three-month events (one target lesion revascularisation [2.1%] and no spontaneous MI or stent thrombosis)13. In addition, a recent study of the BiOSS LIM in LM treatment suggested that this device may reduce resource utilisation (guidewire, balloon, contrast media) versus conventional DES14.
Based on these results, we generated the hypothesis that one-year clinical outcomes after BiOSS LIM C implantation in patients with distal unprotected LMCA disease are non-inferior to the best-in-class stent (XIENCE). Therefore, we designed a single-arm prospective study – the POLish Bifurcation Optimal treatment Strategy study for Left Main bifurcation PCI (POLBOS LM study) with a pre-specified performance goal based on the results of the EXCEL trial as recommended by the ESC/EAPCI task force on devices15.
Methods
STUDY DESIGN
The POLBOS LM study is a prospective, multicentre, single-arm study in patients with an indication for distal unprotected left main revascularisation. The treatment strategy consists of contemporary PCI of the left main bifurcation following diagnostic angiography, on which a significant distal left main disease (diameter stenosis [%DS] ≥50%) is confirmed by using dedicated bifurcation quantitative coronary angiography (QCA) software, and a local Heart Team discussion applying the anatomical SYNTAX score (<33) (Supplementary Figure 1),16. This single-arm study is designed with a pre-specified performance goal based on the EXCEL trial; therefore, the patient selection and event definitions of the current trial are formulated to be comparable to those of the EXCEL trial (NCT01205776). The POLBOS LM study has been registered at www.clinicaltrials.gov (NCT03508219).
The BiOSS LIM C will be used for the treatment of the left main bifurcations. For the potential additional treatment of proximal and distal left main lesions, the ALEX® PLUS cobalt-chromium sirolimus-eluting single stent (Balton) will be used in order to avoid the unexpected interaction of the XIENCE stent in the LMCA lesion. Other non-LMCA lesions will be treated with XIENCE stents for the sake of comparability with an objective performance index trial such as the EXCEL trial, in the present case (Supplementary Appendix 1).
The primary hypothesis of the current trial is that the BiOSS LIM C is non-inferior to the pre-specified performance goal in terms of the 12-month patient-oriented composite endpoint (POCE) consisting of all-cause mortality, stroke (modified Rankin Scale [mRS] ≥1), any MI and any unplanned revascularisation for ischaemia.
PATIENT POPULATION AND INDICATION FOR LMCA STENTING
Patients with silent ischaemia or chronic stable angina who have a de novo lesion in the distal unprotected LMCA and whose anatomical SYNTAX score is less than 33 are eligible if they fulfil the following objective criteria: 1) %DS of the target lesion in the LMCA is ≥50% confirmed by off-line QCA analysed by the independent core laboratory (CORRIB Core Lab, Galway, Ireland) using dedicated bifurcation software (CAAS; Pie Medical Imaging, Maastricht, the Netherlands) prior to the treatment17 with documented ischaemia (e.g., fractional flow reserve [FFR] ≤0.80)18 (in case preprocedural IVUS is available, a left main minimum lumen area [MLA] ≤6.0 mm² is considered equivalent to the DS ≥50% by the core lab) (Figure 2),19, 2) Medina classification for the target lesion in the LMCA is confirmed by off-line QCA, 3) the reference vessel diameter of the distal LMCA is ≥3.0 mm and ≤4.5 mm, and the distal main branch vessel diameter is ≤3.75 mm by visual estimation, 4) clinical and anatomical eligibility for PCI as agreed by the local Heart Team. Patients with stabilised acute coronary syndromes with elevated troponin (high-sensitivity troponin, troponin I or troponin T) at baseline (within 24 hrs pre-PCI) may also be included in the current study, if all of the following conditions are fulfilled: 1) the values of creatine kinase (CK) and creatine kinase myocardial band (CK-MB) are within the normal range; 2) the value of troponin at follow-up should be within a 20% range of the value of the first sample or have dropped; 3) ECG is normal.
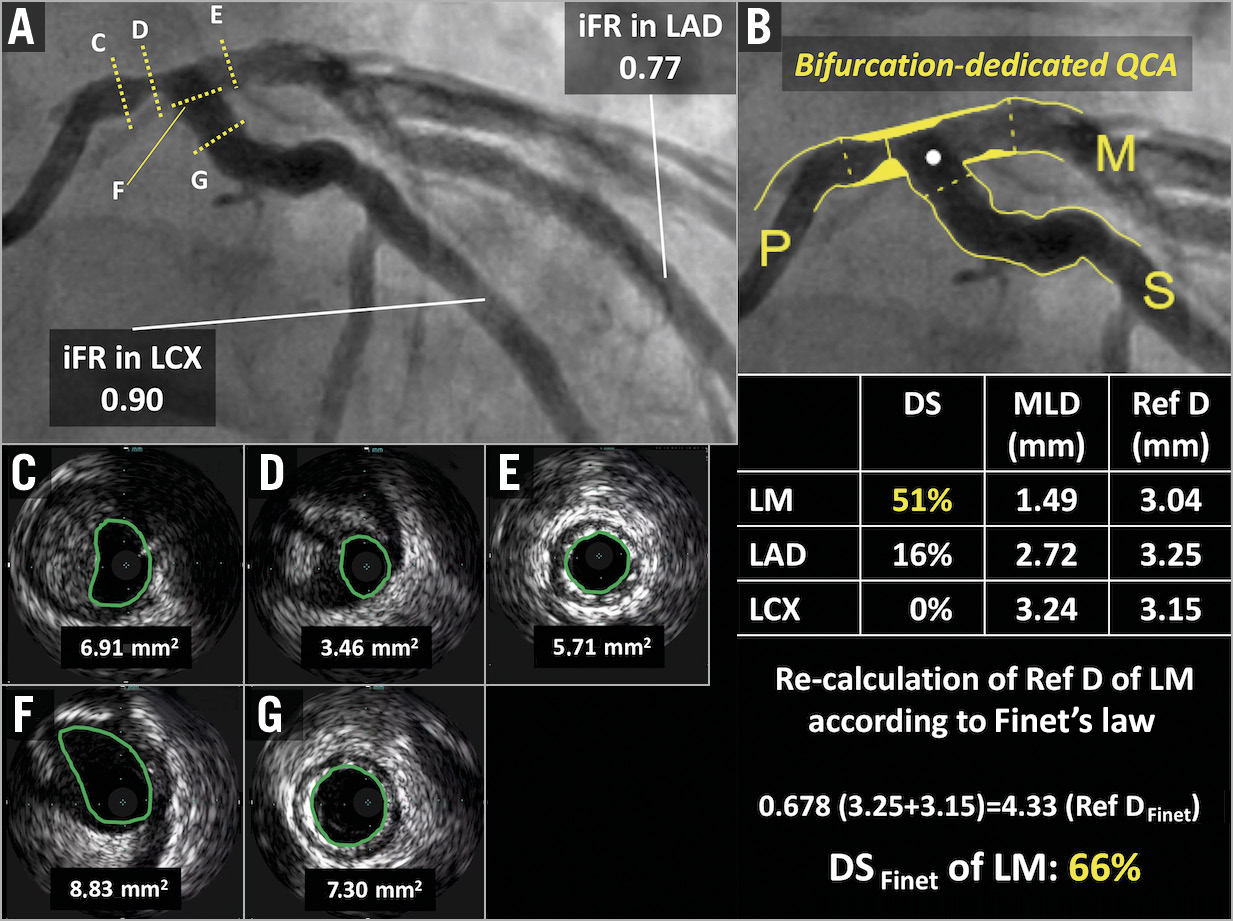
Figure 2. Quantitative coronary angiography of a left main bifurcation using dedicated bifurcation software. A) The first patient of the POLBOS LM study had a Medina 1,0,0 left main bifurcation lesion. B) QCA analysis using dedicated bifurcation software showed significant stenosis (DS of left main: 51%). However, the reference diameter (Ref D) of the left main was underestimated due to diffuse left main disease. Therefore, the Ref D of the left main was recalculated using Finet’s law, resulting in a DS of the left main of 66%. After enrolment, preprocedural MLA measured by IVUS was 3.46 mm2 in the distal left main (C-G). The preprocedural iFR value in the LAD and LCX was measured (A). DS: diameter stenosis; IVUS: intravascular ultrasound; LAD: left anterior descending; LCX; left circumflex; LM: left main; MLA: minimal lumen area; QCA: quantitative coronary angiography; RD: reference diameter
Patients who have a lesion in the LMCA with Medina classification (0,0,1) or with chronic total occlusion/visible thrombus in any bifurcation segments are not eligible for the current trial. Detailed inclusion and exclusion criteria are presented in Supplementary Table 1. Patients will be included at approximately 15 international sites located in Poland, France, and Italy.
iFR MEASUREMENT PRIOR TO PCI
The importance of physiology-guided PCI has become increasingly evident recently. However, few data are available on the use of coronary physiology to guide management in unprotected left main coronary artery disease1. It was reported that, in the assessment of LMCA disease, there were discrepancies in pressure indices between FFR and resting indices such as iFR and Pd/Pa, because the change in coronary flow from rest to maximal hyperaemia is greater in vessels supplying greater amounts of myocardium, such as the LMCA20.
In the current study, irrespective of QCA results, all patients will be interrogated with iFR (Verrata® and PrimeWire Prestige®; Volcano Corp., San Diego, CA, USA) prior to PCI for exploratory purposes. iFR is measured distal to the target lesion in both the proximal LAD and the LCX (two measurements). For the sake of three-dimensional angiography reconstruction, which will be used for the substudy, wire positions will be recorded in two angiographic views at least 30 degrees apart preceding iFR measurement and followed by iFR pullback.
IMPLANTATION OF THE BiOSS LIM C IN THE LMCA
As mentioned above, the protocol mandates that the distal LM bifurcation is treated with the BiOSS LIM C stent. Whenever additional stenting for the proximal or distal left main lesion is needed, the ALEX PLUS stent will be used.
The recommended BiOSS stent implantation strategies as described in the protocol are derived from the MADS classification (Figure 3),21. However, considering the anatomical variability of the left main bifurcation, the selection of the stenting technique strategy is left to the operator’s discretion.
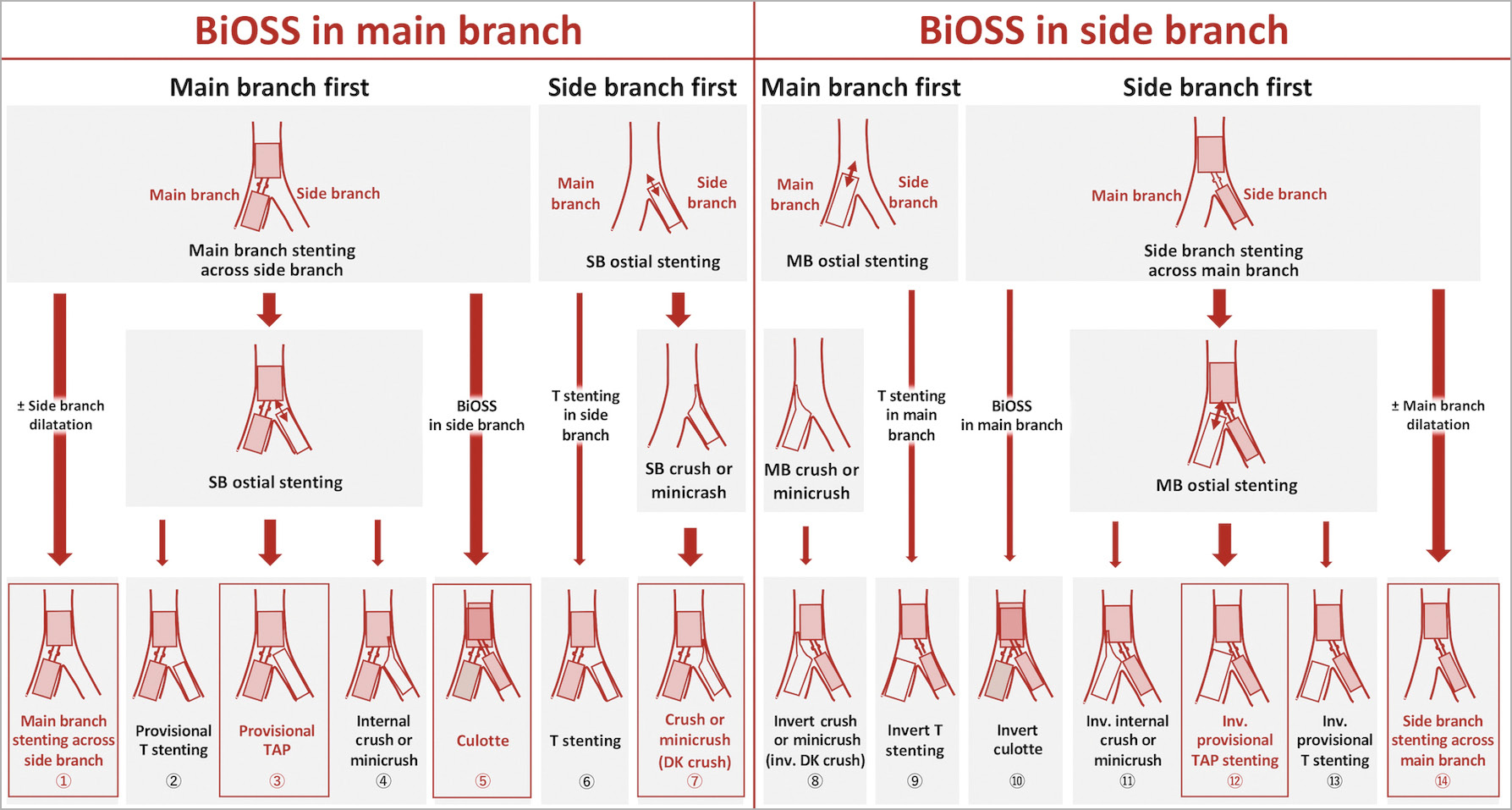
Figure 3. BiOSS stent implantation strategies. The techniques with a red framed box are recommended in the protocol. In case of usage of two BiOSS stents (culotte technique: ⑤, ⑩), the sizes of the MB and SB should be comparable. TAP techniques ③⑫ are not recommended in cases with a bifurcation angle >70° where T stenting ②⑬ is recommended. DK: double kissing; MB: main branch; SB: side branch; TAP: T-stenting and small protrusion
Whenever there is a low possibility of side branch occlusion after BiOSS stent implantation, “main branch stenting across side branch” is recommended. If the ostium of the side branch has a significant residual stenosis after BiOSS implantation (DS >50% by visual estimation, or FFR ≤0.80/iFR ≤0.89 or obvious flow deterioration [TIMI flow <3]), additional side branch dilatation by kissing balloon inflation (KBI) is recommended4,22. In case of residual issues in the ostium of the side branch after KBI, a second BiOSS or ALEX PLUS stent implantation in the side branch is recommended (“provisional T stenting and protrusion [TAP]” and “culotte stenting”). A case example with culotte stenting using two BiOSS stents is presented in Supplementary Figure 2.
The decision to use an upfront two-stent technique rather than a single crossover stent technique should be considered when the side branch is large (>3 mm), with significant disease (DS >50% by dedicated bifurcation QCA/DS >70% by visual angiography with a length >5 mm, or confirmation of a large plaque burden [>60%] on IVUS), or when there are other special anatomic considerations (e.g., heavy calcification)23. In that case, “DK crush stenting” is recommended in combination with BiOSS for the main branch and the ALEX PLUS stent for the side branch24.
Apart from the stenting procedure described above, the interventional procedure, intraprocedural anticoagulation, and dual antiplatelet therapy (DAPT) are according to the current clinical guidelines18.
POST-PROCEDURAL IVUS
In the current study, usage of IVUS for optimisation of the stent implantation is highly recommended, according to the ESC guideline (IIA)18. Performing post-dilation according to the criteria of minimum stent areas (MSA) is recommended based on the criteria adopted in the EXCEL trial4. In the IVUS criteria of the current study, MSA or MLA in the LMCA, LAD and LCX are preferably dilated with MSA/MLA >8.5, 6.0, and 5.5 mm2, respectively (Supplementary Figure 3). POT and balloon dilatation in the distal branch (the so-called distal optimisation technique) are systematically recommended.
STAGED PROCEDURE
A staged procedure is defined as a planned elective second PCI procedure at a separate setting for optimal completion of the PCI. The criteria for staging are left to the operator’s best judgement. Given the complexity of unprotected LMCA patients, it is anticipated that a substantial number of patients may fall into the category of staged procedures. If the patient requires a staged procedure, this is documented at the time of the index procedure. The reasons for staging and the specific lesions planned to be treated in the staged procedure are documented in the electronic case report form (eCRF). Stented segment(s) treated during the index procedure should not be treated by “retouching” again during the staged procedure.
The recommended timing of a planned staged procedure is optimally within four weeks (28 days), and it is strongly recommended that it is completed within 45 days. A staged procedure will not affect the original follow-up schedule.
The residual SYNTAX score is an objective measure of the degree and complexity of residual stenosis after PCI25. In the POLBOS study attempting to achieve a residual SYNTAX score ≤8 post PCI is recommended.
CONCOMITANT MEDICATIONS
Preloading with aspirin 300 to 325 mg is required at least two hours before PCI. Pre-PCI loading of the P2Y12 inhibitors is mandatory, where the selection of either clopidogrel, prasugrel or ticagrelor is left to the discretion of the investigator. After PCI, DAPT consisting of aspirin and one of the P2Y12 inhibitors is mandated for at least one year after PCI; the status of DAPT will be carefully documented in the eCRF. Optimal medical therapy with strict control of LDL cholesterol (target of ≤1.8 mmol/l) by using a statin or a PCSK9 inhibitor is strongly recommended along with optimisation of all medical therapies26. At least one daily dose of atorvastatin 80 mg or rosuvastatin 40 mg should be administered, as performed in the EXCEL trial, before the PCI (within 12 hours), regardless of LDL level and history of prior statin use27. The use of other medications prior to PCI (e.g., beta-blockers, angiotensin-converting enzyme inhibitors) is left to the discretion of the treating physicians, but should be applied as recommended by the ESC guideline18.
CLINICAL FOLLOW-UP
Hospital visits are planned at one month (±7 days) and one year (±30 days). A phone contact is scheduled at six months (±14 days). An assessment of the angina status (Canadian Cardiovascular Society [CCS] grading or Braunwald classification), compliance to protocol-required medications, other cardiovascular drug use and any serious adverse events will be recorded during clinical follow-up visits. The enrolled patients will be followed up until a maximum of three years after the index procedure. Laboratory testing and other tests are described in Supplementary Appendix 2. Data will be entered into a web-based eCRF. Data entry will be monitored according to a pre-specified monitoring plan (CORRIB Core Lab).
ENDPOINTS
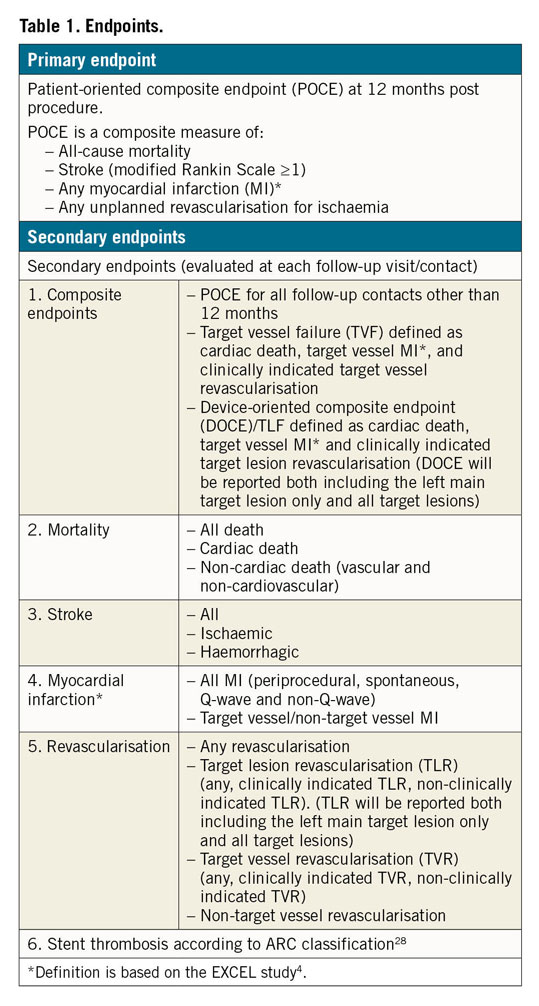
The primary endpoint of the current study is defined as POCE at 12 months post procedure. POCE is a composite of all-cause mortality, stroke (modified Rankin scale [mRS ≥1]), any MI and any unplanned clinically indicated revascularisation including all target and non-target vessels (Table 1),28. To keep consistency with the EXCEL trial, the primary endpoint in the current study applies the same definition of MI as the EXCEL trial. In particular, periprocedural MI is defined as the occurrence within 72 hours after PCI of either CK-MB ≥10x ULN or CK-MB ≥5x ULN in combination with any of the following: 1) new pathological Q-waves in at least two contiguous leads or new persistent non-rate-related left bundle branch block (LBBB), 2) angiographically documented native coronary artery occlusion or new severe stenosis with thrombosis and/or diminished epicardial flow, or 3) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality. Although the definition of the EXCEL trial did not comprise cardiac troponin (cTn), CK-MB ≥5x and ≥10x are considered equivalent to cTn ≥35x and ≥70x, respectively, in the current study29. Stent thrombosis is defined according to the ARC definition28. All definitions of the study endpoint are described in Supplementary Appendix 3. Clinical events will be adjudicated by an independent clinical events committee.
STATISTICAL CONSIDERATIONS AND SAMPLE SIZE CALCULATION
The primary efficacy endpoint is based on comparison to the pre-specified performance goal based on the EXCEL trial. The study is powered at 80% to show non-inferiority of the BiOSS LIM C compared with the XIENCE stent in terms of one-year POCE. The primary analysis will be based on an intention-to-treat patient population. By using the POCE rate of the XIENCE arm with distal LMCA disease in the EXCEL trial (16.7%, as referring to data on file only available to the primary investigators of the EXCEL trial, not available in the public domain), the non-inferiority margin was calculated as 6.3%. A one-sided 95% upper confidence bound will be calculated for the POCE rate at 12 months, using Kaplan-Meier estimates and their standard deviation. In the sample size calculation with PASS software, 256 analysable patients are required based on the assumptions described above. In total, 260 patients will be included from 17 European centres in the current study, accounting for some attrition. Current enrolment status is shown in Supplementary Table 2.
Discussion
Pre-specified subgroup analyses will be performed for the one-stent versus the two-stent technique for unprotected LMCA disease. For these subgroups, the primary endpoint and secondary endpoints will be evaluated. The subgroups will not have significant power, meaning that the results are considered exploratory (hypothesis-generating) only.
Several other substudies are planned, taking advantage of the current study using multimodality assessments (angiography, iFR and IVUS). The impact of anatomical (QCA) and physiological information (iFR) in the LMCA on the stenting procedure and procedure outcome will be assessed. The correlation between quantitative flow ratio (QFR), which is angiography-derived FFR, and iFR in LMCA disease including their pullback index curves will be investigated. Additionally, we will assess QFR for LMCA disease and computed flow dynamics simulation with 3D reconstruction by using angiography and IVUS to investigate the impact of the stenting strategy on the shear stress.
Limitations
The present study has several limitations. First, this is a non-randomised study comparing a dedicated bifurcation stent with a best-in-class DES (XIENCE). Second, the maximum nominal length of the BiOSS LIM C is 24 mm. If the lesion is longer than 24 mm, additional stent (ALEX PLUS) implantation will be required proximally or distally.
Conclusions
The POLBOS LM study will indicate the efficacy of the BiOSS LIM C stent with contemporary PCI for distal left main bifurcation lesions in comparison with the XIENCE stent from the recent EXCEL trial, as a performance index.
|
Impact on daily practice The aim of the POLBOS LM study is to demonstrate the non-inferiority of the BiOSS LIM C bifurcation-dedicated stent to the best-in-class XIENCE stent in the EXCEL trial in patients with unprotected left main bifurcation lesions. A favourable result might provide us with an alternative option for a challenging left main bifurcation treatment in clinical practice. |
Guest Editor
This paper was guest edited by Antonio Colombo, MD; Interventional Cardiology, Centro Cuore Columbus, Milan, Italy.
Funding
This investigator-initiated trial receives unrestricted research grants from Balton Corporation and Philips/Volcano. The grant givers are not involved in the study design, data collection, data interpretation or writing of the manuscript.
Conflict of interest statement
Y. Onuma is a member of the advisory board of Abbott Vascular. J. Legutko is a member of the advisory board of and has received lecture fees from Philips/Volcano and Abbott Vascular and received a lecture fee from Balton. P.W. Serruys is a consultant for Volcano and a member of the advisory board of Abbott Vascular. R. Gil has received a lecture fee from Balton. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

