
Introduction
The wide variation in bifurcation anatomy has generated ongoing research for stents designed specifically for coronary bifurcations; results to date have been unsatisfactory1,2. Earlier, we presented 12 months of pooled data from the POLBOS I and II trials (ClinicalTrials.gov Identifiers: NCT02192840, NCT02198300)3; the present study extends the follow-up to four years.
Methods
OUTCOME MEASURES
The primary endpoint was the cumulative rate of major adverse cardiovascular events (MACE) consisting of cardiac death, myocardial infarction (MI), and target lesion revascularisation (TLR) within 48 months. The secondary endpoints were defined in the primary publication3.
STATISTICAL ANALYSIS
Detailed methods have been described previously3. The significance level was set at 0.05. Statistical analyses were performed using R version 3.0.2 for Mac OS (R Foundation for Statistical Computing, Vienna, Austria).
Results
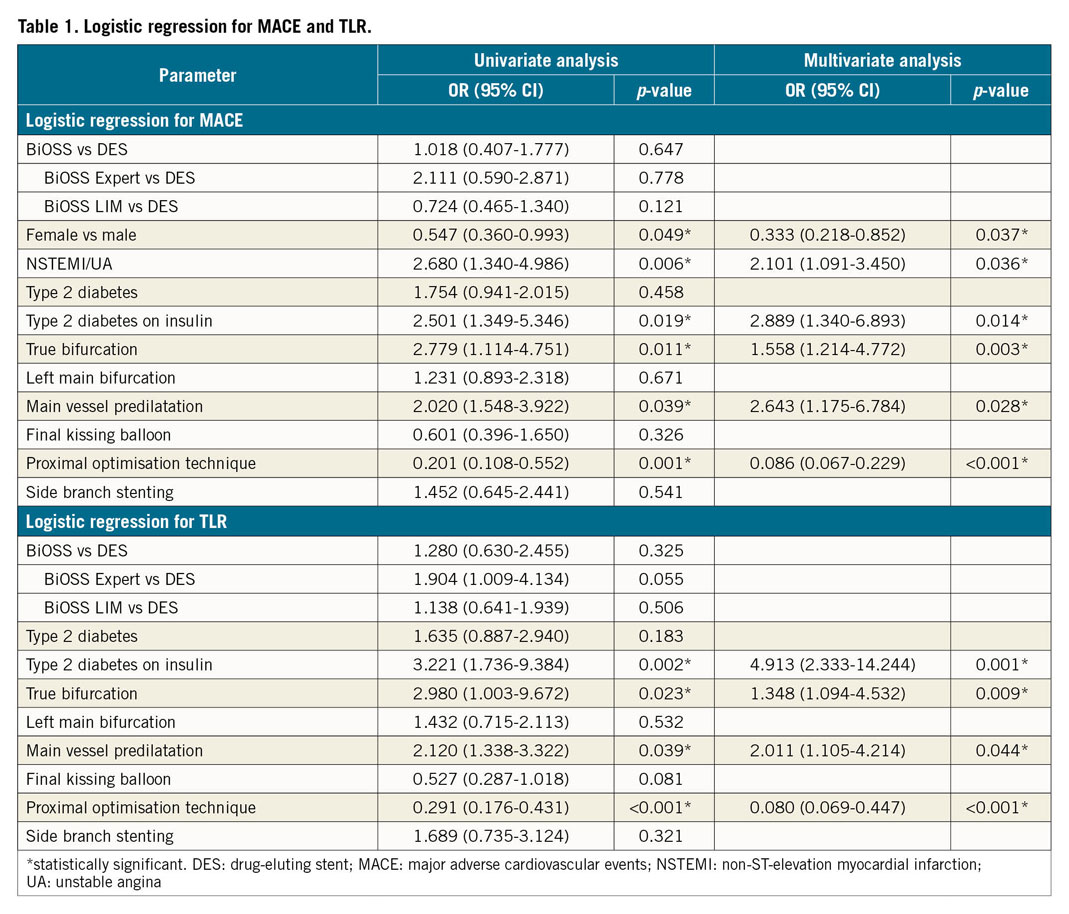
As reported earlier, our population of 445 patients (222 patients in the BiOSS® [Balton, Warsaw, Poland] group and 223 patients in the regular drug-eluting stent [rDES] group) was analysed3. There were no statistical differences between the BiOSS and rDES groups at 12 months in terms of MACE (BiOSS 12.6% vs rDES 13.5%, p=0.43), TLR (10.8% vs 8.1%, p=0.14), MI (1.8% vs 3.1%, p=0.68), or cardiac death (0% vs 2.2%, p=0.79). Similarly, no differences were observed after 2, 3, and 4 years of follow-up. At 48 months, the MACE rate was 19.8% in the BiOSS group and 18.8% in the rDES group (p=0.64), whereas TLR rates were 15.3% and 12.1%, respectively (p=0.34). The Kaplan-Meier curves for MACE and TLR for the BiOSS and rDES subgroups are shown in Figure 1. Further, to determine the prognostic factors for coronary bifurcation treatment, univariate and multivariate regression analyses were performed (Table 1).
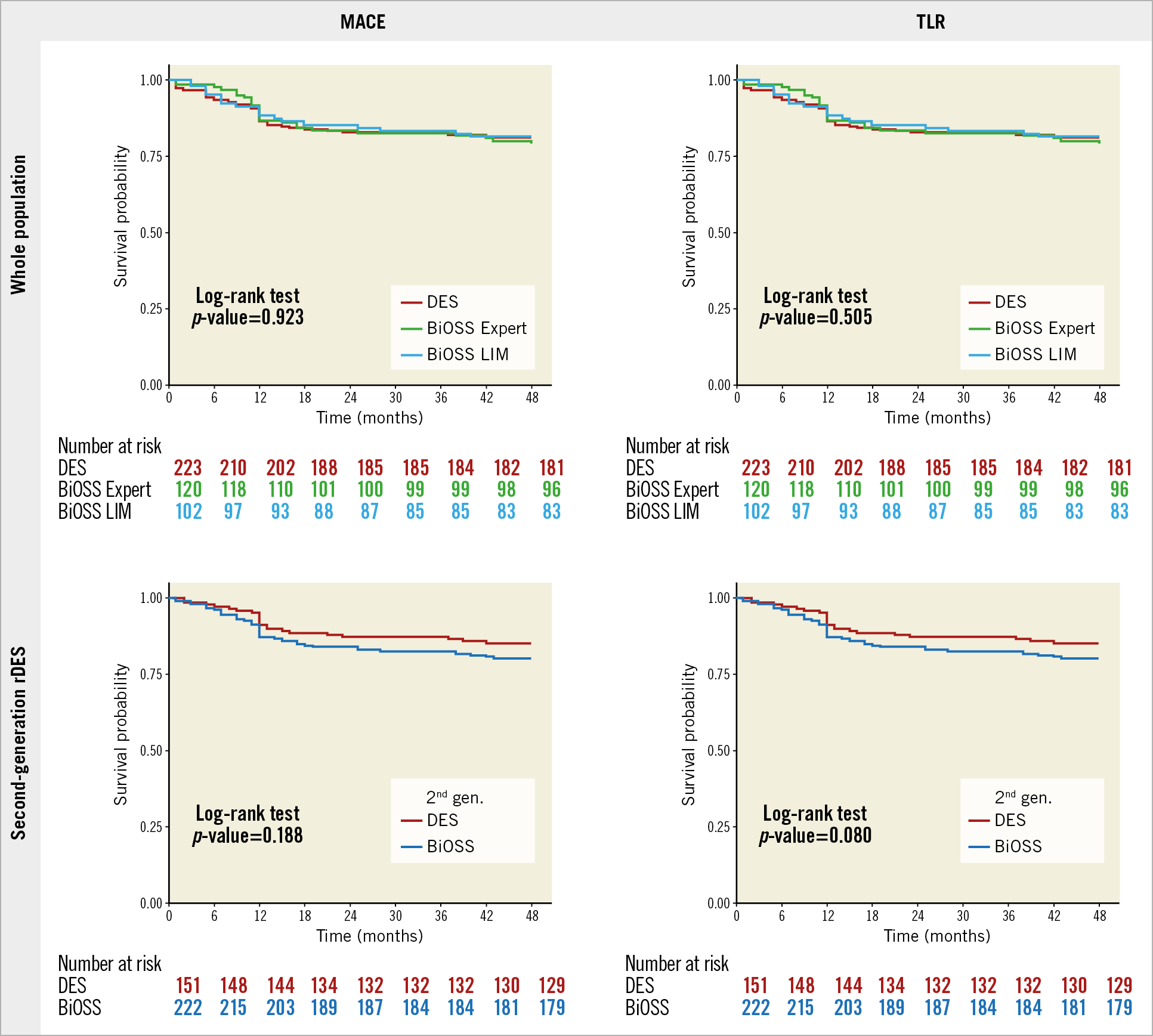
Figure 1. Kaplan-Meier curves showing 48-month outcomes. Kaplan-Meier curves showing event-free survival in the whole population (BiOSS vs rDES) and in the second-generation rDES (BiOSS vs second-generation rDES) for MACE and TLR.
Discussion
The data obtained for the BiOSS and DES groups were comparable with those observed in the literature4,5. Also, the five-year outcomes from the LEADERS trial were as follows: MACE 35.3%, cardiac deaths 8.5%, MI 11.9%, and TLR 14.9%6. In regression analysis, we found that female sex and proximal optimisation technique (POT) decreased the chance of MACE, whereas MV predilatation, non-ST-elevation myocardial infarction (NSTEMI)/unstable angina (UA), true bifurcation, and diabetes treated with insulin increased the chance of MACE. These findings are similar to those of other studies7. For example, in the Milan and New-Tokyo (MITO) Registry, independent predictors of main branch in-stent restenosis (MB-ISR) were calcification (HR 2.284, p=0.016), true bifurcation (HR 2.331, p=0.024), and insulin-dependent diabetes (HR 2.259, p=0.048). Furthermore, POT (HR 0.548, p=0.077), full left main cover approach (HR 0.605, p=0.093), and greater minimal luminal diameter (HR 0.611, p=0.062) showed a tendency to reduce MB-ISR7.
Although there were no statistically significant differences in long-term outcomes between the whole BiOSS group and the whole rDES group, we think that there is still a place for BiOSS stents on cath lab shelves. BiOSS stents ensure easy access to the side branch, no problem with the choice of the proper strut for the SB and enable completing the procedure with provisional T-stenting (PTS) or any double-stent technique. Moreover, we think that BiOSS stents still provide an attractive alternative in coronary bifurcations with a pronounced diameter difference between the proximal and distal parts, minimising the risk of carina and plaque shift.
Limitations
The use of multiple stent types and drugs in the control group was a limitation, although this aspect of the design was intended to replicate real-world clinical practice. Finally, the differences in final kissing balloon and POT strategies between the study groups might also have affected the results.
Conclusions
There were no statistically significant differences in long-term (48 months) outcomes between the whole BiOSS group and the whole rDES group. The regression analysis showed that POT had the largest influence on lowering MACE and TLR rates in both groups.
|
Impact on daily practice Similar results between the BiOSS Expert (stainless steel/paclitaxel; Balton) and BiOSS LIM (stainless steel/sirolimus; Balton) stents suggest that the stent design might play an important role, and therefore the new thin-strut version of the stent (cobalt-chromium platform) has been introduced onto the market – BiOSS LIM C (Balton). |
Conflict of interest statement
R. Gil is a medical consultant for Balton. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

