Abstract
Aims: This study investigated the differences in clinical outcomes between patients with bifurcation lesions (BL) treated with a biolimus-eluting stent (BES) with a biodegradable polymer, and a sirolimus-eluting stent (SES) with a durable polymer.
Methods and results: The clinical outcomes were assessed in the 497 patients (BES 258, SES 239) enrolled in the multicentre, randomised LEADERS trial who underwent treatment of ≥1 BL (total=534 BL). At 12-months follow-up there was no significant difference in the primary endpoint of MACE, a composite of cardiac death, myocardial infarction and clinically indicated target vessel revascularisation (BES 12.8% vs. SES 16.3%, p=0.31). Patients treated with BES had comparable rates of cardiac death (BES 2.7% vs. SES 2.9%, p=1.00), numerically higher rates of myocardial infarction (BES 8.9% vs. SES 5.4%, p=0.17), and significantly lower rates of clinically indicated target vessel revascularisation (4.3% vs. 11.3%, p=0.004) when compared to those treated with SES. The rate of stent thrombosis at 12-months was 4.3% and 3.8% for BES and SES, respectively (p=0.82).
Conclusions: In the treatment of BL the use of BES lead to superior efficacy and comparable safety compared to SES.
Abbreviations
ACS: Acute coronary syndrome
BES: Biolimus-eluting stent
BL: Bifurcation lesion
CABG: Coronary artery bypass graft surgery
DES: Drug-eluting stent
MACE: Major adverse cardiovascular events
MB: Main branch
MI: Myocardial infarction
MLD: Minimum luminal diameter
NSTEMI: Non-ST-elevation myocardial infarction
PCI: Percutaneous coronary intervention
RVD: Reference vessel diameter
SB: Side branch
SES: Sirolimus-eluting stent
ST: Stent thrombosis
TIMI: Thrombolysis in Myocardial Infarction
TLR: Target lesion revascularisation
TVR: Target vessel revascularisation
Introduction
Bifurcation lesions (BL) account for up to one third of coronary lesions and are associated with lower procedural success, and poorer clinical outcomes.1 The previously high rates of target lesion revascularisation (TLR) and major adverse cardiovascular events (MACE) observed after the treatment of BL with the use of bare metal stents1,2 have improved significantly following the introduction of drug eluting stents (DES),3,4 however safety concerns with respect to stent thrombosis (ST) have emerged.5 One of the potential causes of ST is delayed re-endothelialisation which may occur as a consequence of a hypersensitivity reaction induced by the presence of a permanent polymer.6,7 The concerns of ST have been greater with first generation DES with durable polymers, and recent studies have demonstrated numerically lower rates of ST with newer generation DES that have polymers which are more biocompatible,8,9 or completely biodegradable.10
The Biomatrix™ Flex biolimus eluting stent (BES) (Biosensors, Morges, Switzerland) elutes biolimus from a polylactic acid (PLA) biodegradable polymer applied to the stent’s abluminal surface. The polymer is fully metabolised to water and carbon dioxide within 6-9 months, and therefore has the potential to cause less long-term inflammatory sequelae. In the randomised LEADERS (Limus Eluted from A Durable versus ERodable Stent coating) trial, BES was found to be non-inferior to the Cypher® sirolimus eluting stent (SES) (Cordis, NJ, USA) in terms of MACE at nine months follow-up (9% vs. 11%, p for non-inferiority=0.003, p for superiority= 0.39).11
The objective of the present study was to investigate whether there were any differences in clinical outcomes between patients with BL treated with a DES with a biodegradable polymer (BES) compared to a DES with a durable polymer (SES).
Method
Study population
The methods of the LEADERS trial have been published previously.11 The study applied an all-comers approach recruiting 1,707 patients with chronic stable coronary artery disease or acute coronary syndromes (ACS) including ST-elevation myocardial infarction (STEMI), who were eligible for enrolment if they had at ≥1 lesion with diameter stenosis (DS) ≥50% and a reference vessel diameter (RVD) 2.25-3.5 mm. The principle exclusion criteria are described elsewhere.11 The study complied with the Declaration of Helsinki and was approved by all institutional ethics committees. All patients provided written, informed consent for participation in the trial.
In this analysis, patients with ≥1 BL were identified using the electronic clinical record form (eCRF), and results from the core laboratory angiographic analysis which identified and classified all BL according to the SYNTAX bifurcation score.12 The angiograms of 497 patients (258 BES, 239 SES) who had a total of 534 BL (282 BES, 252 SES) identified using either source were reviewed by two investigators (SG and JW), who were blinded to outcomes and stent type. During review of the digital angiogram films, the presence of a BL was confirmed if a lesion of ≥50% DS on visual estimation was present in a main branch (MB) and/or a contiguous side branch (SB) of ≥1.5 mm in diameter. Other information pertinent to the BL recorded during angiographic review was the number of guidewires used; stenting technique; use and site (MB, SB or both) of pre- and post-stenting dilatation; pre- and post-stenting TIMI flow and total number of stents used. Clinical outcomes were compared according to stent type, whilst procedural technique was compared between stents after dividing BL into “true” or “partial” BL. Those BL with a Medina classification13 of 1,1,1; 1,0,1; 0,1,1 (i.e., those with lesions involving both the MB and SB) were defined as “true” BL, whilst those with a Medina classification of 1,0,0; 0,1,0; 1,1,0; 0,0,1 (i.e., those where either the MB or SB was involved) were defined as “partial” BL.
Randomisation and procedures
Patients were randomly allocated on a 1:1 basis to treatment with either a BES or SES, and to active angiographic follow-up at nine months or clinical follow-up only on a 1:3 basis with a factorial design. Percutaneous coronary intervention (PCI) was performed according to standard technique, and direct stenting was allowed. The choice of bifurcation stenting strategy and use of post stenting dilatation was left to the operator’s discretion. No mixture of DES was permitted within a given patient, unless the operator was unable to insert the study stent, in which case crossover to another device of the operator’s choice was possible. Procedural anticoagulation was achieved with unfractionated heparin 5000 IU or 70-100 IU/kg, whilst the use of glycoprotein IIb/IIIa inhibitors was left to the operator’s discretion. Pre-procedure all patients enrolled into the study received ≥75 mg of acetylsalicylic acid, and ≥300 mg of clopidogrel. All patients were discharged on ≥75 mg of acetylsalicylic acid indefinitely, and clopidogrel 75 mg for ≥12 months following the index procedure.
Follow-up
Adverse events were assessed in-hospital, and clinical follow-up was performed at 1, 6, 9, and 12 months. One in four patients was asked to return for angiographic follow-up at nine months.
Study endpoints
The primary endpoint of this sub-study was MACE, defined as the composite of cardiac death, myocardial infarction (MI), and clinically-indicated target vessel revascularisation (TVR) within 12-months. Secondary endpoints were death from any cause, cardiac death, MI, any TLR (both clinically and non-clinically indicated); any TVR, and ST.
A blinded independent clinical events committee adjudicated all endpoints, and independent study monitors verified all case reports from data on-site. The operators were by necessity aware of the assigned study stent during PCI and angiographic follow-up, but patients and staff involved in follow-up assessment were blinded to the allocated stent type. Angiography films were centrally assessed at one angiographic core laboratory (Cardialysis, Rotterdam, The Netherlands) with assessors unaware of the allocated stent.
Definitions
Definitions of all endpoints are provided in full elsewhere.11 MI was defined using the electrocardiographic criteria of the Minnesota code, or by a measured level of creatinine kinase (CK) two times the upper limit of normal (ULN), with either a positive concentration of CK-myoglobin fraction, or troponin I or T. Periprocedural MI was defined as any MI ≤48 hours of the index procedure. Revascularisation was regarded as clinically indicated if on quantitative coronary angiography (QCA) the lumen DS of the treated lesion was ≥50% in the presence of ischaemic signs or symptoms, or ≥70% in the absence of ischaemia. TVR was defined as any repeat PCI or surgical bypass of any segment within the entire major coronary vessel proximal and distal to a target lesion, including upstream and downstream branches and the target lesion itself. TLR was defined as a repeat revascularisation due to a stenosis within the stent or within a 5 mm border proximal or distal to the stent. ST was defined according to the Academic Research Consortium definitions.14
Statistical analysis
Continuous variables are expressed as mean±standard deviation; categorical data is presented as frequency (percentages). Patient demographic data was compared using the Student t-test, whilst χ2 was used for categorical data. Angiographic outcomes were analysed using SAS v8 Proc Mixed for continuous, and Proc Genmod for binominal outcomes, taking into account the within-patient correlation structure of these data. Survival curves were constructed for time-to-event variables using Kaplan-Meier estimates, and compared by the log-rank test. The piecewise Cox proportional hazards model was used to compare clinical outcomes between the groups. All analyses were performed using SAS 8.02 by a dedicated statistician. All p-values and confidence intervals were two-sided; p<0.05 was considered statistically significant.
Results
Baseline characteristics (Tables 1 and 2)
A total of 1,707 patients were enrolled in the LEADERS study of which 29.1% (497 patients, 534 BL) had ≥1 treated BL (Figure 1).
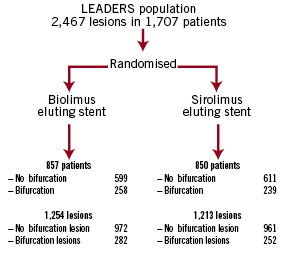
Figure 1. Flow chart indicating the number and type of bifurcation lesions, categorised according to make of stent.
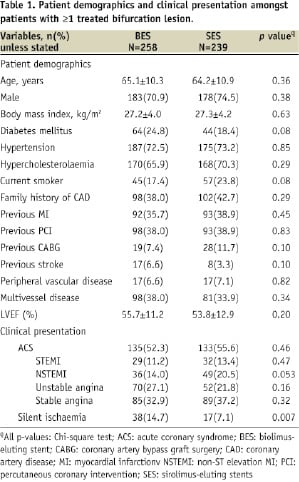
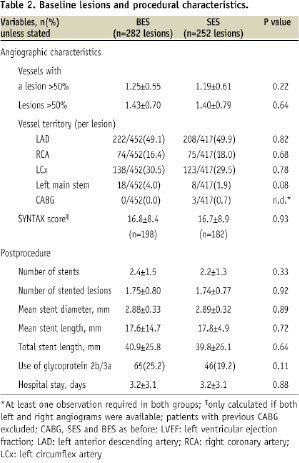
The baseline clinical and lesion characteristics were well matched between those patients with BL treated with BES (258 patients) and SES (239 patients) as indicated in Tables 1 and 2.
Procedural technique (Table 3)
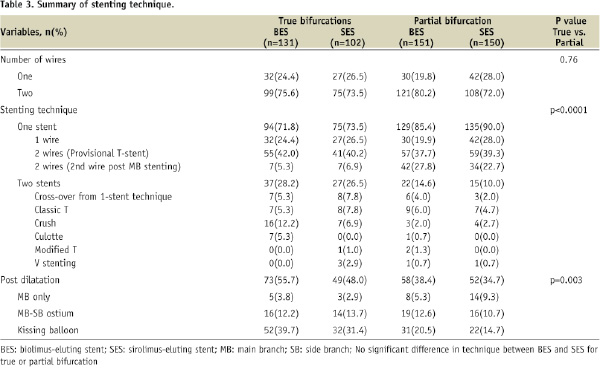
The procedural technique employed to treat the 534 BL is summarised in Table 3. There were no significant differences in technique when comparing BES to SES for patients with a true or a partial BL. Differences in technique did exist however when comparing true BL to partial BL; those patients with a true bifurcation were significantly more likely to be treated with a two-stent strategy (27.5% vs. 12.3%, p<0.0001) and receive post-stenting dilatation (52.4% vs. 36.5%, p=0.0003).
Clinical endpoints (Table 4)
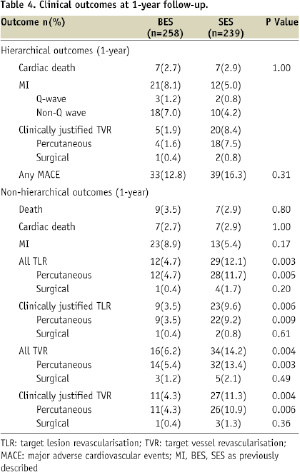
The hierarchical and non-hierarchical clinical outcomes at 1-year follow-up are shown in Table 4, and the Kaplan Meier survival curves are shown in Figure 2.
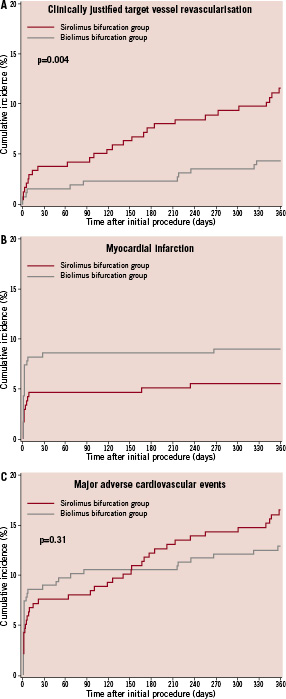
Figure 2. Kaplan Meier survival curves for (A) clinically indicated target vessel revascularisation, (B) myocardial infarction, and (C) MACE.
There was no significant difference in the primary endpoint of MACE between BES and SES at 12-month follow-up (BES 12.8% vs. SES 16.3%, p=0.31). The rate of death was comparable between stents, whilst the rate of clinically-indicated TVR was significantly lower in those treated with BES (11.3% vs. 4.3%, p=0.004). MI occurred more frequently in those treated with BES (8.5% vs. 4.6%, p=0.10), and this was driven by the significantly higher incidence of periprocedural MI (MI 0-2 days: HR 2.53, 95% CI 1.1-6.0, p=0.03; MI 3-360 days: HR 0.64, 95% CI: 0.18-2.27, p=0.49, Figure 2B).
The primary endpoint at 12-months was not influenced by the type of BL treated (true or partial), or the stenting technique used (one or two-stent strategy); however compared to SES, the use of BES was associated with significantly lower rates of percutaneous revascularisation (TLR and TVR) amongst those patients with a true BL, and those treated with one-stent (p<0.05 for all).
Stent thrombosis (Table 5)
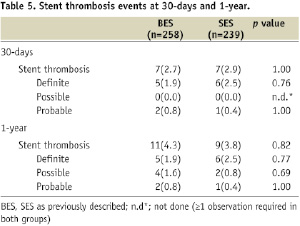
The overall rates of early and late ST were similar between all patients treated with BES or SES, which was irrespective of the type of BL treated (True BL: BES 5.6% vs. SES 4.0%, p=0.76; Partial BL: BES 3.0% vs. SES 3.6%, p=1.00); or the number of stents used (one stent strategy: BES 4.9% vs. SES 3.6%, p=0.62; two stent strategy: BES 1.9% vs. SES 4.8%, p=0.48).
Discussion
This is the first analysis comparing the management of patients with BL using a DES with a biodegradable polymer to a DES with a durable polymer, and demonstrates similar overall clinical outcomes between both patient groups, irrespective of the type of BL treated or the stenting strategy used.
Clinical outcomes
The use of DES have improved outcomes in patients with complex coronary artery disease, with significant reductions in restenosis, however “off-label” use of DES, such as in BL, is still associated with higher rates of restenosis and ST compared to “on-label” use.15,16 Encouraging evidence from this study suggests newer DES, such as BES, may have the potential to improve some of these adverse clinical outcomes. In this study the significantly lower rate of repeat revascularisation in those patients treated with BES was achieved despite any significant differences between stent groups in baseline clinical, angiographic and lesion characteristics, or in procedural technique. This suggests other factors such as differences in stent design, strut thickness, cell size and the drug polymer may have had an influential role on restenosis, as indicated by previous studies comparing different DES in patients with BL treated with the same stenting technique. For example Pan et al reported a significantly lower rate of TLR with Cypher (Cordis, Johnson & Johnson, Warren, NJ, USA) compared to the TAXUS (Boston Scientific, Natick, MA, USA) stent (4% vs. 13%, p<0.05) in 205 patients undergoing provisional T stenting,17 whilst more recently, in patients undergoing culotte stenting, Adriaenssens et al reported restenosis rates of 18%, 29% and 35% with Cypher, Endeavor (Medtronic, Minneapolis, MN, USA) and TAXUS stents, respectively (p=0.12).18 These repeated observations warrant formal assessment in dedicated randomised trials.
In contrast to this reduction in repeat revascularisation, those patients treated with BES had a numerically higher incidence of MI, which was irrespective of the type of BL treated or the stenting strategy employed. Additional analysis indicates that these events were driven by a significantly higher rate of periprocedural MI with BES, which in the vast majority was triggered by the detection of a rise in cardiac enzymes.
Although these periprocedural MIs are a concern, their overall significance is questionable when considering that the rate of death amongst patients who sustained an MI was 0.0% at 30-days. However, setting this, and the on-going discussion regarding the significance of periprocedural MIs aside for a moment,19 there is no disputing that these events did occur, and with a greater frequency in those patients treated with BES. Enzyme rises may be secondary to procedural factors20 however in this study amongst those patients experiencing a periprocedural MI there were no significant differences between stent groups in TIMI flow (MB or SB) either pre- or post-PCI, or plaque shift. Notably however lesion pre-dilatation was significantly higher in the group of patients with periprocedural MIs who were treated with BES (88% vs. 43%, p=0.03).
The physical properties of the stent may also influence enzyme release. For example a smaller cell size can increase the chances of side branch occlusion; however bench studies indicate that the maximum cell circumference of a 3 mm BES is 10.8 mm compared to 9.5 mm in a similarly sized SES. Another physical stent property which merits discussion is the integrity of the polymer coating. Basalus et al recently evaluated the biodegradable coating on BES in vitro using electron microscopy, and observed cracks in the polymer after high pressure balloon inflation, which could potentially lead to the formation of free polymer fragments, capable of embolising and causing subsequent enzyme release.21 These observations however must be interpreted with caution because these assessments were performed in vitro which may have affected the polymer’s stability, and without the use of vascular phantoms which may have stabilised the polymer. In addition, the significant reductions in TLR and TVR with BES are unlikely to have been observed in the presence of polymer fragmentation which ultimately would have reduced the dose of biolimus that could be eluted.
Stent thrombosis
A DES with a biodegradable polymer offers the potential to reduce the risk of late/very-late ST, which is pertinent in patients with BL, as these lesions represent an independent risk factor for ST, and have higher rates of ST when compared with non-BL treated with the same DES (p=not significant).5,22 The cause of this increased risk of ST is likely to be multi-factorial, but stent malapposition, and incomplete stent expansion, particularly in angulated bifurcation lesions, are likely to be two major contributing factors.23 Reassuringly recent studies have dispelled the initial concerns that rates of ST are higher with the use of complex as opposed to simple stenting strategies, or between different complex strategies.24-28 Following on from this, the rates of ST in this study were similar irrespective of stent type (BES vs. SES), type of BL (partial vs. true) or stenting strategy used (one vs. two). Encouragingly provisional results from 2-year follow-up of all patients enrolled in the LEADERS trial does suggest a reduction in very late ST events in patients treated with a stent with a biodegradable polymer;29 however the current study is not powered in isolation to draw any definitive conclusions regarding ST.
Stenting technique for bifurcation lesions
Despite the frequent occurrence of BL, the optimal procedural strategy remains to be established. In the current study a single-stent strategy was preferred for BL, being used to treat over 80% of cases, with a respectable cross over rate from a one to a two stent strategy of 5.3%, and comparable MACE rates of 14.0% and 16.7% for one and two stent strategies, respectively. Historically a two stent strategy was considered the ideal method of dealing with a BL as this produced the best angiographic result, however data from multiple randomised studies3,24,30-33 and three recent meta-analyses indicate that a provisional stenting strategy is as efficacious as a two stent strategy.25,26,34 The current study supports this data, and demonstrates that these results are achievable in an unselected population where ≥50% of patients were treated for ACS.
Limitations
This sub-group analysis is limited by its post-hoc nature. The initial study was not a dedicated bifurcation study, and therefore angiographic analysis of BL was only available using conventional QCA. It is widely recognised that this is limited in its ability to accurately assess a BL, and as a consequence no QCA data is presented here.35 In view of the results obtained a more detailed assessment of BL is warranted using dedicated bifurcation software; however the number of patients with BL returning for follow-up angiography is also a potential limiting factor of the analysis.
Conclusion
In the treatment of BLs, the use of BES lead to superior efficacy and comparable safety compared to SES.

