Abstract
Aims: Dedicated bifurcation stents seem to be the most promising solution for treating bifurcations. The aim of our study was to present the 12 months results of a new dedicated stent for coronary bifurcation lesions –the paclitaxel-eluting stent– BiOSS® Expert (Bifurcation Optimisation Stent System, Balton, Warsaw, Poland).
Methods and results: Sixty-three patients with 65 lesions were enrolled in the registry. Forty-six % of the patients were classified as NSTEMI or unstable angina, 27% were diabetics, 30% had previous myocardial infarction and 48% had a history of previous revascularisation. In addition, hypertension and dyslipidaemia were the most common risk factors (58% and 40%). Sixty-five stents were successfully implanted (100% device success rate). The analysis of 30 days follow-up for 63 patients revealed good clinical results showing lack of death, target lesion revascularisation procedures (TLR) and target vessel revascularisation procedures (TVR). There were six (9,5%) cases of in-hospital raised troponin, however, only one showed an additional increase in CK-MB levels and was qualified as non-Q myocardial infarction (MI). There was a need for percutaneous coronary intervention (PCI) in a non-index vessel in one patient due to exertional angina. The analysis of 12-month follow-up for 63 patients revealed good clinical results. There were two (3.2%) cases of death (three and 10 months after index procedure). The first patient, in good physical shape, drowned, while the second was found dead by his family. There were no incidents of MI or stroke in the rest of the population. At 12 months there were seven (10.8% per lesion; 11.1% per patient) cases of TLR and nine (13.8% per lesion; 14.3% per patient) TVR. There were also 15 (23.8%) cases of PCI on vessels not related to BiOSS® Expert stent implantation.
Conclusions: Our registry showed that bifurcation treatment with a single dedicated paclitaxel-eluting bifurcation stent, BiOSS® Expert is feasible and successful. The long-term clinical results are satisfactory in this high-risk patient population.
Introduction
Coronary bifurcations are predisposed to atherosclerosis lesion formation and progression, due to low shear stress conditions predisposing plaque accumulation1,2. Taking into account the extremely high frequency of vessel branching it is logical that coronary bifurcation stenoses are frequent and relatively often responsible for anginal complaints from patients with coronary artery disease.
Interventions in coronary bifurcation lesions are still challenging for many interventional cardiologists due to the relatively high risk of side branch (SB) closure and long-term restenosis2-5. Experience gained in recent years has led to an increase in percutaneous coronary interventions (PCI) in bifurcation lesions (BL). A strong trend to apply provisional side branch stenting (PTS) strategy is clearly noted. However, with the deployment of classical stents in approximately 30% of these procedures a need for a second stent for the side branch is noted due to significant stenosis at its ostium after main vessel stenting3-5. As a consequence, this approach prolongs the time and complexity of the procedure as well as being associated with high costs and varying patient outcomes.
The concept of the dedicated bifurcation stent (DBS) was proposed as a solution for the problems associated with the treatment of BL lesions when employing classical stents. Currently available DBS may be classified as main vessel (MV) stents ensuring SB access, SB stents implanted before MV stenting, and dedicated for whole bifurcation region stents6-12. However, the majority of the proposed DBS systems are bulkier than conventional stents and require a larger sized guide catheter. Most stents are introduced over two guidewires, which predisposes to both wire crisscrossing and wire biasing (improper device orientation). The systems are more rigid and difficult to advance in the target lesion in case of evident significant proximal tortuosity13.
The aim of our study is to present the initial results of a new dedicated stent for coronary bifurcation lesions the paclitaxel-eluting stent - BiOSS® Expert (Bifurcation Optimization Stent System, Balton, Warsaw, Poland).
Methods
DEVICE DESCRIPTION
The bifurcation optimisation stent system was developed at the end of 2007. After the development of the system, a series of experiments in animal were performed. On the basis of good experimental results, the ethics committee of the Central Hospital of the Internal Affairs and Administration Ministry (Warsaw, Poland) permitted the start of human implantations (N 518 333 435).
The BiOSS® Expert (bifurcation optimisation stent system) is a coronary bifurcation balloon-expandable stent made of 316L stainless steel with a strut thickness of 120 µm and covered with a mixture of biodegradable polymer and an antiproliferative substance – paclitaxel. The profile of the device premounted on the balloon catheter is 1.08 mm (for the 3.75×3.0 mm diameter device). The stent strut/vessel area ratio varies between 15-18%. The smoothness of the surface was achieved by polishing using electromechanical methods. There were two lengths of stents available (15 and 18 mm) at the time of patient enrolment. The proximal part length is 8 mm for both lengths and distal part length is 7 mm for the 15 mm BiOSS stent and 9 mm for the 18 mm long stent. Two sizes were available at the time of the current registry (proximal/distal diameters): 3.75/3.00 mm and 3.25/2.50 mm. The nominal foreshortening of the stent is less than 0.5%.
The stent consists of two parts connected with two connection struts mean length 1.2 mm at the step-up, middle zone. The proximal part of the stent has a larger diameter in relation to the distal part. The stent is crimped on a bottle-shaped semi-compliant balloon (Bottle®; Balton, Warsaw, Poland). After deployment, the contralateral to side branch wall is covered with struts to the same extent as the proximal main vessel part of the bifurcation (14.6%). This is achieved by the “closure” configuration between proximal and distal parts of the stent, following the natural bifurcation angulation between proximal and distal main vessel parts. At the same time, the side-facing side branch at that moment is in “opening” configuration and does not contain struts or in the worst situation there is only one connection strut. These changes result in an adequate configuration of the proximal and distal parts, matching vessel exact sizes. The specific configuration of stent system ensures a “kissing-like” effect. This was discussed earlier in a paper concerning the animal experimentation14.
The Bottle® balloon was available at the beginning of the study at a diameter size of 3.75 mm, proximal, and 3.00 mm, distal, with a single length of 10 mm. The proximal and distal parts have equal lengths of 4.5 mm. The balloon nominal pressure is 10 atmospheres, with rated burst pressure of 18 atmospheres. The balloon is semi-compliant with an increase in diameter size of +0.25 mm at 14 atm, both proximally and distally. The proximal end of a mid-marker is again positioned exactly at the beginning of the narrower distal part for correct positioning.
The BiOSS® Expert has a unique delivery system with three markers (proximal, middle and distal), which assures exact stent placement at the point of the bifurcation. This system is a rapid exchange system compatible with 0.014” guidewires and even 5 Fr (1.63 mm internal diameter) guiding catheters.
The coating process of the stent by biodegradable polymer and paclitaxel uses the same technology as the Luc-Chopin2 stent (Balton, Warsaw, Poland). It enables the use of the most flexible layer of biodegradable polymer as a paclitaxel carrier15. The polymer layers release paclitaxel in a time-controlled process due to their slow biodegradation (lasting around 8 weeks), thus inhibiting a neointimal formation process.
The device itself and principle of the work are demonstrated in Figure 1.
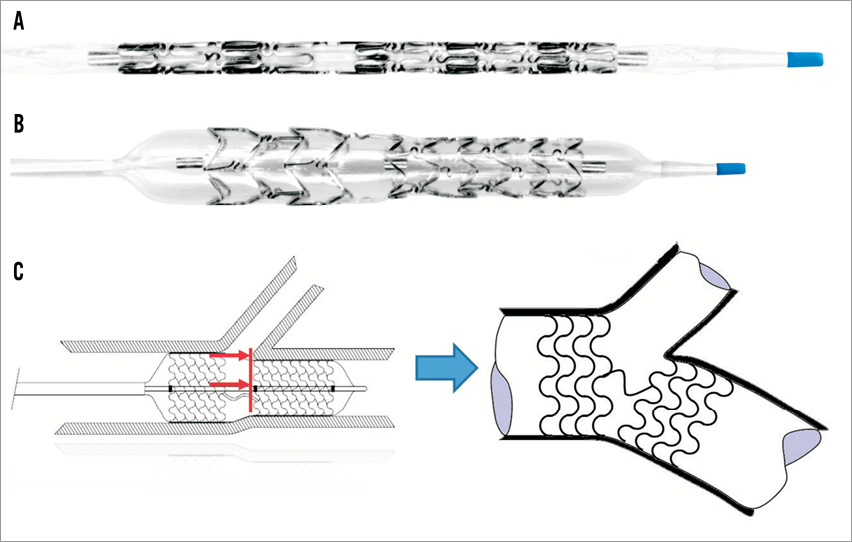
Figure 1. The Bifurcation Optimisation Stent System (BiOSS®). A) Basic view crimped on a balloon dedicated to bifurcation region optimisation (Bottle®); (B) Inflated system – the proximal and distal different diameter parts are clearly defined. The mid-marker position shows the exact placement of the beginning of the distal smaller diameter part. This marker must be positioned precisely against the carina tip; (C) BiOSS principle of work – after balloon deflation the stent conforms according to proximal and distal vessels diameters as well as between vessel angulations, permitting curve fitting according to MV angulation. This leads to good apposition against the SB ostium.
ANGIOGRAPHIC ANALYSIS
Bifurcation lesions were classified according to the Medina classification using an index of 1 for stenosis greater than 50% and 0 for no stenosis.
Quantitative angiographic analysis was performed using commercially available software (Medis QCA version 5.0; Medis, Leiden, The Netherlands). Catheter calibration was used in all cases. The main vessel (MV - the artery before SB delivery), the main branch (MB - artery beyond the ostium of the SB), and the SB (the smaller vessel at the point of vessel divergence) were analysed separately. The following were calculated: reference vessel diameter (RVD) and minimal lumen diameters (MLD) before and after stenting, acute lumen gain at the proximal and distal limb of bifurcation (MLD at proximal or distal limb after stent implantation minus MLD in MV or MB), and the percent diameter stenosis (%DS) in the MV, MB, and SB before and after stent implantation. All reference diameters were measured 5 mm from the end of angiographically visible plaque in all three segments of the bifurcation, without use of interpolations (user-defined reference diameters). Percent diameter stenosis (using parameters from each segment) was measured for each vessel segment separately by using the following formula: %DS= [1 – (MLD/RVD)] ·100.
STUDY POPULATION
To evaluate the safety and feasibility of the paclitaxel-eluting BiOSS Expert stent, patients were enrolled into the multicentre registry (BiOSS® Expert registry) by nine high volume (>1,500 PCI per year) centres located in Poland. The patients were recruited from June 2009 until January 2011. Provisional T-stenting strategy was mandatory.
The classification of bifurcation lesions according to Medina classification was made by visual estimation of lesion severity by the operators during procedure and as such was included in the analysis. The patients were included in the BiOSS® Expert registry if their serum creatinine level was below 2.0 mg/dl and if they were able and willing to take a dual antiplatelet therapy for 12 months. The ST-segment elevation myocardial infarction (STEMI) and lack of an informed consent were additional exclusion criteria. There was no stent implanted that varied from the predetermined protocol of this registry. There was no restriction regarding lesion length in patient selection. In case of necessity, an additional paclitaxel-eluting stent (LUC-Chopin2; Balton, Warsaw, Poland) from the same company was implanted. The distal main vessel diameter was required to be more than 2.5 mm and the proximal main vessel diameter to be less than 4.25 mm. In case of proximal left anterior descending or left circumflex artery disease it was always intended that at least a 7 mm landing zone be available for stent placement.
The primary endpoint was major adverse cardiac events (MACE) including death, myocardial infarction with or without ST-segment elevation or need for revascularisation of the target lesion. The secondary endpoints included device procedural performance and periprocedural safety (periprocedural myonecrosis and complications). All deaths were deemed cardiac unless proved otherwise. All patients had troponin I (TnI) and creatine-phosphokinase MB fraction (CK-MB) levels examined immediately before procedure and 24 hours later. Myocardial infarction was defined as troponin I and/or CK-MB elevation more than three times above the normal limit (0.04 ng/ml for TnI, 24 U/ml for CK-MB).
The angiographic endpoints evaluated at 12-month follow-up included late lumen loss (LLL), percent diameter stenosis (%DS) at follow-up, and binary restenosis rate. The device success was calculated as a ratio of device implanted number to lesion number. The angiographic success was assessed as, at the end of the procedure, MB diameter stenosis of less than 20% and SB ostial stenosis of less than 70%. The value for the SB angiographic endpoint was chosen for two reasons: firstly, based on previous fractional flow reserve data demonstrating that, in the setting of short ostial side branch stenosis post-main vessel stenting, all ostial SB stenoses of less than 70% in diameter (and shorter than 5 mm) are functionally not significant16; secondly, based on our own observations with magnetic resonance delayed gadolinium enhancement before and after bifurcation PCI, which demonstrated a lack of necrosis at the area of the SB of ostial stenosis after stenting, if the SB ostial stenosis after main vessel stenting is less than 73%17. All patients were asked to return for a 9-12 months control angiography.
IMPLANTATION PROTOCOL
The main points of the implantation protocol were as follows:
– wiring of both branches;
– MV predilatation and/or SB predilatation according to operator decision;
– BiOSS implantation – balloon inflation at 10-12 atm for at least 20 sec;
– stent post-dilatation with the Bottle balloon (Balton, Warsaw, Poland) at the operator’s discretion;
– SB post-dilatation if SB ostial had a %DS >70%;
– final kissing balloon inflation (KBI) was not required and left at the operation’s discretion;
– intravascular ultrasound examination (IVUS) was mandatory for all LM cases.
Dual antiplatelet therapy (aspirin 75-300 mg plus clopidogrel 75 mg) was prescribed for 12 months. The patients were clinically evaluated (history, physical examination, electrocardiogram [ECG]) at 30 days, 6, 9 and 12 months. Angiographic control was planned at 12 months.
STATISTICS
All data are presented as mean±standard deviation. The differences between groups were examined with paired or unpaired t-tests, as appropriate, with normal distributions. If distribution was not normal, Wilcoxon signed-rank tests and Mann-Whitney U-tests were performed. Analysis of variance (ANOVA) was used for multiple comparison data, when parameters were distributed normally. Otherwise a Kruskall-Wallis test was performed.
Results
A total of 63 patients with 65 lesions were enrolled and 65 BiOSS® Expert (Balton, Warsaw, Poland) stents were successfully implanted at the sites of bifurcation lesions (100% device success rate). Patient characteristics represented a very diseased population, not always seen in this type of registry, with hypertension and dyslipidaemia as the most common risk factors 56% and 38%, respectively. Almost half (46%) of the patients were classified as having NSTEMI or unstable angina, 27% were diabetics, 30% had previous myocardial infarction and 48% had a history of previous revascularisation (Table 1).
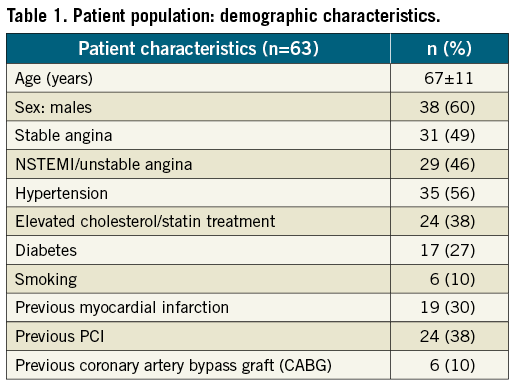
Twenty-six percent of patients (n=16) had triple vessel disease, 58% (n=37) had double vessel and only 16% (n=10) had single vessel disease (Figure 2). In thirteen patients, unprotected left main (LM) interventions were performed. The left anterior descending artery was the dominantly affected vessel in 35% of cases (Figure 3).
Figure 2. Number of diseased vessels. 1 VD: one-vessel disease; 2 VD: two-vessel disease; 3 VD: three-vessel disease; LM: left main significant stenosis
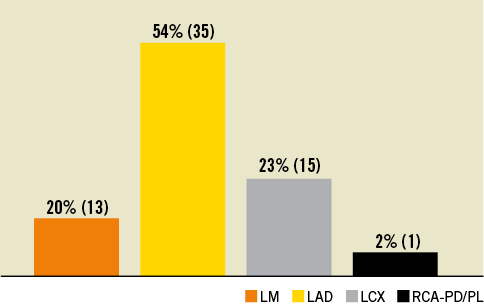
Figure 3. Target lesion location. LM: left main significant stenosis; LAD: left anterior descending artery; LCX: left circumflex artery; RCA: right coronary artery, PD: posterior descending, PL: posterolateral branch. In one patient two LAD bifurcations were treated and counted as one affected territory.
With regard to Medina classification almost half (48%) of patients represented lesions with morphology type 1,1,1 (see Figure 4 for detailed distribution of different types of bifurcation lesions [Medina type X,X,1]). The percentage of true bifurcation lesions was 70%.
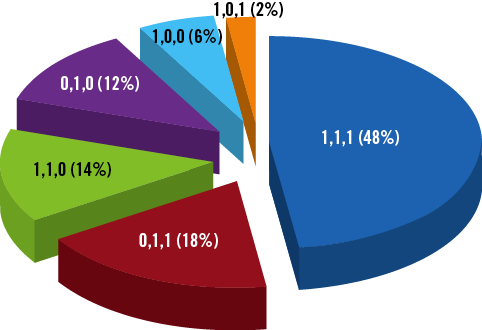
Figure 4. Medina classification bifurcation lesion distribution.
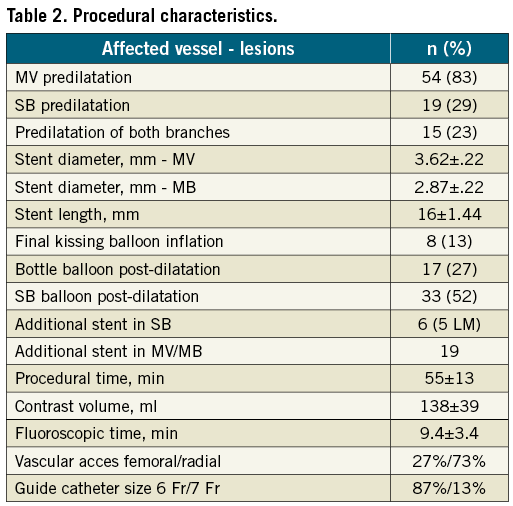
The main procedural aspects are presented in the Table 2. The device success rate was 100%. There was no need for additional BiOSS® Expert stent implantation, because of impossibility to implant the device. The MB was pre-dilated in the majority of cases (87%). The final kissing balloon inflation rate was rare (13%), reflecting a good result in the SB after stenting. However, the SB required additional balloon dilatation in more than a half of lesions (53%). We found that to achieve an optimal result in the SB ostium as well as stent expansion, in 27% of cases an additional Bottle® balloon was necessary. The Bottle® balloon length was 10 mm; proximal/distal diameters 3.75/3.0 mm for all cases.
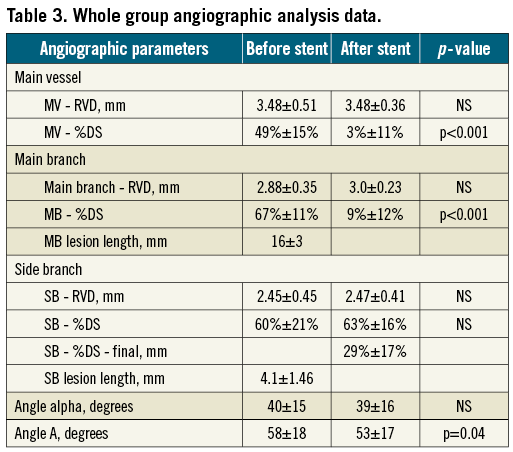
The angiographic data are presented in Table 3, Table 4 and Table 5. It is important to underline that the mean pre-procedural MV, MB and SB stenosis were all well above 50% diameter stenosis. Neither in the left main nor in the non-left main group was there a significant worsening of SB ostial stenosis after stent implantation in the main vessel. The final SB ostial diameter stenosis was at the 30% range, which we assess as a good result, taking into account a very low rate of additional SB stent implantation (16%).
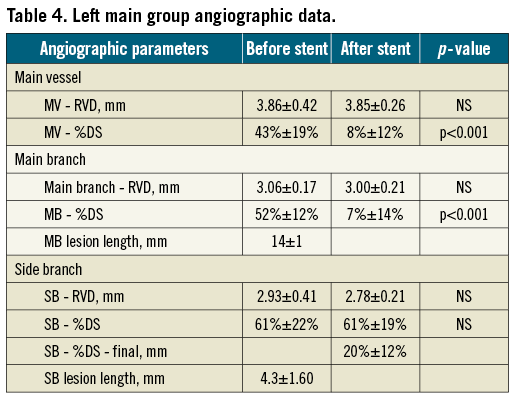
The angiographic success rate was 97%, because in two SB, it was not possible to achieve final percentage diameter stenosis less than 70% (in both cases due to ostial dissection with the wire during SB re-crossing). In both cases, even though balloon dilatation at the side branch ostium was performed, there was persistence of ostial dissection, however without compromising branch flow, both SB were not stented. When it was attempted, there was no case of a wire or balloon unable to re-cross. It is remarkable that in our group with 70% true bifurcation lesions and around 50% SB post-dilatation after BiOSS® implantation, more than 75% of the lesions had a final diameter stenosis of less than 50%.
30-DAY CLINICAL RESULTS
The analysis of 30-day follow-up for 63 patients (65 lesions) revealed good clinical results showing no death, target lesion revascularisation procedures (TLR) and target vessel revascularisation procedures (TVR) (Table 6). There were six (9.5%) cases of in-hospital increase of troponin; however, only in one was an additional increase of CK-MB levels found and qualified as a non-Q-wave MI. There was a need of PCI in a non-index vessel in one patient due to exertional angina.
CUMULATIVE 12-MONTH CLINICAL RESULTS
The analysis at 12-month follow-up for 63 patients revealed good clinical results (Table 6). There were two (3.2%) cases of death (three and 10 months after index procedure). The first patient (55 yrs) drowned while on holiday and in good physical shape (negative stress test before incident). This could be classified as possible stent thrombosis according to the Academic Research Consortium (ARC) definitions, despite lack of any data18. The second patient (82 yrs) was found dead by his family after three days with no contact. There were no incidents of myocardial infarction or stroke incidents in the rest of the population. At 12 months there were seven (10.8% per lesion; 11.1% per patient) cases of target lesion and nine (13.8% per lesion; 14.3% per patient) target vessel revascularisations. There were also 15 (23.8%) cases of PCI on vessels not related to the BiOSS® Expert stent implantation.
ANGIOGRAPHIC FOLLOW-UP
From the 63 initially included patients (65 lesions), 61 patients were followed by personal contact or by telephone for a mean period of 12±4 months (97% follow-up rate). Forty-eight (51 lesions) patients (76%) agreed to have control angiography at 9-12 months. Seven (13.7%) had significant main vessel-main branch in-stent restenosis. The restenosis in side branches was more frequent – 15 (29% from 51 bifurcation lesions in angiographically-followed patients). In five cases (9.8%) additional intervention was needed on the SB because the operator estimated that the patient had symptom-related ostial restenosis. Figure 5, Figure 6 and Figure 7 present changes in minimal lumen diameters, percentage diameter stenosis and late losses in the three bifurcation segments during follow-up. It is an interesting observation that there was a net lumen gain at the side branch ostium in non left main bifurcation lesions during follow-up.
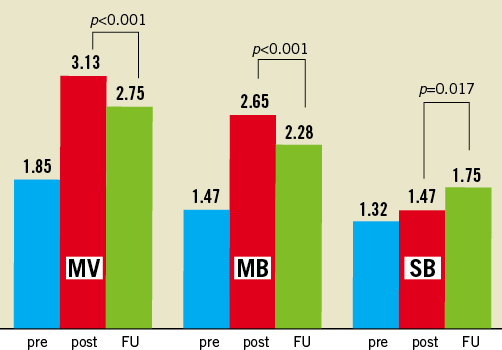
Figure 5. Changes in MLD during follow-up.
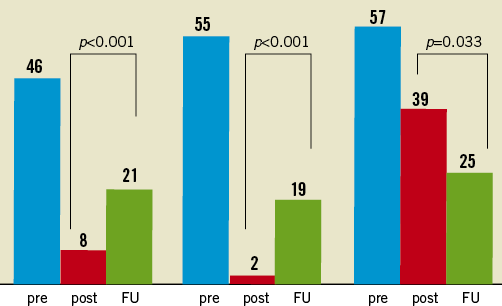
Figure 6. Changes in %DS during follow-up.
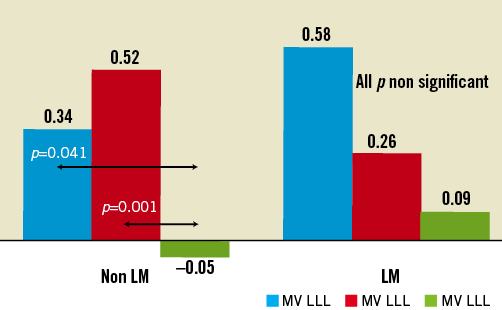
Figure 7. Late loss in LM and non-LM groups during follow-up.
Discussion
The BiOSS® Expert stent (Balton, Warsaw, Poland) should be classified as a dedicated bifurcation stent (DBS) for treating the main vessel (MV) and ensuring side branch (SB) access. Our experience showed that this device is user friendly, especially for a single operator. The vast majority of implantations was possible using radial access (more than 90%) and 6 Fr compatible equipment (also including LM cases). Moreover, such a stent ensured ideal immediate efficacy (100% device success rate). It is worth stressing the fact that it is easy to rewire the SB after BiOSS® Expert implantation. There were only two cases with some difficulties due to a SB ostium dissection. In one patient with NSTEMI at presentation, a total SB closure occurred after balloon predilatation and was complicated with non-Q-wave myocardial infarction, despite the fact that the vessel was additionally dilated, opened and stented before BiOSS implantation. This device success rate is clearly superior to all reported in the literature about procedural success rates of other dedicated coronary bifurcation stents (100% for BiOSS Expert vs. 75-95% for other types of devices6-12,19-24). This high success rate is the result of avoidance of the problems that frequently occur with other dedicated devices –guidewires that criss-cross, improper device orientation and larger device profiles.
The quantitative coronary analysis performed in the BiOSS® Expert registry population revealed that BiOSS® implantation in the main vessel caused a significant increase of MLD and decrease of %DS in MV and MB. Interestingly, such a procedure did not affect proximal main vessel-side branch angle alpha –a good predictor of immediate and late patient outcome and initial SB diameter stenosis25,26. The last parameter was improved significantly after additional balloon dilatations.
It is also noted that according to our criteria an additional stent in the SB was necessary in only six patients (five of them had the procedure in the LMS), which confirms that the BiOSS® stent applies well to PTS strategy. This good angiographic result was achieved with a low rate of KBI (only in 13% cases), which is sometimes very demanding, especially for operators with little experience. This suggests, that the bottle-like shape of the stent delivery balloon and the balloon used for post-dilatation (Bottle®; Balton, Warsaw, Poland) is associated with a “kissing-like” result.
Angiographic restenosis in the MV/MB was found in seven patients. In side branches, angiographic restenosis was found in 10 lesions (15%), but it required re-intervention only in one case, together with intervention in the main vessel. From these patients, only one was inside the stent while all remaining cases were located at stent margins. It is quite possible that during the index procedure some geographical miss occurred and this resulted in restenosis. This then led to the development of an additional length (23 mm) of the currently available system. We realise that the length of the stent system is probably the only significant limitation of the device during the registry period. In three patients, apart from restenosis, a significant lesion progression occurred in another vessel territory and therefore they were scheduled for CABG. In two patients, focal restenosis was treated with plain old balloon angioplasty (POBA) and in the last two patients, due to SB restenosis, an implantation of DES was necessary.
The analysis of 12-month angiograms revealed a rather small (p<0.01) reduction of MLD for MV and MB (12.1% and 14%, respectively) and a significant increase of MLD for SB (19%). In association with this process, the %DS increased both in MV and MB while it decreased in the SB. The late lumen loss was significantly different for the MV and MB (0.46 mm and 0.39 mm, respectively) while it was very low (<0.1 mm) for the SB. It is not easy to explain that phenomenon; however, it seems to be rational to believe that optimised flow conditions are responsible, due to BiOSS® stent design, which has a lesser shear stress effect than that of a conventional stent.
The clinical results obtained in the BiOSS® Expert registry are satisfactory, in particular because the enrolled population was unselected but rather gathered in nine Polish centres. The two incidences of death in the population of the BiOSS® Expert registry should be discussed. It is important to stress that the first one occurred in a relatively young patient who appeared to be in very good shape, while the second occurred in a very mature patient with a low LV ejection fraction. In the first patient, the index lesion was located in LAD/diagonal complex, while in the second it was located in the LMS. According to published data, total biodegradation of the carrier and paclitaxel mixture occurs in eight weeks15. Therefore, it seems unlikely that in the young patient late stent thrombosis might have occurred. It is rather more likely that he overestimated his strength and simply drowned while swimming in the sea. In the second patient restenosis in the index stent was possible, moreover because there was atherosclerosis progression.
The relatively high rate (6/9; 9%) of periprocedural infarctions found in our population is strictly associated with the universal myocardial infarction definition that we took (troponin elevation more than three times the norm27). However, it should be stressed that only in one patient, a significant increase of both necrotic markers (troponin and creatine phosphokinase [CPK] MB) occurred and non-Q-wave MI was diagnosed. Of the five remaining patients, only in one did the troponin level increase more than five times above the norm, which may probably have impacted on the late patient`s outcome according to the literature. It is important to mention that the operators of those two patients had a problem with SB rewiring due to ostial dissection. Our definition of myocardial infarction was much more stringent in comparison with other studies, where only CK-MB or CK were used6-12,19-24.
The fact that in 19 patients it was necessary to implant an additional stent in the MV or MB during the index procedure indirectly proves that the population of the BiOSS Expert registry had a high level of atherosclerosis progression. The necessity of additional PCI (24%) in the vessels is not related to index procedure and inflated additionally the MACE events rate. For the moment, in no other study has there been such a high number of multivessel diseases and LM patients (84%). This patient cohort has inherited a much more aggressive disease course, with a generally more unfavourable overall outcome, no matter what type of treatment is used. This differs greatly from all other studies with dedicated coronary bifurcation devices, where more than a half of the patients have single vessel disease with a low rate of general morbidity6-12,19-24.
Conclusions
In conclusion, we can say that the simple and fast bifurcation treatment with a single, dedicated paclitaxel-eluting bifurcation stent is feasible and successful (100% implantation rate). The BiOSS® stent implanted in the main vessel has almost no affect on the side branch (minimal SB compromise). The long-term clinical results are satisfactory, however, and are closely related to the studied high-risk profile of the patient population.
Funding
This study was financed in the greater part by an official research grant sponsored by the Polish Ministry of Science and Higher Education (N 518 333 435). In addition the study was partially financially supported by Balton Company, Warsaw, Poland.
Conflict of interest statement
R.J. Gil is the medical consultant of Balton Company. The other authors have no conflicts of interest to declare.

