Device description
Name and manufacturer: There are two versions of the BiOSS® stent: paclitaxel-eluting BiOSS Expert® and sirolimus-eluting BiOSS LIM® (Balton, Warsaw, Poland).
Approval status: BiOSS Expert received CE mark in 2008, and BiOSS LIM in 2011.
Platform: 316L stainless steel.
Strut thickness: 120 µm.
Coating: A mixture of biodegradable poly(lactide-co-glycolide) copolymer and an antiproliferative drug (polymer and drug layer thickness: 5 µm). The biodegradation process lasts around eight weeks. BiOSS Expert elutes paclitaxel (1 µg/mm2) and BiOSS LIM sirolimus (1.4 µg/mm2).
Specific stent design: The stent consists of two parts, proximal and distal, joined with two connecting struts (depending on size stent: 0.9-1.5 mm in length) at the middle zone. The proximal part of the stent has a larger diameter - the proximal/distal diameter ratio is 1.15-1.3 (Figure 1A).
Guiding: 5 Fr.
Classification according to MADS: Type “A” (provisional T-stenting [PTS]).
Delivery system: The stent is balloon-expandable and mounted on a stepped delivery Bottle® balloon (Balton). There are three radiopaque markers to guide positioning (RX delivery system) (Figure 1B).
Stent sizes: Proximal diameter: 3.25-4.5 mm; distal diameter: 2.5-3.75 mm.
Stent length: 15, 18 and 23 mm.
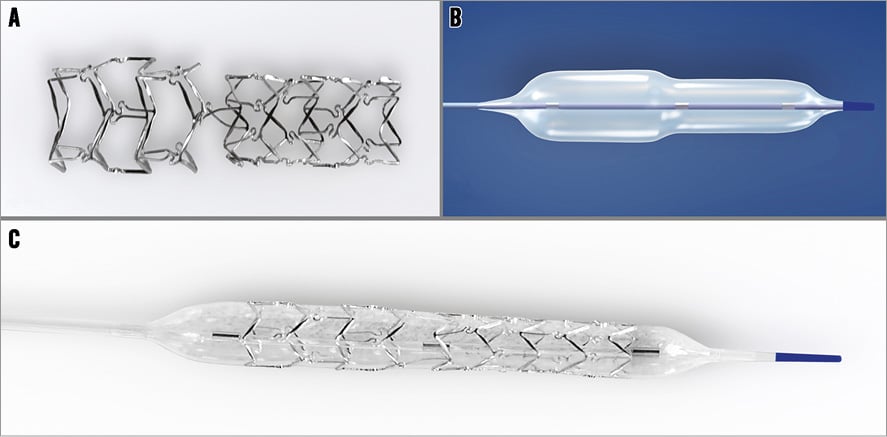
Figure 1. BiOSS stent structure. A) The structure of the BiOSS stent. B) Bottle balloon. C) The BiOSS stent crimped on the Bottle balloon.
Procedural details
First, both the main branch and the side branch are wired, thereafter predilations can be performed according to the operator’s preference. The BiOSS stent’s delivery balloon has three markers: distal and proximal indicating stent edges, and one mid marker showing the mid zone (Figure 1C). The mid marker should be placed exactly at the tip of the carina (Online Figure 1A, Online Figure 1B, Moving image 1). This ensures that after deployment (Online Figure 1C, Online Figure 1D) the contralateral to side branch wall is covered with struts to the same extent as the proximal main vessel part of the bifurcation. This is achieved by the self-centring properties of the device (due to the special shape of the connecting struts) and the “closure” configuration between the proximal and distal parts of the stent (Online Figure 1E, Moving image 2). The BiOSS design reduces the risk of carina shift as well as side branch ostium compromise1. Additionally, the Bottle balloon shape ensures the proximal optimisation technique (POT)-like effect at once after BiOSS implantation2; however, post-dilation of its proximal part (real POT) seems to improve clinical results3.
Clinical data
BiOSS EXPERT
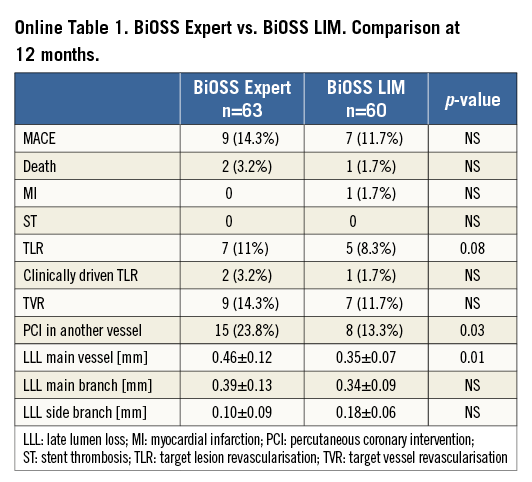
The first-in-man (FIM) study enrolled 63 patients in nine Polish centres. The device success rate was 100%. The side branch was treated with an additional regular DES (rDES) implantation in 9.2% of cases. After 12 months, the rate of major cardiovascular events (MACE) was 14.3% (Online Table 1). There were seven (11.1%) cases of target lesion revascularisation (TLR) and nine (14.3%) cases of target vessel revascularisation (TVR)4.
In the IVUS study, although there was a smaller minimal lumen area in the middle zone in the BiOSS group (compared to the reference segment in the rDES), there was a better preserved inflow into the side branch. Mean window length (the largest diameter at the level of the ostium of the side branch) was similar at baseline between the BiOSS and rDES groups, but in the BiOSS group it was significantly longer post procedure (2.21±0.37 vs. 1.76±0.52 mm, p=0.01). Moreover, analysis of the restenosis pattern revealed that the middle zone of the BiOSS stent was not a “weak point” prone to restenosis1,5.
Also, favourable results were obtained in a study on 12-month clinical and angiographic follow-up of 54 patients with distal LM disease. The BiOSS Expert stent proved to be a feasible device, with promising safety and long-term clinical effectiveness5,6.
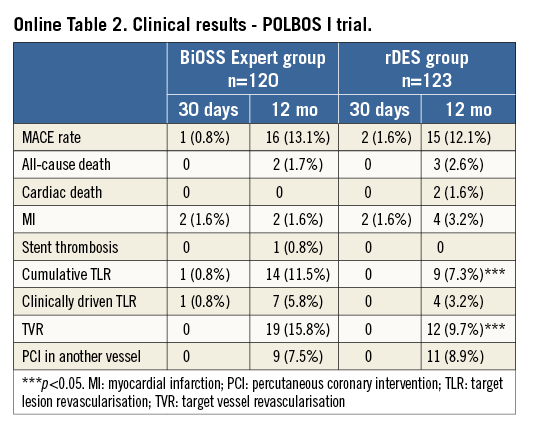
Based on these results, the BiOSS Expert stent was explored in the randomised POLBOS I (POLish Bifurcation Optimal Stenting) trial. Patients with stable coronary artery disease or non-ST-elevation acute coronary syndrome were assigned 1:1 to one of two treatment strategies: BiOSS Expert stent (n=120) or rDES implantation (n=123). The primary endpoint was the composite of cardiac death, MI and TLR at 12 months. There were three stent implantation failures (1.7% in rDES, 0.8% in BiOSS group). Side branch treatment with rDES was required in 10% of cases in both groups. At 12 months, cumulative MACE incidence was similar in both groups: 13.3% vs. 12.2% (p=0.7) (Online Table 2). The TLR rate was significantly higher in the BiOSS group compared to the rDES group, 11.5% vs. 7.3% (p=0.02). However, when considering only paclitaxel-eluting stents in the rDES group, TLR was 10.6%. Moreover, the TLR rate was significantly lower in the BiOSS group compared to the rDES group in the treatment of distal LM stenosis (TLR 7.4% vs. 11.1%, p=0.04)3.
BiOSS LIM
The FIM trial enrolled 60 patients in Bulgaria, Poland and Spain. The device success rate was 100%. The side branch was treated with an additional rDES in 23.3% of cases. Overall TLR was 8.3% (clinically driven TLR 1.7%). Clinical results obtained with the BiOSS LIM seem to be a little bit better compared to the BiOSS Expert stent (cumulative MACE rate 11.7% vs. 13.7%, respectively) (Online Table 1)7.
Ongoing studies
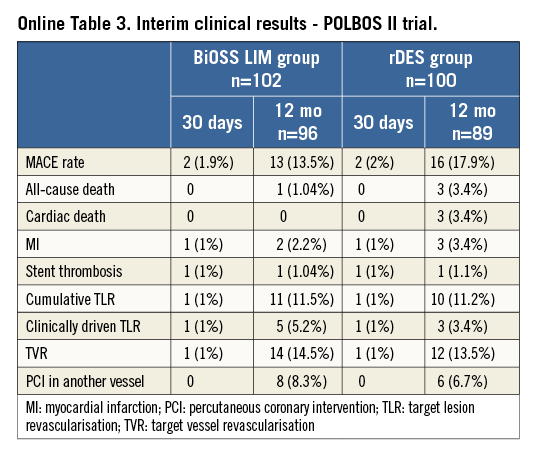
In the POLBOS II study patients were randomised to either the BiOSS LIM group (n=102) or to the rDES group (n=100). Except for one (1%) case in the rDES group and one (0.9%) in the BiOSS group all stents were implanted successfully without any serious periprocedural complications. The rDES in the side branch was implanted in the BiOSS group and the rDES group in 8.8% and 7% of cases, respectively (true bifurcations were present in 65% of patients).
Preliminary 12-month follow-up data (92% completed) show a cumulative MACE rate in the BiOSS group of 13.5% and 17.9% in the rDES group. Detailed results are presented in Online Table 3.
Furthermore, a registry assessing the performance of the BiOSS LIM in distal left main stem stenosis is currently ongoing.
Unique features
– Its structure mimics bifurcation geometry and prevents carina displacement
– It ensures final kissing balloon and proximal optimisation technique-like effects
– It ensures easy access to the side branch
– It fits well to PTS strategy
Potential improvements
– Changing the stent platform to cobalt-chromium to decrease the strut thickness
– Changing the drug to everolimus or zotarolimus
Conflict of interest statement
R. Gil was the medical consultant of Balton. The other authors have no conflicts of interest to declare.
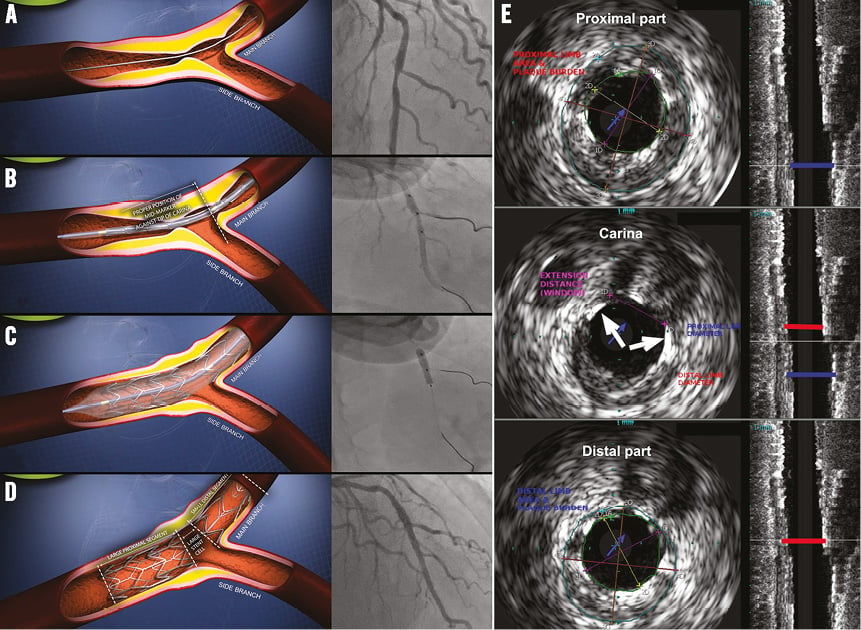
Online Figure 1. BiOSS stent implantation steps. A) Bifurcation lesion in LAD pre-procedure, B) BiOSS LIM stent positioning, C) BiOSS LIM stent deployment, D) final view after BiOSS LIM stent implantation, E) IVUS image showing only two connecting struts at the level of carina (white arrows).
Online data supplement
Moving image 1. BiOSS LIM - the mechanism of action.
Moving image 2. BiOSS LIM implantation in the left main - left circumflex artery.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. BiOSS LIM - the mechanism of action.
Moving image 2. BiOSS LIM implantation in the left main - left circumflex artery.