Abstract
Aims: The aim of the POLBOS II randomised trial was to compare any regular drug-eluting stents (rDES) with the dedicated bifurcation sirolimus-eluting stent BiOSS LIM for the treatment of coronary bifurcation lesions. The secondary aim was to study the effect of final kissing balloon inflation (FKBI) on clinical outcomes.
Methods and results: Between December 2012 and December 2013, 202 patients with stable coronary artery disease or non-ST-segment elevation acute coronary syndrome were randomly assigned 1:1 to treatment of the coronary bifurcation lesions either with the BiOSS LIM stent (n=102) or with an rDES (n=100). Coronary re-angiography was performed at 12 months. The primary endpoint was the composite of cardiac death, myocardial infarction (MI), and target lesion revascularisation (TLR) at 12 months. The target vessel was located in the left main in one third of the cases (35.3% in BiOSS and 38% in rDES). Side branch treatment was required in 8.8% (rDES) and 7% (BiOSS). At 12 months, the cumulative MACE incidence was similar in both groups (11.8% [BiOSS] vs. 15% [rDES, p=0.08]), as was the TLR rate (9.8% vs. 9% [p=0.8]). The binary restenosis rates were significantly lower in the FKBI subgroup of the BiOSS group (5.9% vs. 11.8%, p<0.05).
Conclusions: MACE rates as well as TLR rates were comparable between the BiOSS LIM and rDES. At 12 months, cumulative MACE incidence was similar in both groups (11.8% vs. 15%), as was the TLR rate (9.8% vs. 9%). Significantly lower rates of restenosis were observed in the FKBI subgroup of the BiOSS group.
Introduction
Drug-eluting stents (DES) started being used in coronary bifurcations in the early 2000s and clearly improved midterm angiographic and clinical outcomes compared with bare metal stents1. However, bifurcation lesions still pose a therapeutic challenge during percutaneous coronary interventions (PCI). Therefore, the use of dedicated bifurcation stents (DBS) is one of the proposed potential solutions to improve short-term as well as long-term outcomes. However, up to now only two randomised trials comparing DES with DBS have been published, one with the BiOSS Expert® stent (Balton, Warsaw, Poland), and one with the Tryton Side Branch Stent (Tryton Medical, Durham, NC, USA)2,3.
The aim of the POLBOS II (POLish Bifurcation Optimal Stenting) trial was to compare the use of any regular drug-eluting stent (rDES) with a dedicated bifurcation sirolimus-eluting stent BiOSS LIM® (Balton) for the treatment of coronary bifurcation lesions. The secondary aim was to study the effect of final kissing balloon inflation (FKBI) on clinical outcomes.
Methods
STUDY POPULATION AND STUDY DESIGN
The POLBOS II trial was a randomised, open-label, controlled study conducted between December 2012 and December 2013 in four centres in Poland and in one centre in Spain. The inclusion criteria were: stable coronary artery disease (CAD) or non-ST-segment elevation acute coronary syndrome (NSTE-ACS), age ≥18 years, de novo coronary bifurcation lesion (including unprotected left main stem), main vessel (MV) diameter ≥2.5 mm and side branch (SB) diameter ≥2.0 mm on visual estimation. Main exclusion criteria were: ST-segment elevation myocardial infarction (STEMI), Medina 0,0,1 bifurcations, baseline serum creatinine level ≥177 µmol/L (2.0 mg/dl), inability to take dual antiplatelet therapy for 12 months, left ventricular ejection fraction ≤30%, and the lack of informed consent. The institutional review board of each participating centre approved the study protocol (88/2010, ClinicalTrials.gov Identifier: NCT02198300).
INTERVENTIONAL PROCEDURE, DEVICE DESCRIPTION AND CONCOMITANT MEDICATION
After signing informed consent, patients were randomly assigned to one of two treatment strategies, BiOSS LIM stent implantation or rDES implantation. Patients randomised to the rDES group underwent a second randomisation: completing the procedure with or without FKBI (Figure 1). Patients were randomised by telephone call to an external office, where the random allocation sequence was generated, and the participants were assigned using a sealed opaque envelope system (randomisation 1:1).
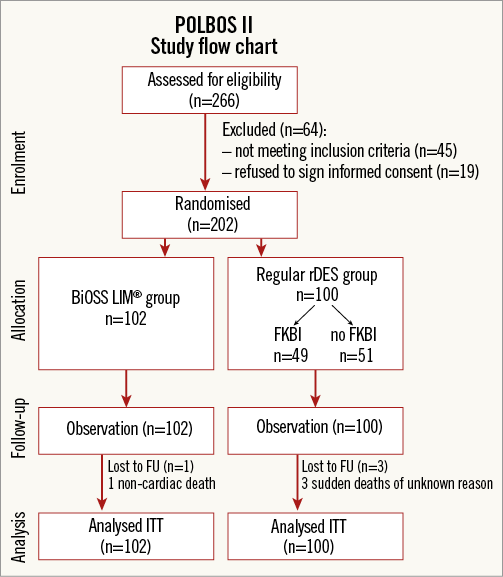
Figure 1. POLBOS II - study flow chart. FKBI: final kissing balloon inflation; FU: follow-up; ITT: intention-to-treat; rDES: regular drug-eluting stent
The BiOSS LIM is a coronary, dedicated balloon-expandable bifurcation stent. The platform is made of 316L stainless steel (strut thickness 120 μm) and is coated with a biodegradable polymer that elutes sirolimus (drug concentration: 1.4 µg/mm2). The rapid exchange delivery system is compatible with conventional 0.014” guidewires and 5 Fr guiding catheters. The BiOSS LIM consists of two parts, proximal and distal, joined with two connection struts at the middle zone (length: 0.9-1.5 mm, depending on stent size)4,5.
In the rDES group, the use of any approved regular DES available in the participating cathlabs was allowed at the discretion of the operator. The following stents were available: paclitaxel-eluting stents (PES) (Luc-Chopin2® [Balton], Coroflex® Please [B. Braun, Melsungen AG, Melsungen, Germany]), everolimus-eluting stents (EES) (XIENCE® [Abbott Vascular, Santa Clara, CA, USA], PROMUS [Boston Scientific Corp., Marlborough, MA, USA]), sirolimus-eluting stents (SES) (PROLIM® [Balton], Orsiro [Biotronik, Bülach, Switzerland], Cre8™ [CID, Saluggia, Italy]), Biolimus A9-eluting stents (BES) (BioMime™ [Meril, Vapi, Gujarat, India], BioMatrix™ [Biosensors, Singapore]), and zotarolimus-eluting stents (ZES) (Resolute Integrity [Medtronic, Minneapolis, MN, USA]).
The default strategy in both treatment groups consisted of the provisional strategy. A single stent was implanted in the MV-main branch (MB) across the SB in all patients. Bifurcation lesions were assessed according to the Medina classification (visual assessment)6. There was no restriction regarding lesion length. MV predilatation and/or SB predilatation was performed according to the operator’s decision. The stent was then implanted in the MV-MB. Next, the proximal optimisation technique (POT) was recommended, utilising a short non-compliant balloon in the proximal part of the MV stent. The balloon for POT was positioned with the distal marker in front of the carina7. After rewiring, SB post-dilatation/stent implantation was performed if indicated. A stent in the SB was implanted only if there was >70% residual stenosis in the SB and/or significant flow impairment (TIMI 0-1) after MV-MB stenting and/or a flow-limiting dissection.
The procedure was completed with FKBI dilatation. In the BiOSS group this step was left to the operator’s discretion, while in the rDES group it was performed according to the result of a second randomisation.
Procedures were performed according to local standards via radial or femoral access using 6 or 7 Fr guiding catheters. Pharmacological treatment was according to the most recent guidelines.
Troponin I (TnI), creatine kinase (CK) and creatine kinase-myocardial band (CK-MB) were measured pre-procedure and after six and 24 hours post procedure in all patients. Periprocedural myocardial infarction (type 4a) was defined according to the third universal definition8.
FOLLOW-UP
Clinical follow-up was performed with office visits or by telephone at one and 12 months after intervention. Adverse events were monitored throughout the study period. Follow-up coronary angiography was performed at 12 months, unless clinically indicated earlier.
ENDPOINTS
The primary endpoint was the cumulative rate of major adverse cardiovascular events (MACE) consisting of cardiac death, myocardial infarction (MI) and target lesion revascularisation (TLR). Secondary endpoints included cardiac death, all-cause death, MI, TLR, target vessel revascularisation (TVR), stent thrombosis (ST), late lumen loss (LLL) and device success. Cardiac death included death resulting from an acute MI, sudden cardiac death, death due to heart failure and death due to cardiac procedures. All deaths were deemed cardiac unless proven otherwise. MI was defined according to the third universal definition8. Clinically driven TLR was defined as reintervention of the target lesion due to presence of a symptomatic ≥50% diameter stenosis during follow-up. Angiographically driven TLR was defined as reintervention due to angiographic detection of significant restenosis (≥70%) in a patient who was clinically asymptomatic. TVR was defined as any revascularisation of any segment of the index coronary artery. LLL was calculated based on quantitative coronary angiographic (QCA) results and was defined as the post-procedural minimal luminal diameter minus the minimal luminal diameter (in millimetres) at 12-month follow-up. Device success was defined as successful deployment of the intended stent in the target site without a system failure.
ANGIOGRAPHIC ANALYSIS
Two orthogonal projections were chosen to visualise the treated bifurcation. All recordings were performed after intracoronary administration of nitroglycerine (200 μg). QCA analysis was performed using the dedicated bifurcation software CAAS 5.11 (Pie Medical Imaging BV, Maastricht, The Netherlands). Calibration was performed using the guiding catheter in all cases. The three bifurcation segments (MV, MB, SB) were analysed separately according to the European Bifurcation Club (EBC) consensus document9. The following parameters were reported: lesion length, reference vessel diameter (RVD), minimal lumen diameter (MLD). Percentage diameter stenosis (%DS), acute lumen gain (ALG) and LLL were calculated as described previously10. The point of bifurcation (POB) was determined automatically by the software and defined as the mid-point of the largest circle that can be fitted in the bifurcation area, touching all three contours, as previously described11.
STATISTICAL ANALYSIS
Continuous variables were presented as mean±SD. Categorical data were presented as numbers (%). Continuous variables were compared using an unpaired Student’s two-sided t-test, and categorical data using the χ2 test or Fisher’s exact test, as appropriate. If distribution was not normal (verified with the Shapiro-Wilk test), Wilcoxon signed-rank tests and Mann-Whitney U tests were used. P-values of <0.05 were considered statistically significant. Statistical analyses were performed using R 3.0.2 for OS (R Foundation, Vienna, Austria).
Results
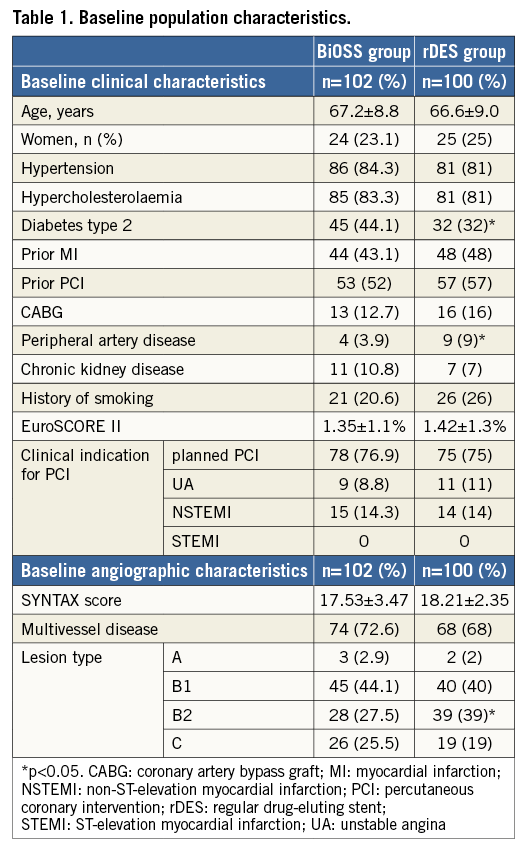
BASELINE CLINICAL CHARACTERISTICS
Between December 2012 and December 2013, a total of 202 patients were enrolled and randomly assigned to either the BiOSS group (n=102) or the rDES group (n=100). Among patients from the rDES group, 49 were randomised to FKBI and 51 to no FKBI (Figure 1). The mean age was 67.2±8.8 years in the BiOSS group and 66.6±9.0 years in the rDES group (p=0.93). Baseline clinical characteristics were well matched between the two groups, although there were more patients with diabetes type 2 in the BiOSS group (44.1% vs. 32%, p=0.01), and more patients with peripheral artery disease in the rDES group (3.9% vs. 9%, p=0.03) (Table 1).
ANGIOGRAPHIC AND PROCEDURAL CHARACTERISTICS
Lesions were located in the left anterior descending artery (LAD) in almost half of the cases (BiOSS vs. rDES: 44.1% vs. 43%, p=0.9), and in the left main (LM) in about one third of the cases (BiOSS vs. rDES: 35.3% vs. 38%, p=0.84). The rest were located in either the left circumflex artery (LCx) (BiOSS vs. rDES: 15.7% vs. 15.0%, p=0.88) or the right coronary artery (RCA) (BiOSS vs. rDES: 4.9% vs. 4%, p=0.9). In the rDES group, EES (46%) were used most frequently, followed by SES (24%). More details are presented in Figure 2A-Figure 2C. No difference between the two groups was observed regarding the distribution of true (i.e., Medina 1,1,1, 1,0,1, 0,1,1) and non-true bifurcations (Table 2).
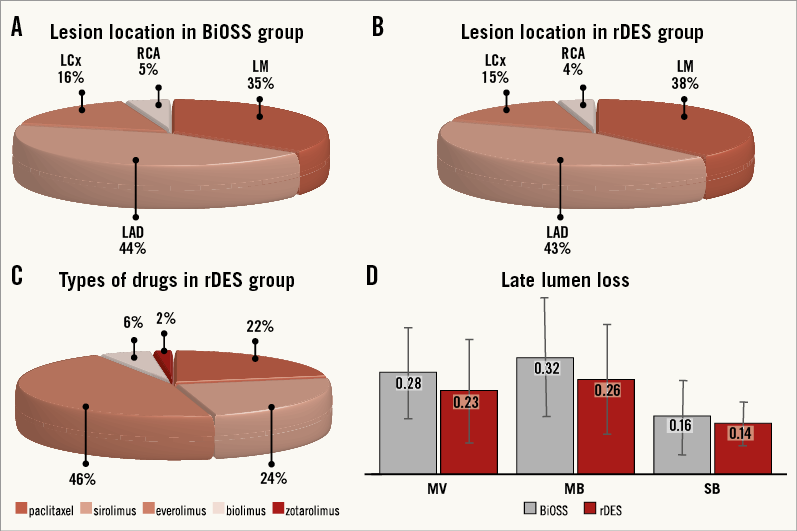
Figure 2. POLBOS II trial results. A) Lesion location in the BiOSS group. B) Lesion location in the rDES group. C) Types of drugs in regular DES group. D) Late lumen loss after 12 months.
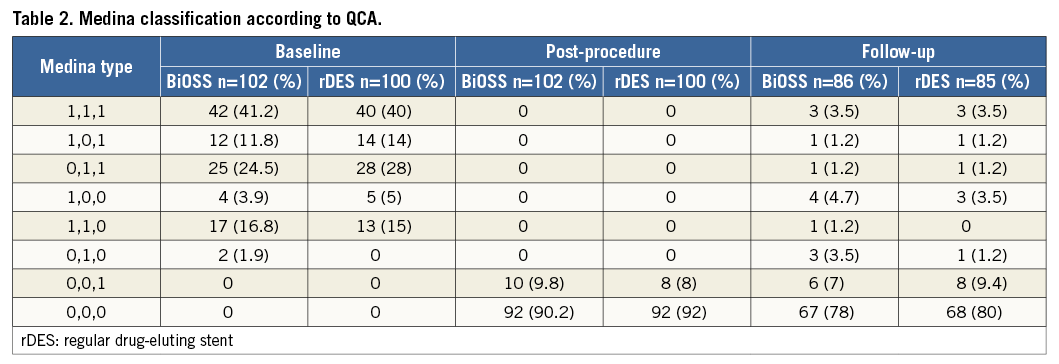
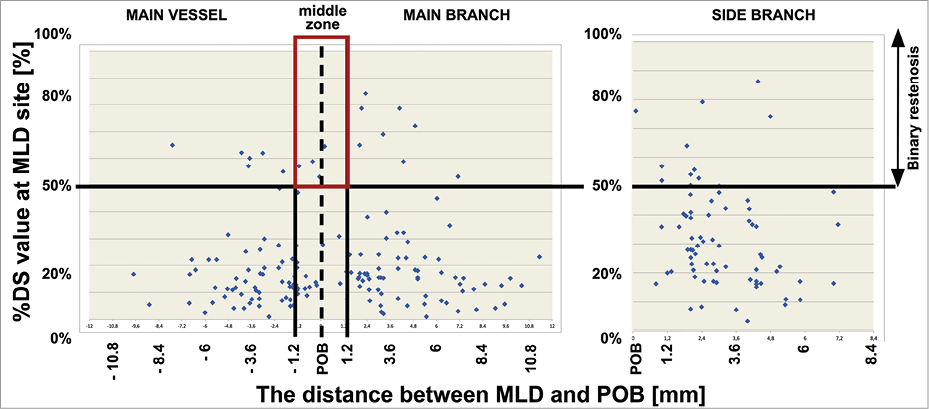
Figure 3. MLD distribution in the BiOSS LIM stent after 12 months.
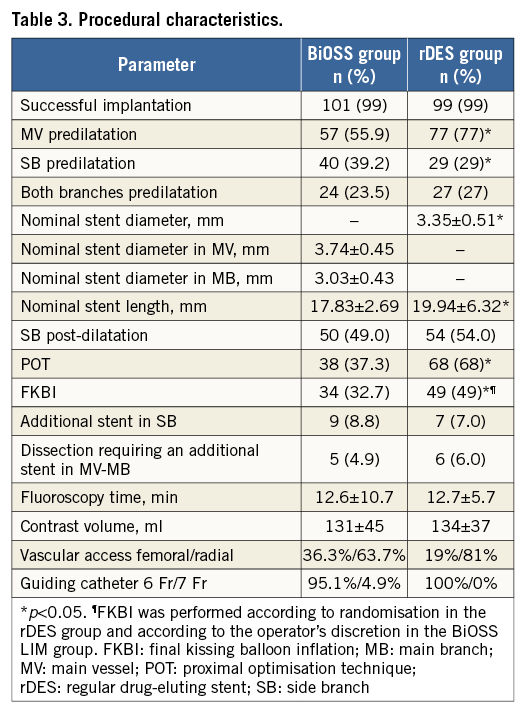
The main procedural variables are presented in Table 3. The device success rate was 99% in both groups. Mean BiOSS Expert nominal stent diameters were 3.74±0.45 mm (MV) and 3.03±0.43 mm (MB). Mean stent length was 17.83±2.69 mm, while the mean maximal implantation pressure was 14.3±4.5 atm. In the rDES group nominal stent parameters were as follows: diameter 3.35±0.51 mm, length 19.94±6.32 mm and mean maximal implantation pressure 17.2±3.7 atm. Procedural characteristics in the two groups were similar, except for rates of FKBI and POT, which were higher in the rDES group, 32.7% vs. 49% (p<0.01) and 37.3% vs. 68% (p<0.01), respectively. In both groups, the MV was predilated in more than half of the lesions (BiOSS 55.9% vs. rDES 77%, p=0.03), and the implantation of an additional rDES in the SB was required in nine cases (8.8%) in the BiOSS group and in seven cases (7%) in the rDES group (p=0.9).
CLINICAL OUTCOMES
There was one (1%) periprocedural MI in each group (due to transient SB occlusion and/or atherosclerotic debris embolisation). Additionally, there were six (5.9%) patients in the BiOSS group and eight (8%) in the rDES group with in-hospital increased TnI levels (max 1.9 ng/ml). These were all asymptomatic, without ECG changes and did not require repeat coronary angiography (i.e., they did not meet the criteria of MI type 4a).
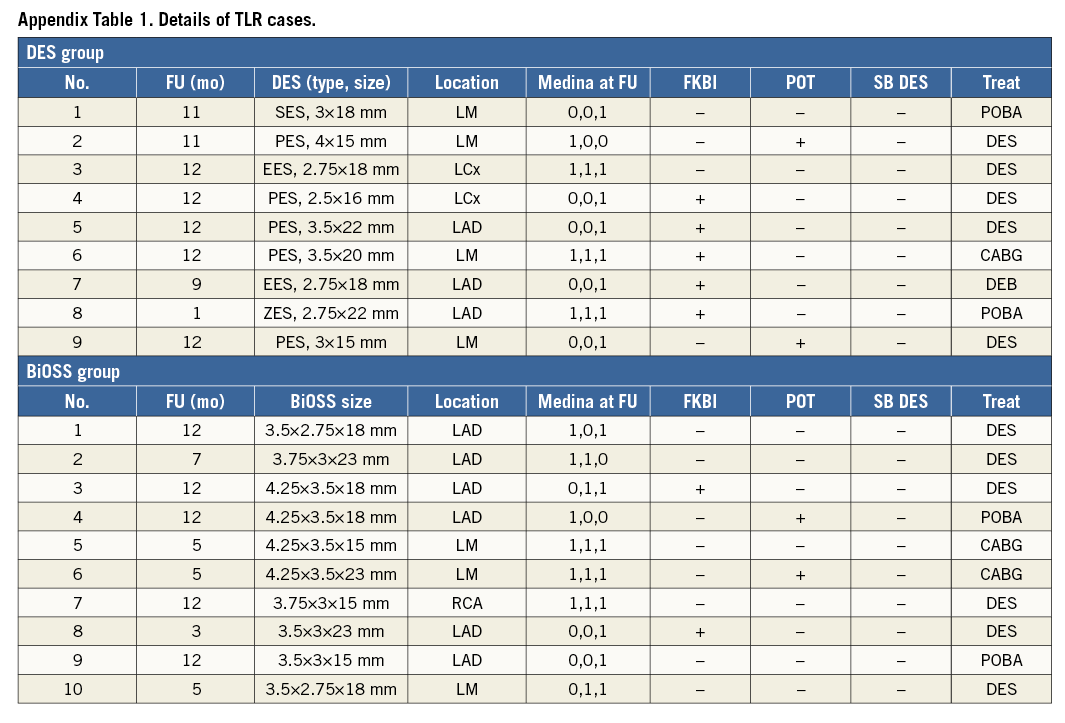
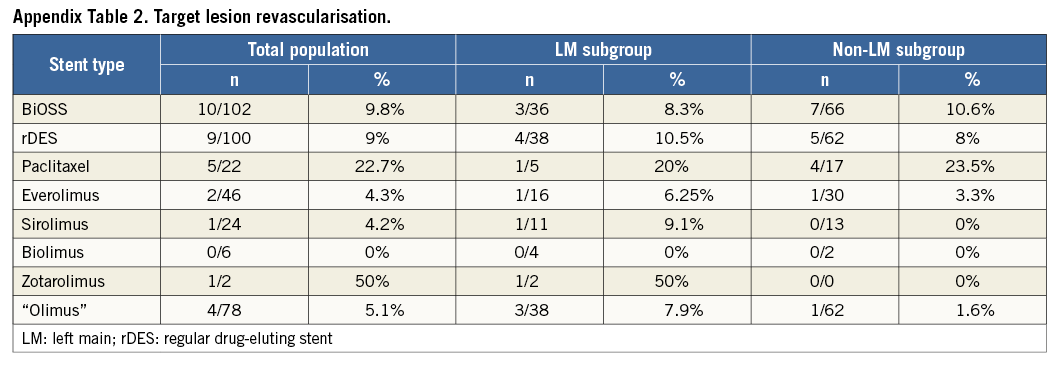
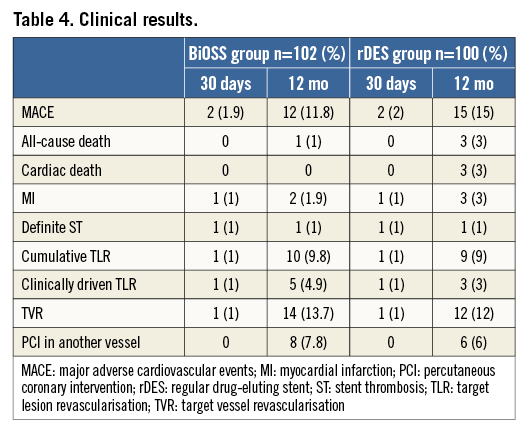
Clinical follow-up at 12 months was available in all patients who were alive (Table 4). The cumulative incidence of MACE was numerically lower in the BiOSS group although it did not reach statistical significance (11.8% in the BiOSS group vs. 15% in the rDES group, p=0.09). In the BiOSS group one non-cardiac death (secondary to a malignancy) occurred, whereas cardiac death occurred in three patients in the rDES group (all sudden deaths of unknown cause). One case of definite ST was observed in each group, both subacute. The TLR rate was comparable between both groups (BiOSS group vs. rDES group: 9.8% vs. 9.0%, p=0.8). When considering only clinically driven TLR there were five (4.9%) cases in the BiOSS group and three (3.0%) cases in the rDES group (p=NS). Also, there was a trend towards a lower TLR rate in the LM subgroup in the BiOSS group (8.3% vs. 10.5%). Moreover, in the PES subgroup of the rDES group the TLR rate was 22.7%, whereas in the “olimus” subgroup it was only 5.1%. Detailed data concerning the type of restenosis with regard to DES type in the rDES group are presented in Appendix Table 1, Appendix Table 2. Figure 3 shows the position of the MLD sites, with their corresponding diameter stenosis values, relative to the POB for each bifurcation segment separately. It shows that the middle zone is not more susceptible to restenosis than the segments more proximal to or distal from the POB.
QCA ANALYSIS
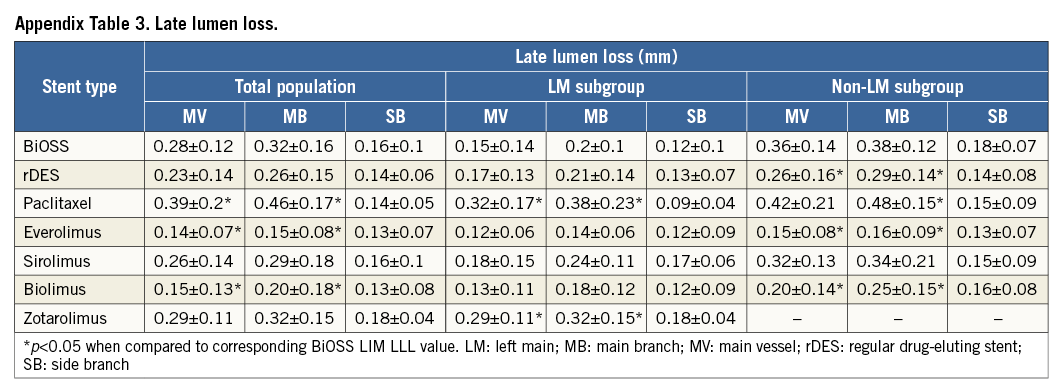
Angiographic follow-up at 12 months was performed in 182 patients (90.1%), of whom 92 (90.2%) were randomised to the BiOSS group and 90 (90%) to the rDES group. Follow-up QCA analysis was performed in 171 (84.7%) cases. Eleven cases had to be excluded due to an inadequate view at the bifurcation lesion, overlapping vessel segments, or the presence of angiographic guidewires. Angiographic data are presented in Table 5. The two groups were well matched in baseline QCA characteristics. Quantitative angiographic analysis revealed that the BiOSS LIM stent as well as rDES implantation caused a significant increase of MLD and decrease of %DS in the MV and MB. However, this procedure did not affect angle alpha between the MV and SB. When comparing LLL values, there were no significant differences in the MV (BiOSS vs. rDES: 0.28 vs. 0.23 mm, p=NS), in the MB (0.32 vs. 0.26 mm, p=NS) as well as in the SB (0.16 vs. 0.14 mm, p=NS) (Figure 2D). Within the rDES group, the smallest LLL values were observed in the EES subgroup, and the largest in the PES subgroup (Appendix Table 3). LLL values were greater in non-LMS bifurcation lesions in all subgroups.

FKBI VS. NO FKBI – SUBGROUP ANALYSIS
Subgroup analysis regarding FKBI versus no FKBI revealed that in both groups (BiOSS group and rDES group) FKBI was related to a higher rate of SB stenting, longer fluoroscopy time and a greater likelihood of the treated lesions being located in the LM. However, in the FKBI subgroup there was a significantly lower rate of restenosis in the BiOSS group (5.9% vs. 11.8%, p<0.05). Such an association was not present in the rDES group (10.2% vs. 7.8%, p=NS). Moreover, the POT technique has a more pronounced effect on the TLR rate than the FKBI and in both groups was significantly associated with a lower rate of TLR (in the BiOSS group: 5.3% vs. 12.5%, p<0.05 and in the rDES group: 2.9% vs. 25%, p<0.01). Additionally, there was a trend towards lower LLL in both the BiOSS group and the rDES group when FKBI and POT were applied (Appendix Table 1).
Discussion
The main findings of this study are as follows: 1) the 12-month cumulative MACE rates were comparable between the BiOSS LIM and the rDES treatment groups; 2) TLR rates were also comparable between BiOSS LIM and rDES; 3) FKBI did not improve outcomes in rDES, although this was underpowered; 4) a subgroup of patients in whom POT with FKBI was performed showed better angiographic and clinical outcomes; 5) the BiOSS LIM provides a single stent bifurcation treatment option with a very high rate of implantation success.
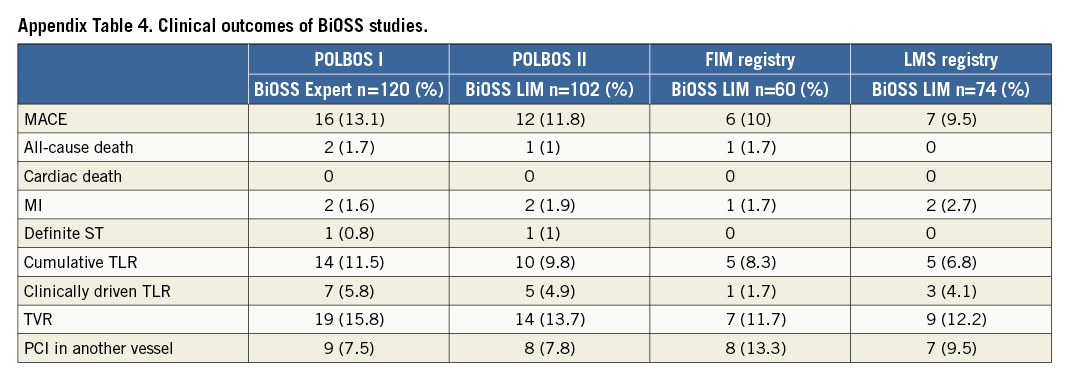
The BiOSS stent is a stainless steel construction based on the physiological concept of the coronary bifurcation anatomy and the coronary blood flow. Results of the BiOSS Expert Registry evaluating the BiOSS paclitaxel-eluting stent were encouraging and, subsequently, the randomised POLBOS I trial comparing BiOSS Expert with rDES was conducted3,12. However, in the meantime many studies have shown paclitaxel-eluting stents to be inferior to “olimus”-eluting stents13. Therefore, a new version of the BiOSS stent, the BiOSS LIM, eluting sirolimus, was designed. After near completion of the POLBOS I trial, the POLBOS II trial was started, in which the sirolimus-eluting BiOSS LIM was compared with rDES.
The results of both POLBOS I and POLBOS II have shown that the BiOSS stent performs very well in distal LM stenosis14. There was no difference between sirolimus- and paclitaxel-eluting BiOSS stents. This suggests that the design of the BiOSS stent itself plays a crucial role. Also, lower LLL values in the LM subgroup suggest that this stent might be better suited for bifurcations with a greater difference in diameters between MV and MB, such as the LMS/LAD/LCX bifurcation (Appendix Table 3). These results were also initially observed in registries of the BiOSS stents in the LM10,15.
The stepped design of the BiOSS stent delivery balloon was created, theoretically, to ensure an FKBI- and POT-like effect, thus allowing operators frequently to omit this part of the procedure. During analysis of the current data we indeed found that the percentages of FKBI and POT were relatively low. These data suggest that the operators also had the perception that the features of the BiOSS stent design might remove the need for POT and/or FKBI as procedural steps during PCI of bifurcation lesions. Unfortunately, in the subgroup of patients treated with BiOSS in whom FKBI and POT were not performed, we found worse clinical outcomes and a trend towards higher LLL values. This unfavourable outcome might be due to the fact that the semi-compliant delivery balloon is not as efficient in POT as the recommended non-compliant balloon catheter (according to the EBC). Also, in the distal part of the stent the semi-compliant delivery balloon might not keep its parameters (it might gain too much in diameter) and cause the plaque shift as well as the carina shift. Therefore, the use of FKBI might restore the correct anatomical conditions in the bifurcation and improve the outcome. The impact of FKBI on clinical outcomes found in both POLBOS trials was in agreement with NORDIC III study findings, in which FKBI reduced angiographic side branch restenosis, especially in patients with true bifurcation lesions3,16. However, to the best of our knowledge, we show for the first time the importance of POT as the final part of coronary stent implantation in bifurcation lesions. It should be stressed that in our study POT had a more pronounced positive effect on TLR rates (5.3% with POT vs. 12.5% without POT, p<0.05, in the BiOSS group; and 2.9% with POT vs. 25% without POT in the rDES group, p<0.01).
Importantly, the pattern of restenosis did not reveal the middle zone (with only the two connection struts) as a “weak point” of the BiOSS LIM stent. The MLD sites were located significantly further away from the POB at follow-up than pre-procedure (Figure 3).
BiOSS stents are made of stainless steel and therefore have relatively thick struts (120 µm without polymer), which might predispose to excessive neointimal proliferation. The ISAR-STEREO trial demonstrated that a thin-strut stent had a lower rate of restenosis than a thick-strut stent of similar design17. Similar results were found in several other trials18-20. During recent years the industry has applied platinum chromium and cobalt-chromium alloys to achieve stents with so-called thin struts (i.e., <90 µm)21.
Although the clinical results obtained with the BiOSS Expert are satisfactory, the BiOSS concept remains under development (Appendix Table 4). The next generation of the BiOSS stent, made of cobalt-chromium with a strut thickness of 70 µm, but with the same sirolimus concentration and biodegradable polymer, is currently under development. The results obtained in the POLBOS I and II trials are very consistent. The results of the BiOSS LIM stent (even without optimisation) were comparable to rDES. It is assumed that only the thin-strut BiOSS version may achieve better results. Therefore, it was decided to prepare a new randomised, international study – POLBOS III, where the newest thin-strut BiOSS version will be compared with the most tested representatives of “olimus” stents (EES, ZES).
STUDY LIMITATIONS
The POLBOS II trial has several limitations that should be acknowledged. First of all, the sample size was relatively small. Furthermore, a 100% angiographic follow-up rate could not be achieved, although a follow-up rate of 90% is in line with similar studies. The use of multiple stent types and drugs in the control group is also a limitation, although this aspect of the design was intended to replicate real-world clinical practice. The predictable randomisation scheme may potentially have biased the decisions of investigators to randomise patients with certain angiographic characteristics. Finally, the differences in FKBI/POT strategies between the two study groups (randomisation in the rDES group and operator choice in the BiOSS group) may also have influenced the results.
Conclusion
The success rate of implantation of the BiOSS LIM stent is very high. The cumulative rate of MACE is comparable between BiOSS and rDES groups. The TLR rate achieved with the BiOSS LIM was comparable to that obtained with rDES. The POLBOS II trial sets an important benchmark for future studies with new generations of BiOSS stents eluting “olimus” drugs and utilising newer stent materials.
| Impact on daily practice This trial has shown that implantation of the dedicated bifurcation BiOSS LIM stent is safe and effective. Also, the results for MACE and TLR rates were comparable between the BiOSS LIM and rDES groups. Therefore, this stent might be an alternative in some cases of coronary bifurcation treatment, especially when there is a large difference in diameter between the main vessel and the main branch. |
Guest Editor
This paper was guest edited by David Hildick-Smith, MD, Sussex Cardiac Centre, Brighton and Sussex University Hospitals NHS Trust, Brighton, United Kingdom.
Conflict of interest statement
R. Gil was the medical consultant of the Balton Company. The other authors have no conflicts of interest to declare. The guest editor is a proctor/adviser for Medtronic, Boston Scientific, Edwards Lifesciences, and St. Jude Medical.
Supplementary data