Abstract
Aims: To report the three-year clinical outcome of the Axxess™ stent, a nitinol self-expanding Biolimus A9™ eluting stent for treatment of de novo coronary bifurcation lesions. The Axxess stent is a new-generation drug-eluting stent that might offer advantages in terms of improved clinical outcomes and safety profile in bifurcation lesion stenting.
Methods and results: The DIVERGE study was a multicentre, prospective, single-arm trial. The primary endpoint was the cumulative rate of major adverse cardiac events (MACE), a composite of all-cause death, myocardial infarction (MI), and ischaemia-driven target lesion revascularisation (TLR) at one, two and three years. Secondary safety endpoints were cumulative stent thrombosis (ST). A total of 302 patients were included across 14 sites: 77.4% had a true bifurcation lesion, with the left anterior descending/diagonal as target vessel in 80.8%. The Axxess stent was placed in 299 patients (99.0%) and scored as optimal in 93.0%. Two hundred and ninety-eight patients (98.7%) returned for the three-year follow-up. The MACE rate was 9.3% at one year, 14.0% at two years and 16.1% at three years. Individual components at three years were 10.1% for ischaemia-driven TLR, 3.0% for death (2.0% cardiac death), and 7.4% for MI. In the secondary safety endpoint at three years, a total of seven patients (2.3%) had ST with six (2.0%) definite and two (0.7%) probable ST events.
Conclusions: The present large study of the Axxess stent reports a good cumulative MACE rate during three years of long-term follow-up. The Axxess stent offers a promising treatment strategy for bifurcation lesions.
Introduction
Significant improvements have been made in percutaneous coronary intervention (PCI) of bifurcation lesions with the introduction of drug-eluting stents (DES). However, challenges remain since bifurcation stenting is technically challenging and is associated with lower procedural success rates and worse clinical outcome, with higher rates of restenosis1 and stent thrombosis2 than non-bifurcation lesions.
Dedicated bifurcation stents are novel technologies that may simplify the technical challenge and improve the clinical outcome of bifurcation PCI3. Major progress in stent design allowing complete bifurcation lesion coverage with minimal strut deformation and overlap, as well as significant innovation in polymer coating and eluting drug compound, reducing the hypersensitivity reaction, endothelial dysfunction and delayed re-endothelialisation, could ultimately improve technical and clinical outcomes.
The Axxess™ stent system (Biosensors Europe SA, Morges, Switzerland) has been developed focusing on these important issues. It is a conical-shaped self-expanding nitinol stent, coated with a biodegradable polylactic acid (PLA) polymer eluting the highly lipophilic semisynthetic sirolimus analogue Biolimus A9 (Biosensors Europe SA, Morges, Switzerland). We previously reported the nine-month clinical, angiographic and intravascular ultrasound results of the DIVERGE (Drug Eluting Stent Intervention for Treating Side Branches Effectively) trial, a multicentre, prospective, single-arm study evaluating the safety and feasibility of the Axxess stent system4. It showed a procedural success rate of 96.7% with a cumulative MACE rate of 4% at 30 days and 7.6% at nine months. Although the low MACE rate at nine months is encouraging, longer follow-up is warranted, since little is known about the long-term clinical outcome of this patient population with their complex lesions in general, and after treatment with novel devices in particular. Here, we report on the complete long-term three-year clinical outcome data of the DIVERGE study.
Methods
STUDY POPULATION
The DIVERGE study is a prospective, single-arm, multicentre study with patients enrolled between June 2006 and October 2007 in 14 clinical sites in Europe, Australia and New Zealand. The methods with inclusion and exclusion criteria have been published previously4. In brief, patients of 18 to 80 years of age with documented stable or unstable angina or positive functional study and a de novo native bifurcation lesion in a major coronary artery in which either the MV or SB had at least a 50% stenosis were eligible for PCI. The reference vessel diameter by visual estimate had to be 2.75 to 3.75 mm in the MV and ≥2.25 mm in the SB. The angle between the distal MV and the SB had to be <70°. All patients provided written informed consent before the procedure and the local ethics committee of all participating hospitals approved the study.
THE AXXESS STENT AND PROCEDURAL STRATEGY
The Axxess stent system has been described in detail previously5,6. In brief, it is a self-expanding, conical-shaped nitinol stent with a strut thickness of 0.16 mm. It elutes Biolimus A9™, a highly lipophilic, semi-synthetic sirolimus analogue (Biosensors Europe SA, Morges, Switzerland). The drug is emulsified into a biodegradable PLA polymer, which is applied primarily to the abluminal stent surface. The PLA biodegradable coating is absorbed after six to nine months. The Biolimus A9 elution half-life is 21 days, and the drug is completely eluted by 196 days (Biosensors, data on file).
The deployment of the Axxess stent is depicted in Figure 1. The stent diameters available for the study were 3.0 and 3.5 mm, with lengths of 11 or 14 mm. All patients who had the study stent introduced into the vasculature were considered enrolled in the DIVERGE study. Depending on the disease status of the distal MV and SB, adjunctive stenting was permitted at the discretion of the operator. An optimal angiographic outcome was strongly recommended to ensure no residual stenosis at the end of the procedure. Additional stents were thus implanted if there was a residual stenosis of >30% in any segment of the bifurcation. The protocol mandated the use of the CYPHER® sirolimus-eluting stent (Cordis, Johnson&Johnson, Warren, NJ, USA) for this purpose. Bail-out stenting with additional CYPHER stents was allowed in case of dissection or incomplete lesion coverage. Aspirin (≥80 mg/day) was given daily and clopidogrel (loading dose ≥300 mg and 75 mg/day thereafter) was administered for at least six months in all patients. A protocol amendment of October 2007 mandated clopidogrel for 12 months post procedure according to the updated AHA/ACC/SCAI recommendations7. Prolonged DAPT after 12 months post procedure was given at the discretion of the treating physician.
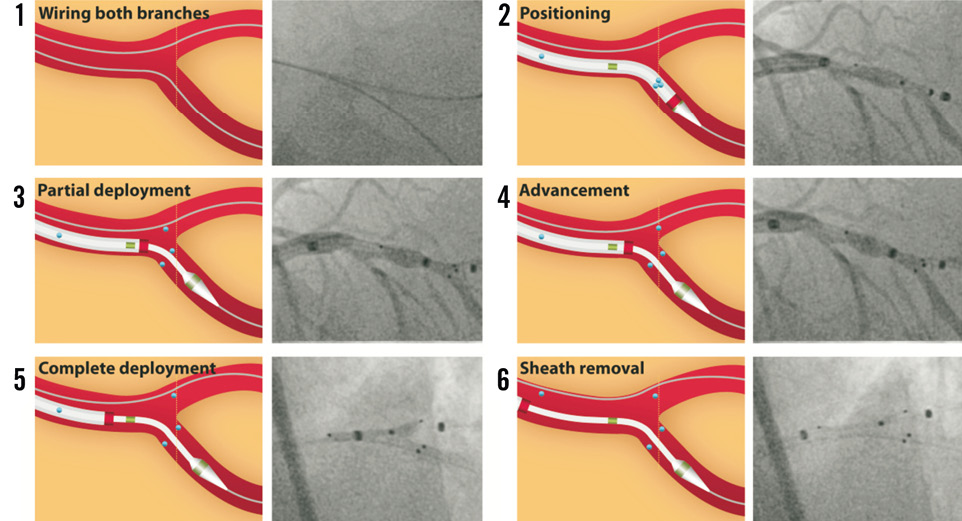
Figure 1. Step-by-step illustration and angiography of the Axxess stent deployment. 1) The Axxess stent is specially designed for the treatment of bifurcation lesions up to an angle of 70°. The procedure typically requires wiring and predilatation of both the MV and the SB. 2) The Axxess stent is loaded on a 7 Fr delivery system and kept in place by a cover sheath. Markers are clearly visible under fluoroscopy, two on the inner catheter (green markers in the illustration) and one on the cover sheath (red marker). Three highly visible radiopaque markers (blue dots) are located at the distal end of the stent and a fourth marker is located at the proximal edge to facilitate placement. The stent is advanced on one of two wires, preferably towards the distal branch presenting the sharpest angle with the proximal MV. This facilitates the stent flare into the opposite vessel. 3) The stent is then gently deployed by retracting the cover sheath once the distal stent markers are aligned at the level of the carina. 4) The stent is carefully advanced over the carina, allowing one or two markers to protrude into the opposite distal branch. As long as the cover sheath contains more than half the length of the stent and does not pass the second catheter marker, further adjustment of the stent position (or removal) remains possible. 5) Once the distal part of the stent is correctly positioned, the stent is completely deployed and (6) the sheath is carefully removed. The stent covers both the MV and the SB without obstructing access to either lumen. This allows additional stent placement in the distal vessels if needed.
STUDY ENDPOINTS
Patients were evaluated clinically in the outpatient clinic or by phone call at one, six, and nine months, and every year after the procedure. The primary endpoint of the present study was the rate of MACE at one, two and three years, consisting of cardiac or non-cardiac death, Q-wave or non-Q-wave MI, and ischaemia-driven TLR, as described previously4. Myocardial infarction (including periprocedural) was defined as: 1) Q-wave MI when chest pain or symptoms consistent with myocardial ischaemia and new pathological Q-waves in two or more contiguous electrocardiogram leads were present; and 2) non-Q-wave MI if creatine kinase was elevated >2x the upper laboratory normal with the presence of elevated creatine kinase-MB in the absence of new pathological Q-waves. Cardiac death was defined as death caused by acute MI, heart failure, cardiac perforation, arrhythmia, stroke within 30 days of the procedure, or complication of the procedure (including bleeding, vascular repair, transfusion reaction, or bypass surgery), or any death for which a cardiac cause could not be excluded.
Secondary safety endpoints of the present study were defined as definite or probable ST at the following time points: one year and annually until three years post index procedure. ST was categorised depending on the timing of occurrence into acute (peri-procedure), subacute (post procedure to 30 days), late (30 days to one year) or very late (>1 year) according to the Academic Research Consortium criteria8.
Coronary angiograms of all ischaemia-driven TLR were reviewed for in-stent restenosis, edge stenosis and stent thrombosis in the Axxess and additional CYPHER stents by an independent core lab (Cardiovascular Research Foundation, New York, NY, USA). In-stent restenosis (ISR) and stent thrombosis (ST) were angiographically assessed using all views provided and coded for the Axxess stent using the radiopaque markers at the proximal/distal Axxess stent edge as a reference point. ST was coded if there was an angiographically visible thrombus (a discrete, mobile intraluminal filling defect with or without defined borders) that was either occlusive, globular, hazy or a filling defect in the stent or within 5 mm of the stent edge. ISR was coded for any restenosis ≥50% in stent or 5 mm from the stent edge and no suggestion of thrombus.
STATISTICAL ANALYSIS
Continuous variables are presented as means±standard deviation and categorical variables are presented as counts and frequencies. All analyses were conducted according to the intention-to-treat principle.
Results
DEMOGRAPHICS AND LESION CHARACTERISTICS
The patient clinical characteristics are shown in Table 1. Most of the patients were male (74.2%) with a mean age of 62.8 years and multiple risk factors for ischaemic heart disease. The location of the stenoses is illustrated in Figure 2. The majority of the lesions were classified as true bifurcations (77.4%) according to the Medina classification9, and most of the lesions involved the left anterior descending – diagonal bifurcation (80.8%, Table 2).
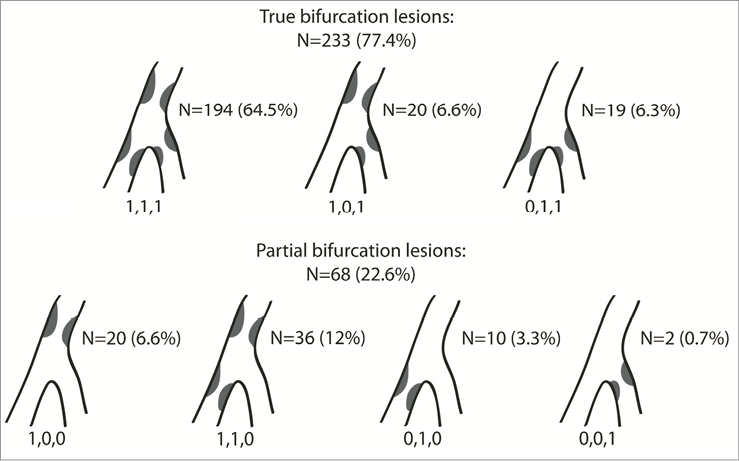
Figure 2. Medina classification of the bifurcation lesions included in the DIVERGE study.
PROCEDURAL OUTCOMES
The Axxess stent was placed in 299 of the 302 patients (99.0%). CYPHER stents were placed in the distal MV and/or SB in 259 patients (85.7%; Table 2 shows individual numbers). Procedure success, defined as attainment of a final angiographic success in the absence of any in-hospital MACE, was obtained in 96.7% of the patients, as reported previously4. After the procedure, 98.0% of the patients were discharged on aspirin and 100% on clopidogrel. At one year, 94.3% and 73.1% were taking aspirin and clopidogrel, respectively. At three years, 91.5% of the patients were on aspirin, while 40.5% of the patients were on clopidogrel (Table 3).
CLINICAL OUTCOMES
Thirty-day and nine-month clinical outcomes have been reported previously4. Three hundred patients (99.3%) returned for the one and two-year follow-up visits and 294 patients (97.4%) for the three-year follow-up. The cumulative MACE rate was 9.3% at one year, and increased moderately to 14.0% at two years and to 16.1% at three-year follow-up (Table 4 and Kaplan-Meier curve in Figure 3 and Figure 4). This was primarily driven by ischaemia-driven TLR, with rates of 6.0% at one year, 8.7% at two years and 10.1% at three years, respectively. There were six cardiac deaths (2.0%) at three years, eight Q-wave MIs (2.7%) and 15 non-Q-wave MIs (5.0%).
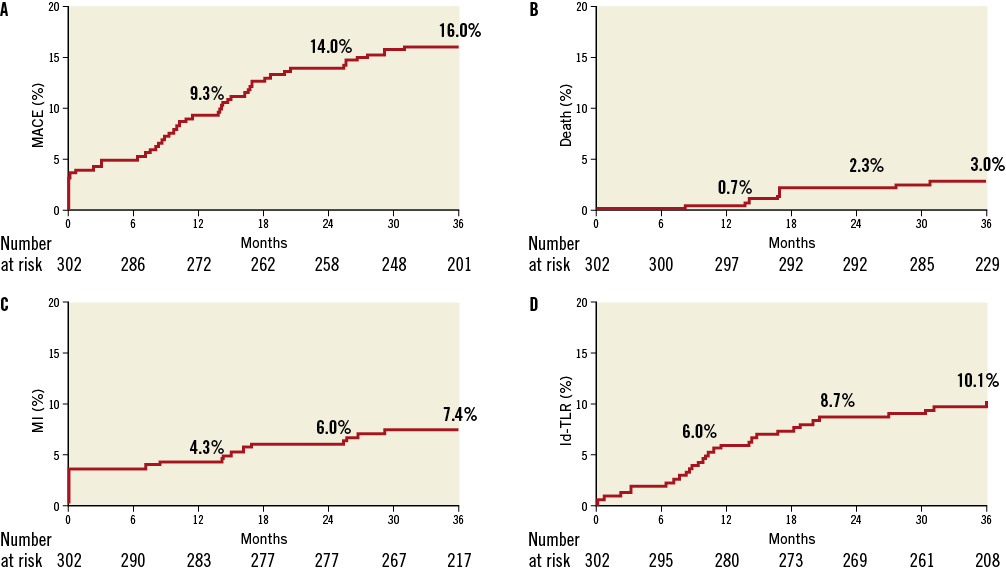
Figure 3. Kaplan-Meier event curves up to three years of follow-up for major adverse cardiovascular events. A) Total MACE, (B) death, (C) MI, and (D) ischaemia-driven TLR. Id-TLR: ischaemia-driven target lesion revascularisation
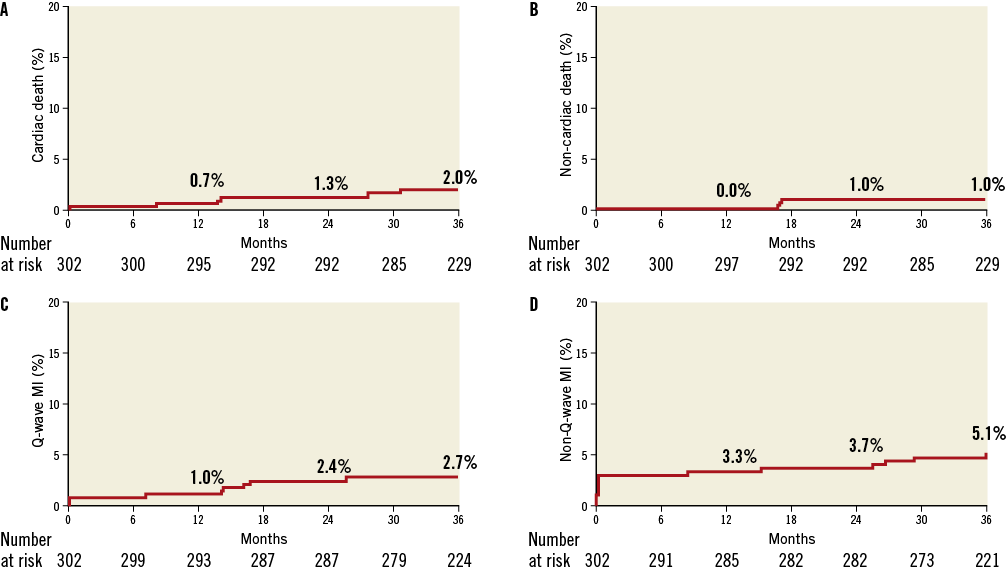
Figure 4. Kaplan-Meier event curves up to three years of follow-up for death and myocardial infarction. (A) Cardiac death, (B) non-cardiac death, (C) Q-wave MI and (D) non-Q-wave MI.
Four ST occurred during the first nine months post procedure (all three definite ST: two subacute and one late)4. While no ST occurred between nine months and one year, one definite and two probable ST occurred during the second year (definite ST: one Q-wave MI with thrombosis of both the Axxess and the two CYPHER stents on the LCX/OM; probable ST: one cardiac death with Q-wave MI in the implanted stent territory, and one Q-wave MI successfully treated with thrombolytics). Two additional definite very late ST occurred during the third year post procedure causing non-Q-wave MIs (one very late ST of the CYPHER stent in the diagonal occurred in a patient 10 days after stopping aspirin and, having already stopped clopidogrel one year after the index procedure, the ST was left untreated; and another occurred in the CYPHER stent of the LAD two days after stopping both aspirin and clopidogrel and was successfully treated with PCI –this was the same patient with a probable ST during the second year successfully treated with thrombolytics). Altogether, at three years, seven patients (2.4%) had one or more ST, with individual numbers of six (2.0%) definite ST events and two (0.7%) probable ST events, with 0 (0%) acute, two (0.7%) subacute, one (0.3%) late and four (1.3%) very late ST events. One patient had both a probable ST during the second year and a definite ST during the third year of follow-up (see above for narrative).
CLINICAL OUTCOMES PER TREATMENT GROUP
For all the patients who received an Axxess stent and had three years of follow-up, we calculated the cumulative MACE rate and individual components per treatment group (Table 5). The 35 patients treated with an Axxess stent only had the lowest MACE rate (5.7%) at three years, with only one edge restenosis (2.9%) of the Axxess stent. This might reflect a selection bias of less severe bifurcation lesions. Of the 52 patients treated with an Axxess stent and an additional stent in the MV, seven (13.5%) had an ischaemia-driven TLR with four restenoses in the Axxess stent (57.1%) and three in the additional stent (42.9%). The majority of these were edge restenoses (3 out of 4 for Axxess, 2 out of 3 for the additional stent). Of the 193 patients treated with an Axxess stent and two additional stents in the MV and SB, 34 (17.6%) had a MACE at three years, with 20 (10.4%) ischaemia-driven TLR. Six (30%) patients had restenosis in both the Axxess and the additional stent, 13 (65%) had a restenosis in the Axxess stent only, and one (5%) had a restenosis in the additional stent only. The majority of the Axxess restenoses were due to edge restenosis (15 out of 19, 78.9%). A small number (11) of patients were treated with an Axxess stent and one additional stent in the SB (Table 5).
Discussion
The DIVERGE study is a large safety and feasibility study of the Axxess stent, a nitinol self-expanding DES dedicated for bifurcation lesions. We were able to implant the Axxess stent successfully in 299 of the 302 recruited patients (99.0%) with complex bifurcation lesions, mostly located in the left anterior descending – diagonal branch (80.8%). We previously reported the short and medium-term clinical follow-up with a low all-cause cumulative nine-month MACE rate (7.7%, including 3.0% periprocedural non-Q-wave MI)4. In the present study, we report on the long-term complete follow-up of the DIVERGE study and detail a consistent low cumulative MACE rate of 9.3% at one year, 14.0% at two years and 16.1% at three years of follow-up. These results during long-term follow-up consolidate the safety of this novel dedicated bifurcation DES.
The cumulative MACE rate of 16.1% in the DIVERGE trial at three years compares favourably to other long-term trials with DES. Further analysis of the patients with ≥1 bifurcation lesion from the LEADERS trial (497 patients or 29.1% of the 1,707 all-comers), comparing a biolimus-eluting stent (BES, n=258) with a sirolimus-eluting stent (SES, n=239) revealed a composite MACE rate at three years of 18.5% for BES and 23.3% for SES, with individual components for BES and SES, respectively, of 3.9% and 5.5% for cardiac death, 9.8% and 9.1% for MI, 8.9% and 16.0% for clinically indicated TVR, and 2.0% and 4.3% for definite ST10. These are in line with the individual MACE components in the DIVERGE study at three years, with 2.0% cardiac death, 7.4% MI, 10.1% for ischaemia-driven TLR and 2.0% definite ST. Likewise, the long-term results of DIVERGE are similar to Axxess Plus, the first-in-man safety and performance study in 139 patients11. The Axxess Plus study reported a final MACE rate of 19.7% at five years, with individual components of 5.1% for death, 3.4% for cardiac death, 9.4% for MI, and 12.8% for ischaemia-driven TLR12. However, different inclusion criteria (e.g., left main in Axxess Plus), trial design (e.g., enhanced lesion coverage in DIVERGE) and endpoint definitions (e.g., ARC definition of stent thrombosis in DIVERGE) preclude firm comparison between the two trials11.
Large randomised bifurcation trials also demonstrated comparable MACE rates, although different MACE definitions among the trials should be acknowledged. The NORDIC trial (single MV stenting strategy versus stenting of both the MV and SB with SES [CYPHER] in 413 patients), reported lower MACE rates of 10.9% in the MV group and 10.2% in the MV and SB group at three years. However, this is partly due to the exclusion of periprocedural MIs from the primary endpoint13,14. In DIVERGE, the MACE rate of 16.1% included 3.0% periprocedural non-Q-wave MI. So far, three-year follow-up results for CACTUS or BBC ONE have not yet been published. However, the CACTUS trial (SES crush stenting versus provisional side-branch stenting in 350 patients) already showed at six months a cumulative MACE rate of 15.8% in the crush group and 15.0% in the provisional group, which is in the same range as DIVERGE, yet at a later three-year follow-up15. This can also be said for the BBC trial (systematic stenting of both vessels with culotte or crush versus provisional stenting in 500 patients using paclitaxel-eluting stents), which observed a 15.2% versus 8.0% MACE rate (composite of death, MI and target vessel failure) at nine months16. Finally, the ARTS II trial reported an incidence of all-cause death, MI or any repeat revascularisation of 17.1% at three years in 324 patients who underwent revascularisation procedures with a sirolimus-eluting stent involving at least one bifurcation (465 lesions)17. In summary, although definite conclusions should only be drawn from randomised trials, the present long-term data in the DIVERGE trial underscore the efficacy of the Axxess stent and warrant further studies.
Several factors could have contributed to the low MACE rates observed in the DIVERGE study. Both minimum stent cross-sectional area and minimum stent expansion are known to correlate with restenosis and stent thrombosis18. However, the self-expanding properties of the Axxess stent prevent stent recoil and late lumen loss, and could thus reduce ST. In addition, recent studies have shown numerically lower rates of ST with newer-generation DES with more biocompatible19, or completely biodegradable polymers20,21, such as Axxess. Although a single provisional stent strategy is the currently preferred stenting approach, SB compromise is not inconsequential. Occlusion of SB >1 mm can be associated with a 14% incidence of MI22, and SB ≥2 mm compromise can be associated with a large periprocedural MI23. However, because the Axxess stent is placed proximal to the carina and since it spans both main and side branch ostia without obstructing access to either, it substantially reduces occlusion of the SB and allows the tailored implantation of additional stents in the distal MV or SB for complete lesion coverage with minimal strut overlap and distortion. There is also no necessity for final kissing balloon angioplasty through the strut, which is known to produce significant stent distortion and detrimental stent geometry, and may fail to ensure circular expansion and full apposition of the stent strut in the MV24. Stent asymmetry has been shown to correlate with ST and a higher risk of adverse outcomes in a recent optical coherence tomography (OCT) study25, while malapposed struts may significantly increase the risk of restenosis and ST. The current consensus is that a one stent –provisional– strategy is the technique of choice for simple lesions in which the side branch is not severely diseased. However, in complex bifurcation lesions where a multi-stent strategy is warranted for complete bifurcation reconstruction, the Axxess stent platform might be preferred over a multi-stent strategy, since there is no crush or double layer, no strut deformation, and no protruding struts or extensive neocarina. Finally, the limited availability of Axxess stent sizes (3.0 and 3.5 mm) restricted the treatment to main vessel diameters between 2.75 and 3.75 mm. The exclusion of patients with smaller vessels, which are known to be at higher risk of restenosis, might have impacted on the low MACE rates observed in this study. However, it should be noted that, in the majority of large bifurcation trials such as NORDIC13, CACTUS15 or BBC ONE16, a main vessel diameter cut-off of ≥2.5 mm was used.
It is noteworthy that patients who were treated with only an Axxess stent had the lowest MACE rate (5.7% at three years) compared to patients treated with additional stents (13.5% to 33.3%, Table 5). Perhaps this is, in part, due to a selection bias: the less severe lesions were probably seen in patients with less severe coronary artery disease and a less severe bifurcation stenosis. While bare metal stents have more shaft restenosis, the majority of the Axxess restenoses in DIVERGE were present at the edge, similar to balloon-expandable sirolimus-eluting stents26. Possible mechanisms contributing to these edge restenoses are so far unclear, but could be: the absence of neointima inhibition due to the reduced or absent drug elution on the stent edges; incomplete coverage of the lesion; vessel margin injury due to eventual post-dilatation outside the stent boundaries. Further studies are needed to determine the different components responsible for these edge restenoses.
Strong points of the study are the enrolment of a large number of patients with de novo bifurcation lesions in a prospective trial with total and independent monitoring of data acquisition, independent evaluation of the events and excellent three-year complete follow-up data for 98.7% of the patients.
Limitations
The design was non-randomised and operator bias could have influenced the decision of stenting the MV and/or SB. First-generation sirolimus DES (CYPHER) were protocol-mandated for stenting the distal MV and/or SB, which might be inferior to current second-generation DES in terms of the MACE rate. Hence, better clinical outcomes can be expected with the use of second/third generation DES. Angiographic follow-up was performed in only 46% of the 302 patients, which might have influenced the rates of TLR and MACE. Although the Axxess stent provides a feasible and safe solution for bifurcation lesion stenting, larger randomised trials are needed comparing the Axxess system to current treatment strategies.
Conclusions
The Axxess stent is a nitinol self-expanding Biolimus A9 eluting stent dedicated for bifurcation lesions with promising long-term follow-up. The cumulative MACE rate was consistently low with 9.3% events at one year, 14.0% at two years and 16.1% at three years of follow-up, with few ST (2.3%) at three years. The Axxess system offers an acceptable strategy for the treatment of bifurcation lesions.
Funding
This trial was supported by Devax, Inc., Lake Forest, CA, USA, whose assets were acquired by Biosensors International Group.
Conflict of interest statement
M. Bichalska and S. Meis are employees of Biosensors Europe SA. All other authors have no conflicts of interest to declare.

