Abstract
Aims: Our aim was to evaluate the five-year clinical impact of side branch (SB) stenting with a drug-eluting stent (DES) following Axxess stent implantation in coronary bifurcation lesions.
Methods and results: Four hundred patients treated with Axxess were pooled from the AXXESS Plus and DIVERGE five-year follow-up studies. We compared unadjusted and propensity-adjusted major adverse clinical events (MACE) between Axxess with no SB stenting (“Axxess provisional”) versus Axxess with SB stenting (“Axxess additional”). “Axxess additional” had no impact on the MACE rate, with unadjusted and adjusted HR 1.59 (95% CI: 0.95-2.64) and 1.37 (95% CI: 0.88-2.13), respectively. No differences were seen in the individual components of death, myocardial infarction and ischaemia-driven target lesion revascularisation, respectively, both in unadjusted (HR 0.92 [95% CI: 0.38-2.19]; HR 1.73 [95% CI: 0.78-3.82]; HR 1.65 [95% CI: 0.84-3.26]) and adjusted analysis (HR 0.92 [95% CI: 0.41-2.09]; HR 1.13 [95% CI: 0.59-2.17]; HR 1.31 [95% CI: 0.74-2.31]). No differences in definite stent thrombosis were seen with unadjusted HR 2.1 (95% CI: 0.45-9.88) and adjusted HR 1.0 (95% CI: 0.32-3.1).
Conclusions: Stenting the SB following Axxess implantation does not impact on long-term clinical outcomes compared to MV stenting only. The Axxess stent system offers a safe and tailored alternative for the treatment of coronary bifurcation lesions.
Introduction
Current consensus recommends a single stenting strategy of the main vessel (MV) with bail-out side branch (SB) stenting as the percutaneous treatment strategy of choice for bifurcation lesions1. However, this strategy does not apply to all bifurcation lesions, especially when the SB is severely diseased over a long segment. In that case, a double stent technique or dedicated bifurcation device might be the preferred approach. A double stent technique, while being readily available, is technically demanding, time-consuming and sometimes cumbersome. Even if the final result is angiographically perfect, a double stent technique has the micro-anatomical disadvantages of significant strut overlap, crush, distortion or malapposition, all of which might ultimately result in an increased risk of in-stent restenosis, and/or myocardial infarction (MI) driven by stent thrombosis (ST)2. Another downside of the double stent technique is the commitment of having to stent both the main and side branches, even if the distal main branch is unaffected.
Dedicated bifurcation devices might solve these issues. However, most devices are complex in nature with asymmetrical designs, requiring a rigid implantation protocol that is often technically demanding, with, for instance, multiple wires, balloons or stents running through the same delivery catheter. The Axxess™ stent (Biosensors Europe SA, Morges, Switzerland), on the other hand, is a symmetrical self-expanding conically shaped Biolimus A9-eluting stent with a rapid exchange catheter running over a single wire. The device is positioned at the level of the bifurcation carina and flares the ostia of both distal vessels, with no prioritisation of either MV or SB and with no need of stent recrossing, allowing the treatment of both distal branches symmetrically as needed.
Although a double stent strategy using balloon-expandable stents has resulted in mixed results with higher rates of short-term major adverse cardiovascular event (MACE) rates in some3, but not all studies4,5, one recent large meta-analysis of all current major studies of percutaneous treatment of bifurcation lesions showed an increased risk of long-term late ST and MI after a routine double stent strategy compared to a single stent strategy2. Given the advantages of the Axxess stent over conventional balloon-expandable stents in terms of symmetry and equivocal access to both MV and SB resulting in no strut deformation or crush, we compared the five-year long-term impact of additional SB stenting following Axxess implantation in the main vessel in the AXXESS Plus6 and DIVERGE7,8 studies.
Methods
STUDY POPULATION
The data from two independent studies were pooled at patient level for the present analysis. Both the AXXESS Plus and DIVERGE studies are prospective, single-arm, multicentre studies of the treatment of bifurcation lesions with the Axxess stent at clinical sites in Europe, Australia, New Zealand and Brazil. The 139 patients in AXXESS Plus were included from July 2004 to February 2005, and the 302 patients in DIVERGE were enrolled from June 2006 to October 2007. Local ethics committees approved the protocols and all patients gave written informed consent before the procedure. The methods with inclusion and exclusion criteria have been published previously6,7. In brief, patients of 18 to 80 years of age with documented stable angina, unstable angina or positive functional study and a de novo native bifurcation lesion in a major coronary artery in which either the MV or SB had at least a 50% stenosis based on visual estimation were included. The reference vessel diameter had to be 2.75 to 3.75 mm in the MV and ≥2.2 mm in the SB by visual estimate, with a bifurcation angle <70° in DIVERGE.
AXXESS STENT AND PROCEDURE
The Axxess stent system has been described in detail previously9,10. It is a self-expanding, conically shaped nitinol stent with a strut thickness of 160 microns, eluting Biolimus A9, a highly lipophilic, semi-synthetic sirolimus analogue (Biosensors Europe SA). The drug is emulsed into a biodegradable PLA polymer applied primarily to the abluminal stent surface. The Biolimus A9 elution half-life is 21 days, and the PLA biodegradable coating is absorbed after six to nine months. A straight configuration of the Axxess stent was available only in AXXESS Plus6. The stent diameters available were 2.5, 3.0 and 3.5 mm, with lengths of 11, 14 or 20 mm, depending on the study. Not all diameters and lengths were available in both studies. Adjunctive stenting was permitted in the distal MV and SB, at the discretion of the operator. In AXXESS Plus6, the protocol allowed the use of commercially available stents in conjunction with the Axxess stent, while the protocol in DIVERGE7 mandated the use of the CYPHER® sirolimus-eluting stent (Cordis, Johnson & Johnson, Warren, NJ, USA). Aspirin (≥80 mg/day) was given daily and clopidogrel (loading dose ≥300 mg and 75 mg/day thereafter) was administered for at least six months in all patients.
POOLED STUDY POPULATION
Given the similar clinical and angiographic inclusion and exclusion criteria and long-term clinical outcome of both the AXXESS Plus and DIVERGE studies, the two data sets were pooled for analysis. For the sake of uniformity, we excluded the AXXESS Plus patients with the following characteristics: (i) additional stent different from CYPHER (11 patients), (ii) left main lesion (six patients), (iii) patients treated with bare metal stents (three patients), and (iv) those treated with a straight-shaped Axxess stent (18 patients). A total of 35 patients with one or more of these characteristics were excluded. In addition, two patients in AXXESS Plus and four in DIVERGE did not receive any Axxess stent, and were therefore also excluded.
STUDY ENDPOINTS
Patients were evaluated clinically in both studies with annual contacts up to five years after the procedure. All data were submitted to a coordinating centre (Cardiovascular Research Foundation, New York, NY, USA). A data safety and monitoring board (DSMB) periodically reviewed safety data, and all clinical endpoints were adjudicated by an independent clinical events committee (CEC).
The primary endpoint of the present report was the rate of MACE at five years, consisting of any death, myocardial infarction (Q-wave or non-Q-wave), and ischaemia-driven TLR, as previously defined6,7. Differences between the two trials in terms of endpoint definition were adjusted and harmonised for the pooled analysis. Secondary safety endpoints were definite or probable ST at five years, according to the Academic Research Consortium criteria11.
Statistical analysis
Continuous variables are presented as mean±standard deviation and categorical variables are presented as counts and frequencies. All reports of the individual AXXESS Plus and DIVERGE studies are according to the intention-to-treat principle.
Unadjusted analyses were performed using a Cox proportional hazards model. Adjustment for differences in clinical variables was performed using propensity score matching. A propensity score for each patient was obtained from a logistic regression by inverse probability of treatment weights, predicted from variables associated with severity of coronary artery disease and including: age, sex, weight, body mass index, diabetes status, hypertension, hypercholesterolaemia, angina status, smoking status, renal insufficiency at screening, heart failure, peripheral vascular disease, history of coronary artery bypass graft (CABG), history of percutaneous coronary intervention (PCI) and history of MI. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
DEMOGRAPHICS AND LESION CHARACTERISTICS
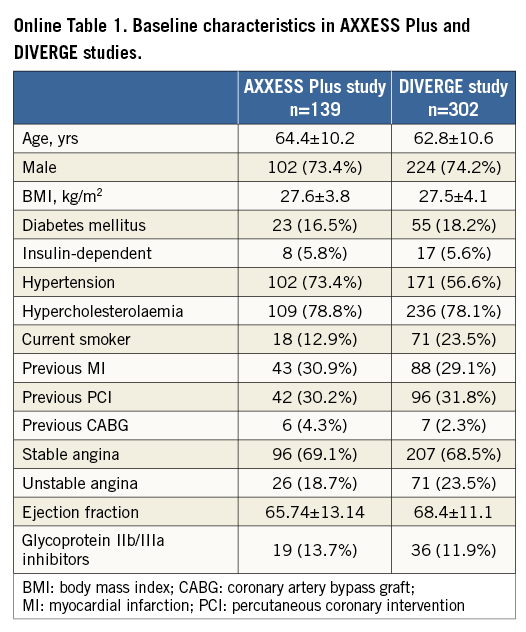
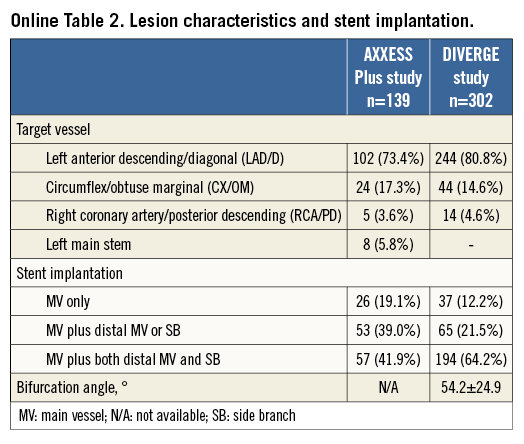
The patient characteristics and locations of stenoses of the complete AXXESS Plus and DIVERGE studies have been published previously6,7 and are summarised in Online Table 1 and Online Table 2.
A total of 102 patients from AXXESS Plus and 298 patients from DIVERGE were pooled for a sample size of 400 patients. Baseline characteristics for the individual studies and for the total group are shown in Table 1. Most of the patients were male (74.9%), with a mean age of 63.1 years, and presence of diabetes mellitus in 16.8%. The majority of the lesions (77.1%) were classified as true bifurcations according to the Medina classification12.
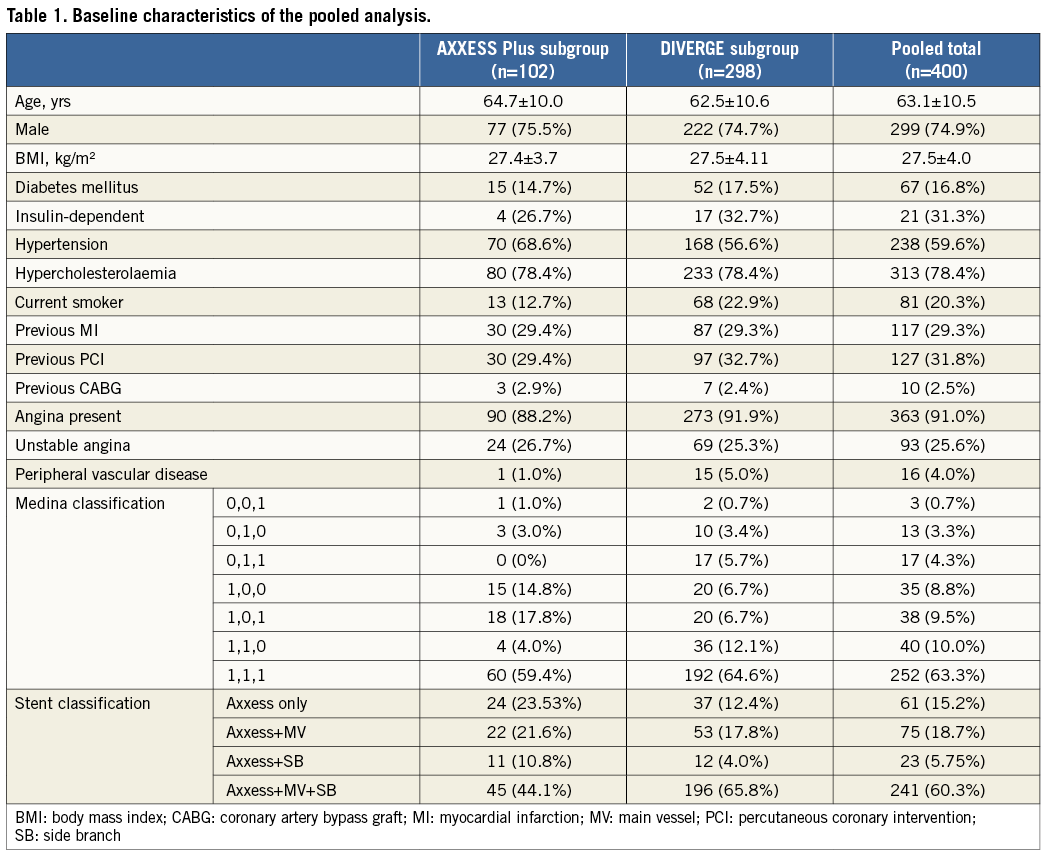
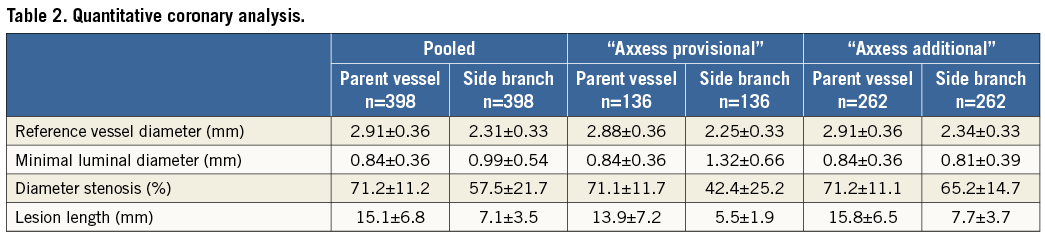
Stenting of the MV only, with either an Axxess stent alone or with an Axxess stent and an additional stent in the distal MV but not in the SB, resembles the provisional approach and was termed “Axxess provisional”. Stenting of the MV with an Axxess stent and an additional stent in the SB, regardless of whether the distal MV was stented or not, resembles the double stent approach and was termed “Axxess additional”. An “Axxess additional” stent implantation was performed in 66.0% of the patients. Lesion characteristics analysed by quantitative coronary analysis (QCA) are shown in Table 2.
LONG-TERM CLINICAL OUTCOME OF IMPACT OF SB STENTING OR “AXXESS ADDITIONAL”
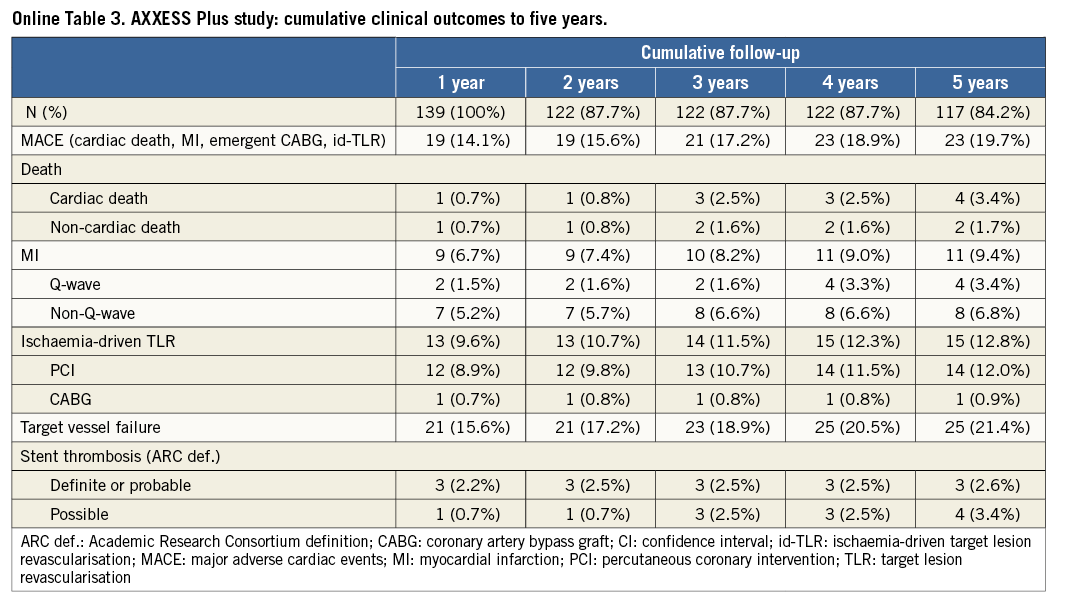
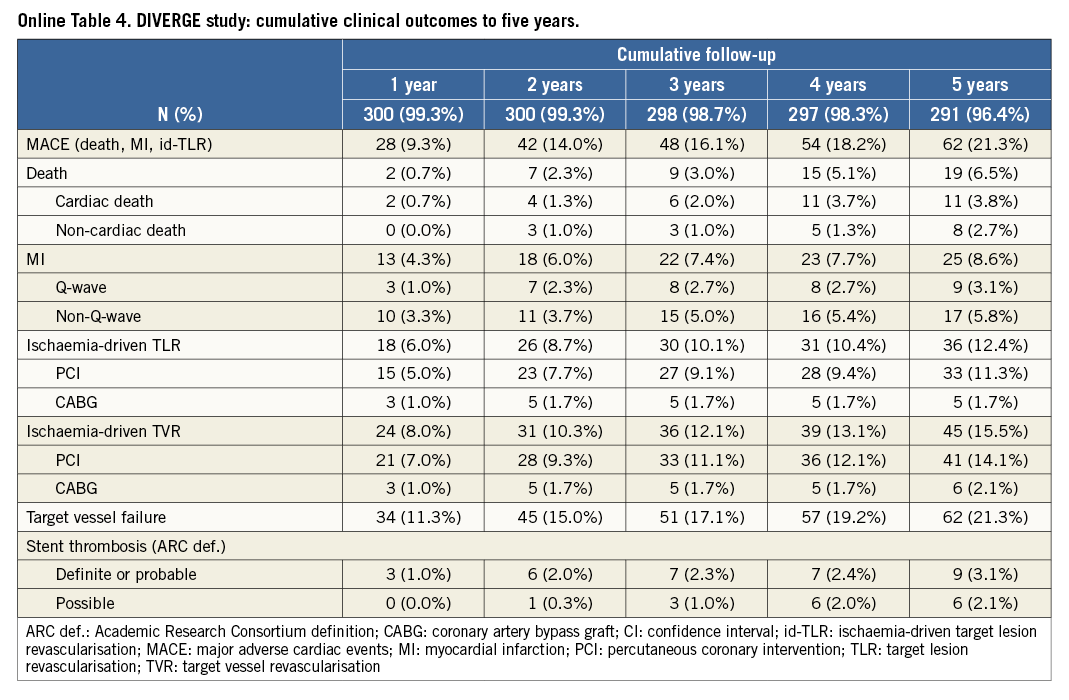
The cumulative long-term clinical outcomes of the complete AXXESS Plus and DIVERGE studies are depicted in Online Table 3 and Online Table 4, respectively. In the pooled analysis, the five-year long-term follow-up was slightly higher for DIVERGE (96.3%) than for AXXESS Plus (86.3%), with an overall five-year follow-up available for 93.7% of the pooled patients. As reported in Table 3, outcome after “Axxess additional” was similar to “Axxess provisional”. Although the rates of MACE and some of its individual components were numerically lower in the “Axxess provisional” group compared to the “Axxess additional” group, none of the differences was statistically significant, both in the unadjusted and propensity analysis, as reported in Table 3. Adjusted Kaplan-Meier curves for both “Axxess provisional” and “Axxess additional” groups are shown in Figure 1. In terms of stent thrombosis, no significant differences were seen, as shown in Table 3. A representative case of “Axxess provisional” with medium-term angiographic follow-up is shown in Figure 2.
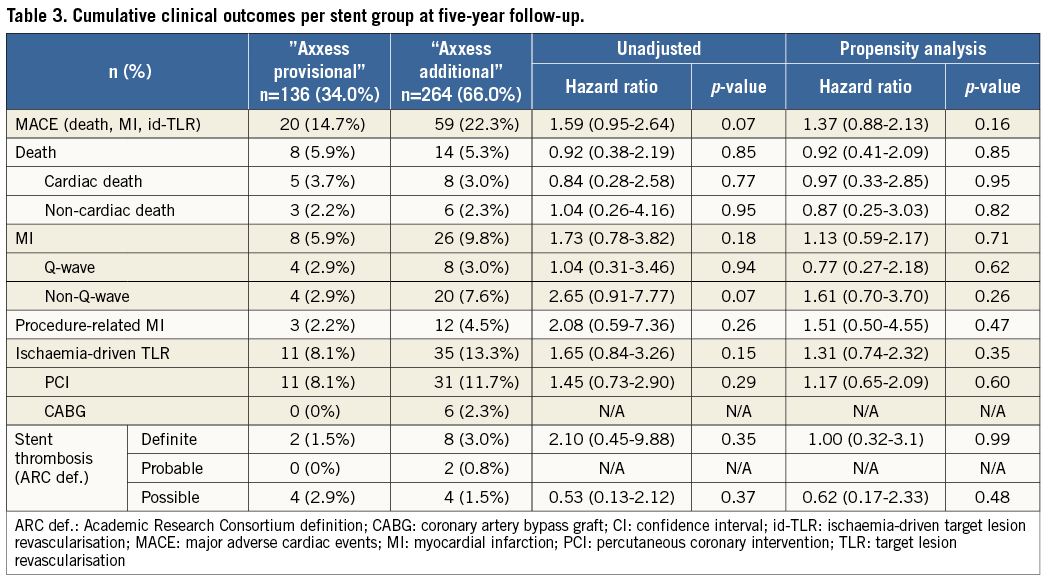
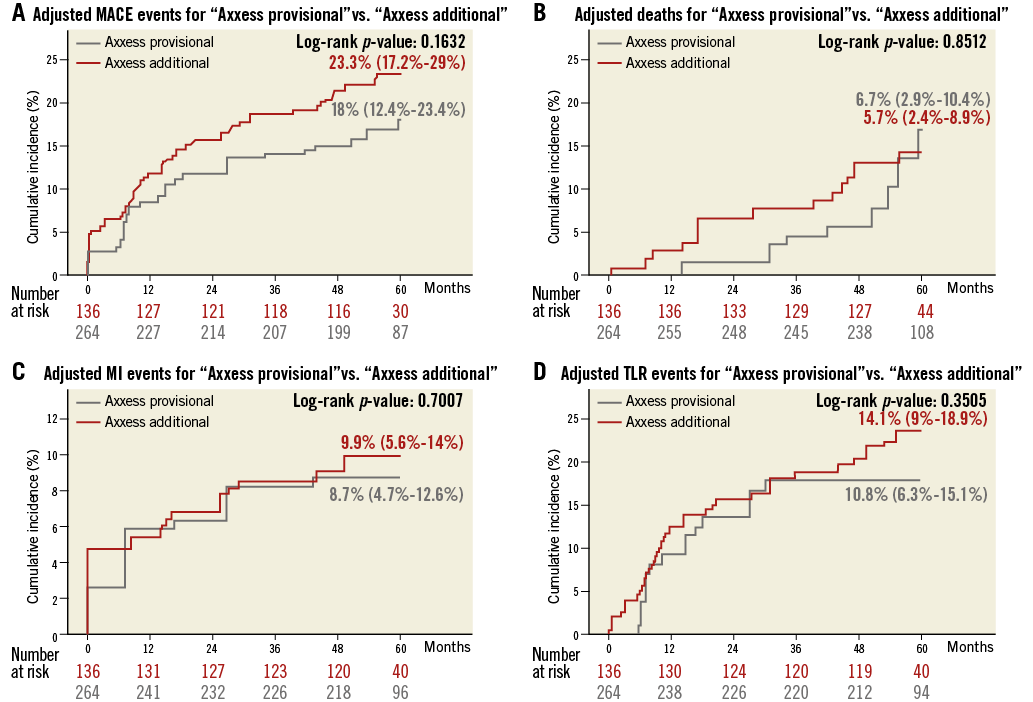
Figure 1. Adjusted Kaplan-Meier curves for “Axxess provisional” versus “Axxess additional” over five years. A) Total MACE. B) Death. C) MI. D) Ischaemia-driven (id)-TLR.
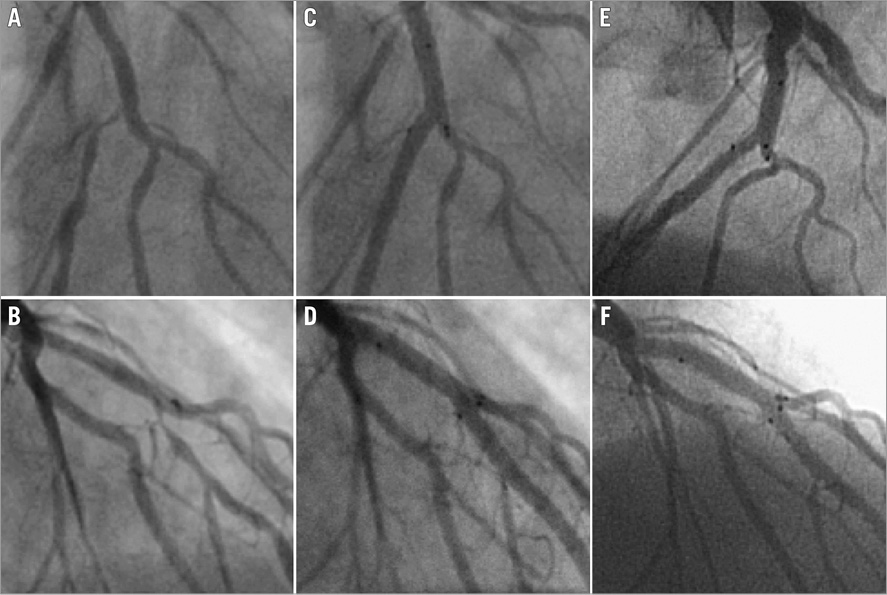
Figure 2. Case example after “Axxess provisional” stent implantation. Sequential coronary angiogram magnifications of a 53-year-old active smoker with dyslipidaemia and presenting with angina due to a severe stenosis of the left anterior descending artery at the bifurcation with the first diagonal branch. The patient was successfully treated with the Axxess stent and an additional CYPHER stent distal in the MV. At 19 months post procedure, he presented with unstable angina. Coronary angiography showed no in-stent restenosis or ST. Diffuse coronary spasms were noted during angiography, which responded to intracoronary nitrates. He was diagnosed with variant angina and treated with calcium antagonists and nitrates. A) & B) Baseline angiograms after intracoronary nitrates showing a severe stenosis of the left anterior descending artery, starting at the bifurcation with the first diagonal branch (Medina 0,1,0). C) & D) Final angiographic results after PCI with Axxess stent (3.0×14 mm), and additional CYPHER stents distal in the LAD (2.75×28 mm). E) & F) Angiographic results at 19 months post procedure showing patent stents with no sign of restenosis or thrombosis. Panels A, C and E are in LAO-cranial projections and lower panels B, D and F are in RAO-cranial projections.
Discussion
The present analysis reports the absence of clinical impact of SB stenting following Axxess stent implantation (or “Axxess additional”) on the five-year long-term clinical outcome when compared to a provisional-like strategy (or “Axxess provisional”) in the percutaneous treatment of de novo coronary bifurcation lesions. Both unadjusted and propensity-adjusted comparison between the two groups in a total of 400 patients pooled from two prospective studies, AXXESS Plus6 and DIVERGE7,8, found no significant differences in terms of MACE and its individual components of death, MI and ischaemia-driven TLR.
PCI of bifurcation lesions with balloon-expandable stents often challenges the operator with the dilemma as to whether to use a single stent or a double stent strategy. While the provisional technique is the preferred strategy whenever possible, bail-out stenting of the SB is sometimes needed, certainly in case of reduced or occluded flow in a large SB following MV stenting. This step is often time-consuming and technically demanding. Recrossing the stent towards the SB, preferably through the most distal cell, can be challenging and sometimes even impossible, while requiring extra resources in terms of wires and balloons to pass and dilate the struts. Another potential problem of this bail-out double stent approach is that not all double stent strategies are applicable once the MV has been stented first. The crush and mini-crush techniques, for instance, can no longer be applied and have to be replaced by an internal crush which leaves crushed struts intraluminally instead of abluminally13.
In contrast to this is the simple and tailored approach with the Axxess strategy. The advantage is that the same technique is used to tackle any bifurcation lesion, with no upfront commitment to additional SB or main branch stenting, and with no prioritisation of any distal branch. The Axxess stent is always deployed first in the parent vessel and will span the carina and flare the ostia of both distal branches. There is no carina shift as in a conventional provisional stenting. Following the Axxess deployment, additional stents can be implanted in any or both distal branches when deemed necessary. There is no need to recross or dilate any struts, and there is no commitment to additional steps once the results are acceptable. This stepwise approach is predictable, with no need for often challenging strut recrossing and ballooning, and results in good short-7, medium-8 and now long-term clinical outcome, as well as on intravascular ultrasound (IVUS) analysis14, as published previously.
While our current long-term clinical outcome results are in line with previously reported randomised comparisons between single and double stent strategies, which reported no clear clinical benefit or detriment from additional SB stenting over provisional stenting4,5, it should be noted that few bifurcation studies have published five-year outcome data so far. The present manuscript is the first to report five-year follow-up data of a dedicated bifurcation device, whereas for the balloon-expandable DES only the NORDIC Bifurcation Study has reported five-year follow-up15. The long-term results in NORDIC, with a MACE rate of 18.3% in the optional SB stenting group and 28.2% in the MV and SB stenting group (p=0.03), were higher than those reported here, with adjusted Kaplan-Meier estimates of 18.0% and 23.3%, respectively. It is worth noting, however, that procedure-related MI were excluded in the NORDIC combined endpoint, while they were not in AXXESS Plus and DIVERGE and in the pooled analysis. When excluding periprocedural MI in our data, we found even lower numbers with a MACE rate of 17.0% in the “Axxess provisional” group and 19.9% in the “Axxess additional” group. Hence, the present report confirms that the easiness of the Axxess stent and its tailored strategy with regard to the additional stenting of the distal vessels also results in excellent long-term clinical outcome with no impact of additional stenting.
The same holds true for stent thrombosis. A recent meta-analysis of randomised and observational studies with a total of 6,961 patients reported that an SB stent strategy was associated with an increased risk of definite stent thrombosis and MI, with, respectively, RR of 2.31 (95% CI: 1.33-4.03) and 1.86 (95% CI: 1.34-2.60)2. However, we found no differences in definite ST after SB stenting, with adjusted HR 1.0 (95% CI: 0.32-3.1) for “Axxess additional” compared to “Axxess provisional”. The Axxess system therefore seems to be safe, again with no impact of SB stenting.
The size of the SB has recently become of interest as a selection parameter for a two-stent approach. It could be argued that we found no differences between the “Axxess provisional” and “Axxess additional” groups because of too small SB reference diameters. While the majority of the earlier bifurcation trials such as CACTUS, BBC and the NORDIC trials found no major differences between a one- and a two-stent strategy in terms of target vessel failure16, the more recent DKCRUSH-II trial showed that the complex DK-crush technique had less need for revascularisation inside the side branches compared to provisional stenting17. These favourable results were attributed to the enrolment of patients with bifurcation lesions with larger visually estimated SB size of ≥2.5 mm. However, mean RVD of the SB in DKCRUSH-II17, 2.29±0.35 mm in one-stent and 2.38±0.32 mm in two-stent, is not larger than other trials (e.g., in NORDIC4, 2.24±0.46 vs. 2.28±0.51 mm) or in our present study (2.31±0.33 mm). Similarly, selection criteria for inclusion in the recent Tryton IDE trial were bifurcation lesions with visually estimated SB size of ≥2.5 mm, while the mean SB size of the effectively included lesions was 2.21±0.33 mm in the one-stent group vs. 2.25±0.30 mm in the two-stent group18. Our observed signal cannot therefore be explained by the selection of bifurcation lesions with too small SB.
Many dedicated bifurcation stents have been developed so far18-20. However, none has been widely adopted in the cathlab as yet. On a general level, most dedicated devices are limited to particular types of bifurcations, and do not adhere to the “one device fits all” principle. In addition, the majority of the dedicated devices are hampered by a complex and asymmetrical design, implicating a more challenging positioning, resulting in a higher operator skills requirement and a lower success rate. Also, a commitment to stent both branches is often made even if not warranted, with some devices only being available in a bare metal version. None of these is applicable to the Axxess stent. It is by nature a single and symmetrical DES, which is implanted on a single wire positioned either in the MV or SB. It can be used for all bifurcation types, with the only limitation being a bifurcation angle of 70° or less. While both distal branches can be treated with additional stents if needed, there is no commitment to doing so if not necessary. Hence, we believe the Axxess stent system offers a flexible alternative for bifurcation stenting.
Study limitations
The strong points of the studies are the prospective, very long-term follow-up, with independent adjudication of the clinical events, and periodic review of the data by an independent data safety monitoring board. The limitations, however, are the non-randomised and non-“all-comers” design, as well as the exclusion of patients treated with BMS or non-commercially available Axxess stents, which implies a degree of selection bias. For instance, patients requiring additional SB stenting might represent a patient subset with a higher degree of disease burden, and the non-balanced groups might increase the number of events in the larger group. Furthermore, the use of the Axxess system required implantation of two stents in 75% of the bifurcation lesions. Although both the AXXESS Plus and DIVERGE studies have a comparable study protocol and patient population, a certain amount of heterogeneity is inherent. In addition, while propensity score matching can correct for potential confounders, it cannot eliminate them completely: this analysis is hence inferior to a randomised trial. Finally, given the relatively small sample size, the present study is underpowered and should be considered as hypothesis-generating.
Conclusions
The treatment of bifurcation lesions with SB stenting following Axxess stent implantation in the MV does not impact on the five-year long-term clinical outcome in terms of MACE, or in terms of ST. The Axxess stent system therefore offers a convincing alternative for the tailored treatment of coronary bifurcation lesions.
| Impact on daily practice Both simple and complex coronary artery bifurcations can be treated with the Axxess stent followed by additional drug-eluting stents in one or both distal branches. The present study showed that five-year long-term clinical outcomes are good, with similar MACE rates compared to randomised bifurcation trials with long-term follow-up. Stenting the side branch following Axxess implantation (resembling a double stent technique) did not impact on the long-term clinical outcomes when compared to main vessel stenting only (resembling provisional stenting). A complete reconstruction of the bifurcation can therefore be achieved, with minimal stent overlap or distortion, and with good long-term clinical outcome. |
Acknowledgements
The contributions of Samuel Copt (statistical analyses) and of Susanne Meis (careful review of the paper), both full-time employees at Biosensors, are gratefully acknowledged.
Funding
The study was supported by Biosensors International, Morges, Switzerland.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data