Abstract
Aims: To demonstrate the acute and early outcomes of the novel Nile PAX dedicated polymer-free paclitaxel-coated stents (Minvasys SAS, Gennevilliers, France) in the treatment of de novo coronary bifurcation lesions.
Methods and results: The Nile PAX device incorporates a cobalt-chromium alloy with a side aperture in the mid-stent designed to optimise scaffold at the bifurcation carina and side branch (SB) ostium, while maintaining SB access during procedure. From December 2008 to February 2010, 101 patients were prospectively enrolled in a non-randomised, multicentre study. Lesion criteria were: vessel size 2.5-3.5 mm in the parent vessel (PV) and 2.0-3.0 mm in the SB, and lesion length <14 mm in the PV, and <5 mm in the SB. Mean age was 63 years, 29% had diabetes, LAD/Dg was involved in 80.4%, and 61.7% had significant involvement of both branches. The study stent was successfully attempted and implanted in 98%. SB received additional stent in 26%; final kissing-balloon inflation was performed in 93%; and lesion (angiographic) success was achieved in 98%. There was only one non-Q myocardial infarction during hospitalisation, and no additional events up to 30 days.
Conclusions: Preliminary results of the prospective, non-randomised, multicentre BIPAX clinical trial demonstrated encouraging results with the novel Nile PAX bifurcation DES in the treatment of coronary bifurcation lesions, including high device and procedural success. Overall, there was only one major adverse cardiac event during hospitalisation, with no additional events up to 30 days follow-up. Long-term follow-up is warranted.
Introduction
Percutaneous coronary intervention (PCI) in bifurcation lesions remains challenging, especially because of problems occurring in the side branch (SB) during procedure, including poor access and transient or sustained complications (vessel closure, dissection, impaired flow, and significant residual stenosis).1 Furthermore, relatively high rates of recurrences have been reported in bifurcations, even with drug-eluting stents (DES), given that most of restenosis is found at the SB ostial location.2 Results from several randomised DES trials including selected lesions suggested that a simplified technical approach with parent vessel (PV) stenting plus provisional SB stenting (“provisional technique”) should be preferable for most bifurcation lesions;3-5 even though more complex subsets may require a complex approach with double stenting so as to achieve an adequate result in the SB.6-8 Such outcomes may be explained by the wide variability of lesion complexity found in bifurcations.9,10 Moreover, because of the asymmetric carina shaping, and diverse anatomical/morphological features, current bifurcation PCI techniques with conventional (straight) stents may be laborious and limited by the degree of complexity encountered.1,11
The novel Nile PAX (Minvasys SAS, Gennevilliers, France) dedicated bifurcation DES system incorporates: a low-profile cobalt-chromium alloy with a side aperture in the mid-stent designed to optimise scaffold at the bifurcation carina and SB ostium, and a polymer-free paclitaxel coating (PAX technology). In general, the Nile device concept focuses on simplifying the procedure with a provisional approach, offering continuous SB access throughout the procedure that allows further treatment of the SB if needed.12,13
Our objective was to report the procedural and 30-day outcomes of a series of patients with coronary bifurcation disease treated with the novel Nile PAX dedicated DES device.
Methods
STUDY DESIGN AND PATIENT POPULATION
The BIPAX clinical trial was a prospective, non-randomised, multicentre study designed to evaluate the safety and efficacy of the novel Nile PAX dedicated bifurcation DES system for the treatment of patients with de novo coronary bifurcation lesions. Key inclusion criteria were: ≥18 years of age; clinical evidence of ischaemic heart disease including stable or unstable angina, or silent ischaemia with a positive function test; native target vessel with a singled de novo bifurcation lesion; vessel size 2.5-3.5 mm in the PV and 2.0-3.0 mm in the SB; lesion length ≤14 mm in the PV with maximum extension of 7 mm proximally and/or distally to the bifurcation carina to allow full lesion coverage (≥2 mm) with the study device, and lesion length ≤5 mm in the SB; bifurcation lesion morphology including all Medina types, except 0,0,1;14 and agreement to undergo all protocol scheduled follow-up (FU) evaluations including angiographic FU. Key exclusion criteria were acute myocardial infarction (MI) within 72 hours of the index procedure; baseline serum creatinine level >2.0 mg/dL; PCI of the target vessel within nine months prior to index procedure; left main (LM) location; heavy calcification; severe tortuosity of the segment proximal to the bifurcation; thrombus; total occlusion; and in-stent restenosis. A complete list of clinical and angiographic inclusion and exclusion criteria is provided in Appendix A.
All patients provided written informed consent prior to enrolment and procedure. The study complied with the Declaration of Helsinki regarding investigation in humans, and was approved by the local Ethics Committee at each participating clinical institution. In addition, the BIPAX clinical trial was registered at the United States National Institute of Health website (www.clinicaltrials.gov: NCT01308229).
DEVICE DESCRIPTION
The dedicated bifurcation Nile PAX DES system encompasses the CE (Conformité Européenne) mark approved Nile Croco uncoated platform. The design and specifics of the Nile CroCo stent had been previously described elsewhere.12,13 Briefly, it is a low-profile (73 μm) open cell cobalt-chromium L605 alloy, 6 Fr compatible system (inner diameter ≥0.070”), that incorporates an unique 3-segment modular design with a side aperture located in the middle of the stent for optimal conformability, coverage, and scaffold at the bifurcation carina and SB ostium (Figure 1A). By design, the Nile stent offers no restrictions with regard to PV-SB angles as measured both proximally and distally to the carina. Furthermore, the stent platform is pre-mounted on a dedicated delivery system with two independent balloon catheters, each with a rapid exchange lumen for independent use of PV and SB 0.0014” guidewires during procedure. Therefore, after implantation of the dedicated stent in the PV, the system allows full access for immediate performance of SB dilatation and/or final kissing-balloon (FKB) inflation with a dedicated SB balloon designed with a conical shape on its proximal end for optimal conformability. Specifically, the SB balloon is already engaged in the delivery system; consequently, avoiding the need for wire re-crossing. Such feature also gives the possibility to easily place an additional stent at the origin of the SB if needed without protrusion in the PV. The Nile PAX device incorporates the PAX technology, in which pure paclitaxel is applied onto the abluminal surface of the stent through microdrop spray crystallisation process. This process adds only 5 µm to the strut thickness and secures drug integrity, providing a controlled release profile. The drug concentration is 0.67 µg/cm2, and the elution kinetics includes a burst release of 33-52% of the drug in the first eight hours. In one week, about 55-75% of the drug is expected to be released, and in 45 days, all paclitaxel is expected to be released from the stent platform, leaving behind only a bare metal stent (BMS).
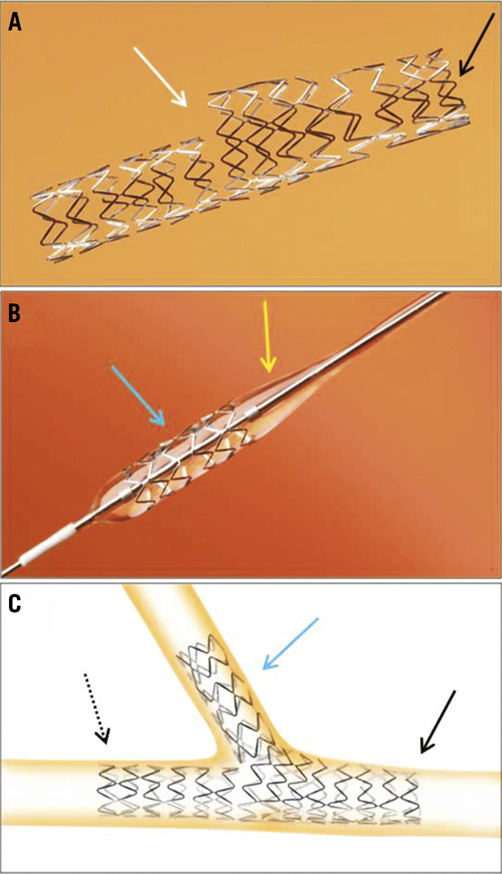
Figure 1. A) The Nile Croco dedicated bifurcation stent platform with SB aperture (white arrow) in the middle of the stent; black arrow indicates the proximal end of the stent. B) The Nile Delta stent (blue arrow) with a dedicated balloon with conical shape at its proximal end (yellow arrow) to optimise scaffolding and conformability at the bifurcation carina and SB ostium; C) Illustration of a double stenting approach with the Nile Croco stent in the PV (black solid arrow indicates proximal end of the stent; black dashed arrow indicates distal end of the stent), and the Nile Delta stent in the SB (blue arrow).
PROCEDURE
Overall, PCI procedure was recommended according to current standard guidelines.15 For bifurcation PCI with the study device, both branches (PV and SB) were initially wired, followed by mandatory predilatation of the PV with a balloon approximately 0.5 mm smaller than the reference diameter (RD) of the distal vessel. SB predilatation was performed according to operator’s discretion. The study device was sized according to the distal RD of the PV, and, for deployment, was gently advanced and positioned at the lesion site. In the event of resistance owing to wire tangling, it was recommended to gently remove one of the wires (SB or PV wire) into the balloon and re-advance it in the vessel distally. The two markers at the proximal and distal ends of the stent, and specially the marker located in the mid-part of the stent (at the level of the SB aperture), were used to accurately position the study device at the target location. After stent implantation in the PV, final kissing-balloon (FKB) inflation was performed without wire re-crossing into the SB. Importantly, single balloon post-dilatation in the PV and/or SB prior to FKB was allowed at operator’s discretion. The study device was available in a length of 18 mm, and in the following set of diameters: 2.5/2.0 mm, 3.0/2.0 mm, 3.0/2.5 mm, 3.50/2.5 mm and 3.5/3.0 mm for the PV and SB balloon, respectively. In case of suboptimal angiographic result in the SB (significant residual stenosis, dissection, TIMI flow <3), an additional straight stent could be deployed. In that case, it was recommended to implant the Delta PAX device, which consists of a tubular stent (Nile Delta stent platform) with a non-polymeric paclitaxel coating available in a length of 8 mm, for 2.5 mm or 3.0 mm diameters (Figure 1B). Hence, the Nile PAX system in combination with the Delta PAX stent allowed a modular treatment of bifurcation lesions, including double stenting approach if needed (Figure 1C). Of note is the fact that during the index procedure, the use of a device other than a standard balloon was not allowed prior to stent placement (including but not limited to cutting-balloon, drug-eluting balloon, atherectomy devices, laser, thrombectomy, etc.). By protocol, PCI procedure in non-bifurcated (non-target) lesion located in non-target vessel prior to target lesion PCI was allowed.
Regarding the antithrombotic regimen, aspirin (100-200 mg daily) was recommended prior to the procedure and prescribed indefinitely after hospital discharge. For thienopyridine (clopidogrel), a loading dose of 300 mg was given at least 24 hours prior to procedure, unless already taken. If <24 hours, a loading dose of 600 mg was given at least 2 hours prior to procedure. At post-procedure, clopidogrel therapy with 75 mg daily was prescribed for a minimum of 12 months. During procedure, intravenous heparin was recommended to maintain an activated clotting time >250 seconds (>200 seconds if glycoprotein IIb/IIIa receptor inhibitors were used). The decision to use and the type of glycoprotein IIb/IIIa receptor inhibitor was left at the operator’s discretion.
A routine 12-lead electrocardiogram was obtained at three time points: before procedure, immediately afterwards and 24 hours later. Blood sample laboratory analysis included CK and CK-MB before procedure (<24 hours), 18-24 hours after, and daily thereafter until hospital discharge.
ENDPOINTS, DEFINITIONS AND FU
The study’s primary efficacy endpoint was angiographic binary restenosis in the PV and SB at nine months FU, as determined by independent quantitative coronary angiography (QCA) analysis. Secondary endpoints included: target vessel failure (TVF), clinically-driven target lesion revascularisation (TLR) and clinically-driven target vessel revascularisation (TVR) at nine months FU; also, acute device, lesion, and procedural success; in-stent late lumen loss (along with other angiographic parameters) at nine months FU, as determined by independent QCA analysis; and major adverse cardiac events (MACE) at nine months FU. TVF was defined as the combined endpoint of cardiac death, MI, and clinically-driven TVR. MACE was defined as the combined endpoint of all cause death, MI, emergent CABG and TLR. MI was defined according to the definitions of the European Society of Cardiology guidelines.16 Stent thrombosis (ST) was defined according to the propositions of the Academic Research Consortium (ARC).17 Lesion (or angiographic) success was defined as attainment of <50% residual stenosis in the target lesion (both PV and SB) with any PCI method. Procedural success included lesion success without the occurrence of MACE during index hospitalisation. A complete list of the endpoint definitions is provided in Appendix B.
Clinical FU was scheduled at one, six and nine months, and annually up to five years FU. All patients were assigned for angiographic re-evaluation at nine months FU. The study was coordinated and managed by an independent Data Management and Coordinating Centre (Cardiovascular Research Center, São Paulo, Brazil). Independent electronic monitoring was performed in all patients, and adverse events were adjudicated by an independent Clinical Events Committee. In the current analysis, we report the procedural and 30 day outcomes of the BIPAX Clinical Trial.
ANGIOGRAPHIC ANALYSIS
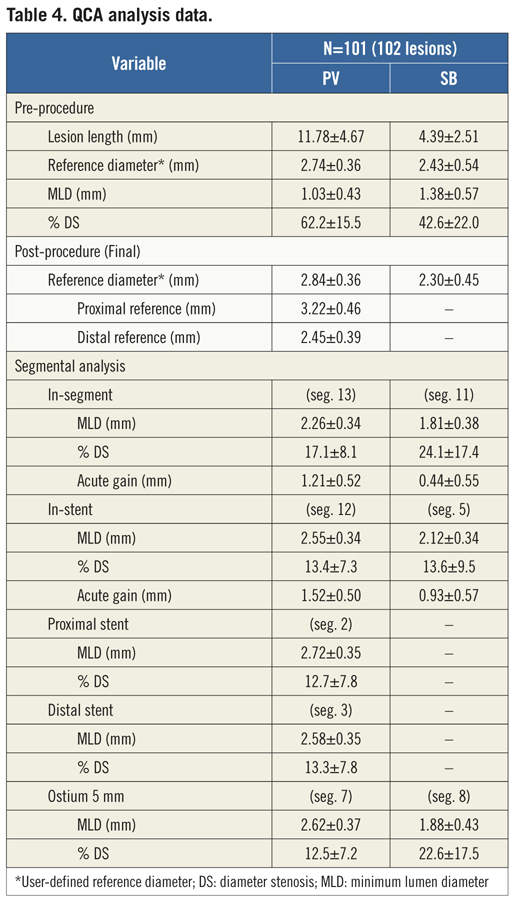
Serial coronary angiographic studies were obtained at baseline (pre-procedure) and final (post-procedure) after intracoronary administration of nitrate (recommended 100-200 μg, unless clinically contraindicated) in two orthogonal matching views with optimal visualisation of the bifurcation carina, specially the SB ostium. The angiographic analysis at all study time-points followed the recommendations of the Consensus Statement from the European Bifurcation Angiographic Sub-Committee.18 All angiographic analysis were performed off-line by experienced operators at an independent Angiographic Core Laboratory (Cardiovascular Research Center, São Paulo, Brazil) with a validated dedicated bifurcation 2D software for QCA analysis (QAngio XA version 7.2, Medis, Leiden, the Netherlands).19 Figure 2 illustrates the method for subsegmental QCA analysis. The contrast-filled non-tapered guiding catheter tip was used for calibration. The minimum lumen diameter (MLD) and the mean RD, obtained from averaging 5 mm segments proximal and distal to the target lesion location (distal reference only for the SB), were used to calculate the diameter stenosis (DS = [1–MLD/RD]×100). When performing subsegmental QCA analysis, the interpolated reference was considered at each analysed segment. Acute gain was the change in MLD from baseline to post-stent implantation. Overall, QCA measurements were reported: “in-stent” within the stented segment; and “in-segment”, spanning the stented segment plus the 5 mm proximal and distal peri-stent areas.
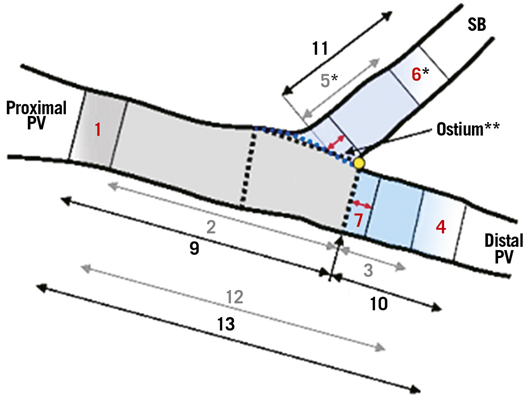
0 - bifurcation core
1 - proximal edge (5 mm) of the PV stent
2 - stented segment (in-stent analysis) of the proximal PV
3 - stented segment (in-stent analysis) of the distal PV
4 - distal edge (5 mm) of the PV stent
5 - stented segment (in-stent analysis) of the SB*
6 - distal edge (5 mm) of the SB stent*
7 - ostium (5 mm) of the distal PV
8 - ostium (5 mm) of the SB*
9 - in-segment analysis of the proximal PV
10 - in-segment analysis of the distal PV
11 - in-segment analysis of the SB
12 - overall stented segment (in-stent analysis) of the PV
13 - overall in-segment analysis of the PV
* If stent implanted in the SB
Figure 2. Illustration of segmental QCA analysis method for coronary bifurcation lesions.18,19 PV: parent vessel; SB: side branch.
STATISTICAL ANALYSIS
Categorical data were presented as frequencies of the overall counts, and continuous variables were presented as mean ±standard deviation (SD). Categorical variables were compared with the Fisher’s exact test or Chi-square test when appropriate, and continuous variables were compared with means of the Student t test (paired and unpaired according to the compared data). A two-tailed p value <0.05 was considered statistically significant.
Results
BASELINE CHARACTERISTICS
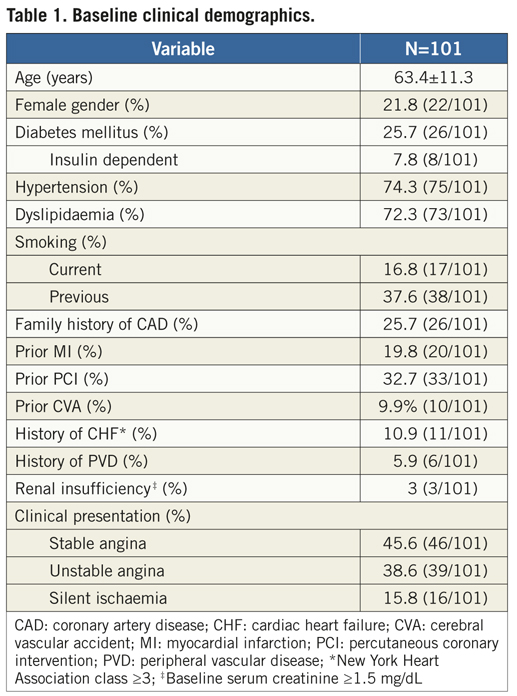
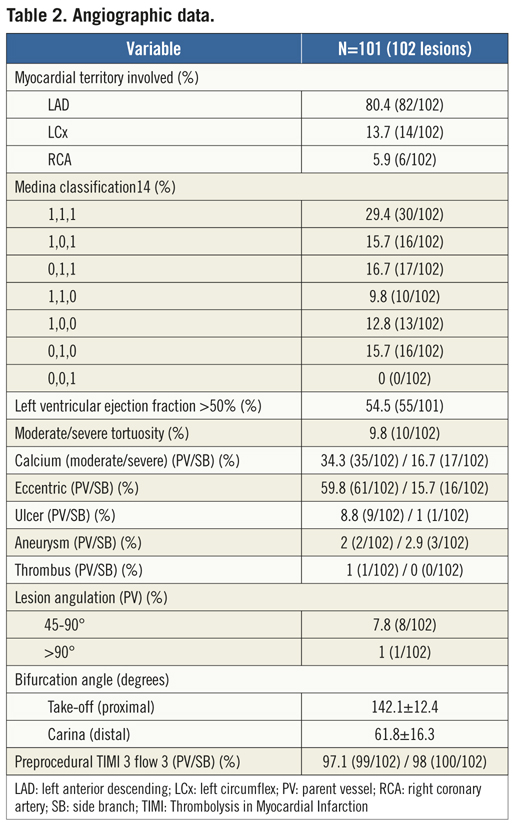
A total of 101 patients were enrolled from December 2008 to February 2010 at the 10 participating clinical sites in Europe (France, Spain, Bulgaria, the Netherlands, Italy, Poland) and South America (Brazil). Overall, mean age was 63.4 years, 25.7% were diabetics, 19.8% had previous MI, and about one third of patients had undergone prior PCI (Table 1). The majority of lesions was located in LAD/diagonal bifurcations and involved the proximal and mid segments of the coronary bed in 50% and 43.1%, respectively. Of the 102 lesions (one patient had two bifurcations treated with the study device at index procedure), 99% were de novo stenosis (there was one in-stent restenosis post-BMS), 61.8% had significant involvement of both branches (“true” bifurcation lesions with Medina types 1,1,1,; 1,0,1, and 0,1,1), and moderate/severe tortuosity of the proximal segment to the bifurcation site was found in 9.8%. Table 2 depicts the baseline angiographic data including complex morphological characteristics in the PV and SB.
PROCEDURAL DATA AND ANGIOGRAPHIC RESULTS
During the procedure (Table 3), radial arterial access was used on a significant proportion of patients (42.6%), given that all procedures were performed with size 6 Fr guiding catheters. Predilatation was performed in most lesions in the PV and in 35.3% in the SB. Noteworthy is the fact that wire tangling was evidenced in 42 cases (41.1%), yet was solved in the majority of attempts (40/42). Consequently, the Nile PAX device was successfully implanted at the lesion site in all but two cases (98%), with a mean deployment pressure of 12.7±3.5 atm. Figure 3 shows a case treated with the Nile PAX dedicated system with provisional approach. In 26 cases (25.5%), an additional stent was implanted in the SB in order to optimise the angiographic result. Final TIMI 3 flow was achieved in 100% in both branches, and overall lesion success was 98% (two lesions had significant residual stenosis in the SB). Bifurcation angles measured at baseline vs. final were not significantly different, including take-off angle (143.1±12.4o vs. 151.0±11.5o, p=0.23) and carina angle (61.8±16.3 vs. 51.5±15.7, p=0.35). At pre-procedure, mean lesion length was 11.78±4.67 mm in the PV, and 4.39±2.51 mm in the SB. Baseline and final subsegmental QCA analysis is shown in Table 4.
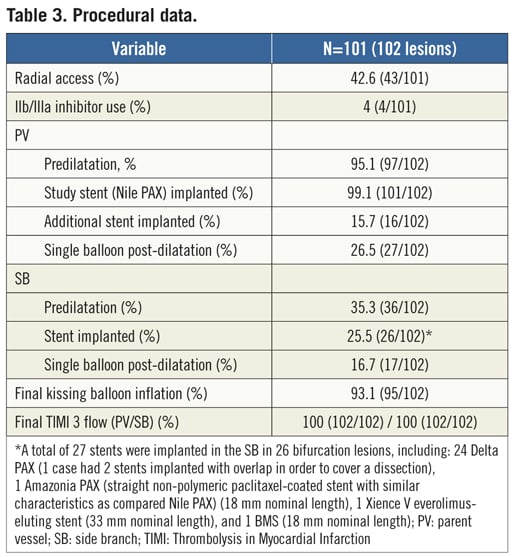
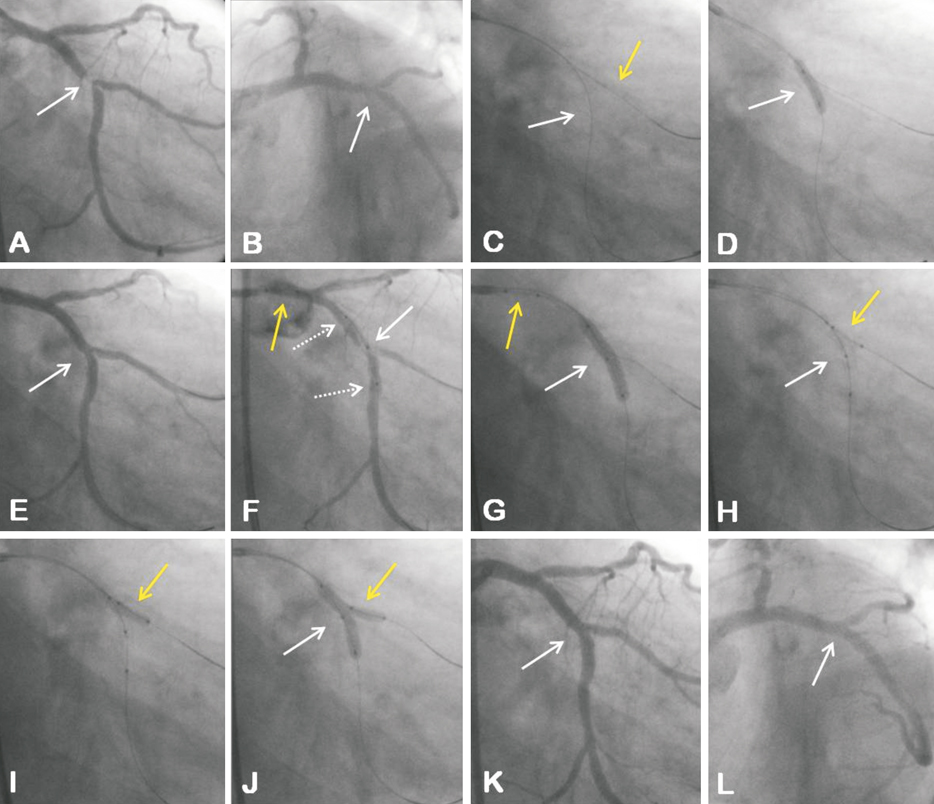
Figure 3. Coronary bifurcation lesion (LCx/OM) with significant involvement of the SB ostium (Medina type 1,1,1) visualised in the RAO caudal view (A) and LAO caudal view (B); C) Both branches of the bifurcation wired (PV white arrow; SB yellow arrow); D) PV predilatation; E) Angiographic result after PV predilatation; F) The Nile Croco dedicated stent positioned at the bifurcation site: white solid arrow indicates optimal positioning of the stent with marker in the middle of the stent (SB aperture) located at the level of the SB take-off; white dashed arrows indicate markers at the proximal and distal ends of the stent; yellow arrow indicates SB balloon with proximal and distal markers, which is already engaged in the delivery system; G) Stent implantation (white arrow); yellow arrow indicates SB balloon which is already engaged in the delivery system; H) SB balloon advanced and positioned at the bifurcation site (yellow arrow) without need for wire re-crossing; white arrow indicates PV balloon positioned within the stent implanted; I) Single SB (post)-dilatation; J) Final kissing-balloon inflation; K and L) Final angiographic result with optimal SB scaffold visualised in the RAO and LAO caudal views, respectively.
IN-HOSPITAL AND 30-DAY CLINICAL OUTCOMES
During index hospitalisation, there was only one non-Q MI occurring post-index procedure and no additional MACE up to discharge; therefore, procedural success was 97% (98/101). At 30 days, there were neither cardiac deaths/MIs nor stent thrombosis (cumulative MACE rate was 1%), and one non-cardiac death in a patient with several comorbidities who was successfully treated with the study device, but presented with infection at post-procedure that evolved to sepsis and renal failure, followed by non-cardiac death on day 12 post-index procedure.
Discussion
In the current analysis, the Nile PAX dedicated device demonstrated encouraging results in coronary bifurcation lesions, including high procedural success (97%) and safety up to 30 days FU. Such outcomes confirmed the acute efficacy of the Nile technology for the treatment of bifurcation lesions. A recent study by del Blanco et al reported the procedural and 6-month clinical outcomes in 151 real world patients/lesions (acute MI in 36%) treated with the uncoated Nile Croco stent platform at one clinical institution. In this study, 64.9% of lesions involved LAD/diagonal (2.6% LM location), 71.5% were classified as true bifurcation lesions, 21.6% had significant calcification and baseline mean lesion length and RD were 10.76±5.63 mm and 2.73±0.53 mm in the PV, and 3.68±2.59 mm and 2.15±0.53 mm in the SB, respectively. At final procedure, angiographic success was 96%; also, the in-hospital MACE rate was 1.3% including one non-Q MI and one cardiac death. At six months clinical FU (91.4%), cumulative MACE rate was 13.9% including 3.6% cardiac death, 2.2% MI, and 7.3% TLR.13 In BIPAX (102 lesions), patients with acute MI were excluded; however, a similar lesion profile was found including target vessel located in LAD/diagonal in 80.4% (LM excluded), 61.8% true bifurcation lesions, 34.3% significant calcification (PV), and mean baseline lesion length and RD of 11.78±4.67 mm and 2.74±0.36 mm in the PV, and 4.39±2.51 mm and 2.43±0.54 mm in the SB, respectively. In addition, angiographic success was 98%, whereas there was only one (1%) in-hospital MACE (non-Q MI) with no additional cardiac adverse events up to 30 days FU. Specifically, additional stents in the PV were needed in 15.7% of cases in BIPAX in order to treated edge dissections (nine of 16 cases, including type B dissection in five cases and type C dissection in four cases) and significant residual stenosis (seven of 16 cases) located either proximally or distally to the stent. In fact, the study protocol recommended lesion length ≤14 mm in the PV in order to be adequately covered by an 18 mm stent. For the SB, lesion length was restrained to its ostium (≤5 mm). In daily clinical practice, such criteria maybe a limiting factor in order to expand the study device’s applicability, as long/diffuse disease involving both PV and SB of bifurcation lesions are commonly found. In order to address these issues, a new version of the Nile PAX device including longer stent length (24 mm) with a side aperture at its centre, has been recently released; also, a combination between Nile PAX with CE mark approved Amazonia PAX (a straight non-polymeric paclitaxel-coated stent with similar characteristics as compared to the Nile PAX, but with a wider range of measures) maybe an option for long lesions extending SB’s ostium. Differently from the uncoated Nile Croco device used in del Blanco series,13 the Nile PAX stent used in BIPAX incorporates a novel non-polymeric paclitaxel coating. Overall, DES have improved outcomes in bifurcations compared to uncoated stents;2 though long-term FU is still needed to assess the impact of the dedicated Nile PAX technology on late clinical outcomes. In BIPAX, all patients were assigned for angiographic and clinical FU at nine months, and final results are expected shortly.
It is well recognised that the fate of the SB during bifurcation PCI is a key factor predicting clinical outcomes. In general, it is recommended to wire both branches in bifurcation lesions undergoing PCI as it has been shown to improve SB patency after PV stenting. A wire in the SB may also serve as a marker of its origin in case of vessel occlusion.20 With standard provisional techniques, SB re-wiring after PV stenting is required in order to restore SB access, and therefore, allow further treatment in the SB including single post-dilatation, FKB, and/or stenting (if needed). Such procedure is commonly achieved by exchanging guidewires, although an additional wire may be required. Nevertheless, it can be laborious and even unfeasible, especially in more complex anatomy/morphology. Specifically, the presence of: a) heavy calcification, b) severe stenosis with large plaque burden in the proximal segment, c) excessive proximal tortuosity, d) severe SB ostium stenosis, e) carina (or distal) angle >90°, and f) impaired flow, have been identified as predictors of SB access/wiring difficulty and failure.10,20,21 By design, the dedicated Nile stent system allows full access to the SB after implantation in the PV as its delivery system contains two separate rapid-exchange balloon-catheters: one for stent deployment in the PV and the other for opening the stent struts towards the SB; for that, the stent is crimped proximally over the tip of the SB balloon-catheter. More importantly, there is no jailed wire, wire exchange or need for additional wire and/or SB balloon if adequate positioning and deployment of the Nile system is attained.12 Nevertheless, wire tangling may occur, which was evidenced in a significant proportion of cases in both del Blanco (33.1%)13 and BIPAX studies (41.1%). Interestingly, such phenomenon did not appear to impact procedural outcomes as untangled wires followed by self-alignment positioning at the lesion site was achieved in the majority of lesions in both series (100% and 95%, respectively). In general, wire tangle is recognised once the study stent is advanced into the coronary vessel. At this point, the recommendation is to withdraw the system to the level of the distal end of the guiding catheter. Following, one of the wires (the one thought to be less difficult to rewire and/or located in the least diseased branch) must be withdrawn (to untangle the wires) and re-advanced in the correspondent distal branch. In BIPAX, all cases with wire tangling followed this procedure to rewire the branch vessel. Furthermore, FKB was performed in 95.1% and 93.1%, and device success was found in 99% and 98%, for del Blanco13 and BIPAX series, respectively.
Another important aspect includes accessibility and deliverability. Most dedicated devices tested to date are 7 Fr compatible systems due to relatively higher crossing profile as compared to straight conventional stents.22 In addition, large guiding catheters, usually 7 or 8 Fr, are commonly used in bifurcation PCI as extra back-up support may be warranted in complex lesion morphology and when targeting a distal coronary location.1 The Nile stent is a 6 Fr compatible system with 0.055-0.059” crossing profile.12 Notably, all procedures in BIPAX were performed with 6 Fr guiding catheter; also, radial access was used in almost half of cases (42.6%). Moreover, significant calcification was found in one third of cases and moderate/severe tortuosity was present in approximately 10%. Interestingly, such features did not impact device and procedural success in the current analysis. Specifically, there were 10 cases with significant tortuosity of the proximal segment, including one severe tortuosity (≥3 bends ≥75 degrees), but no failures associated with it. Of the two unsuccessful cases in BIPAX, one had the study device implanted proximally to the bifurcation site due to an apparent “pseudo-wrap” associated with a difficulty to advance and rotate the Nile stent into alignment because of severe vessel calcification. In this case, further treatment was performed with tubular stents implanted in both PV and SB (lesion Medina type 1,1,1), with optimal angiographic result after FKB. In the other case, the study device was retrieved completely out of the guiding catheter as the operator was not able to advance and position it at the bifurcation site (mid-LAD) because of unsolved wire tangle. In this case, the lesion was treated with a non-study stent without FKB, and the patient evolved asymptomatic up to 30 days. Lastly, only 6.9% of lesions were located in distal coronary segments; thus, new analyses are necessary to further evaluate the performance of the Nile PAX technology in some complex anatomies.
Several dedicated bifurcation stents have been developed to date, including dedicated PV devices, dedicated PV with SB access, dedicated SB devices, and dedicated PV plus SB device systems.22 The TAXUS® Petal™ bifurcated stent system (Boston Scientific, Natick, USA) incorporates a platinum-chromium tubular platform that has a side aperture in the mid-stent (petal), which provides scaffold and drug delivery at the SB ostial location. The delivery system contains a dual-side shaft for PV and SB wires (controlled rotational alignment).23 The first human use (FHU) of the TAXUS Petal trial was a prospective, multicentre, single-arm safety and feasibility study that included 28 patients with de novo bifurcation lesions in non-LM location. Regarded as a whole, the study device was successfully implanted in 89.3%, and additional stents were implanted in 28% in the PV, and 25% in the SB (all TAXUS Liberté). Overall technical (angiographic) success (residual stenosis <30% in the PV, and <50% in the SB with final TIMI 3 flow in both branches) was achieved in 96.4%.24 Compared to the Petal device in the FHU trial, the Nile PAX attained higher device success, even though there was relatively more complex lesions treated in BIPAX. Comparing SB lesions treated with stent versus without stent in BIPAX, stented SB had more complex lesions, including true bifurcation lesions (84.6% vs. 54%, p=0.004), significant (moderate/severe) calcification (46.2% vs. 30.3%, p=0.12) and longer lesions (5.92±2.1 mm vs. 2.8±1.3 mm, p=0.0008). The provisional arms of several randomised DES trials comparing single (provisional) versus elective double stenting, demonstrated SB stenting rates ranging from 2% to 32%.3-5,7 Such discrepancies are associated with the greater variance of lesion complexity and also different criteria for crossover. Importantly, common predictors for SB stenting are: long lesions (extending from the SB ostium), severe ostial stenosis and presence of heavy or significant calcification.1 In the prospective, randomised CACTUS trial, crossover criteria were residual stenosis >50%, dissection >type A, and TIMI flow <3.7 Similar criteria were used in BIPAX, and the majority of lesion requiring a stent in the SB had dissection (types A-C) and/or significant residual stenosis post-FKB.
Limitations
This study describes the preliminary results of a novel dedicated device evaluated in a prospective, multicentre registry including a relatively low number of patients (n=101); therefore, even though there was only one case (1%) of MACE up to 30 days FU, no definite conclusions regarding safety can be drawn from the current analysis. In addition, a few patients did not fully meet inclusion criteria as cases of in-stent restenosis (one case), heavy calcium and relatively long lesions in both PV and SB were found. Furthermore, PV predilatation and FKB – procedural steps considered critical to facilitate stent delivery and optimise scaffolding, were not performed in some cases per operator discretion. Even though such features did not impact on procedural outcomes, the late outcomes of these subsets are yet to be determined.
Conclusions
Procedural and early clinical results of the prospective, non-randomised, multicentre BIPAX clinical trial demonstrated encouraging results with the novel Nile PAX bifurcated DES in the treatment of coronary bifurcation lesions, including excellent acute performance as demonstrated by high device and procedural success. Overall, there was only one major adverse cardiac event during hospitalisation, with no additional adverse events up to 30 days follow-up. Longer-term follow-up is warranted.
Conflict of interest statement
R. Costa has received a research grant from Minvasys SAS. All the other authors have no conflict of interest to declare in relation to this paper.

