Abstract
Aims: This first human use (FHU) study in bifurcation lesions evaluated safety and feasibility of the TAXUS Petal paclitaxel-eluting dedicated bifurcation stent.
Methods and results: This prospective, single-arm, multicentre study had a composite primary endpoint of 30-day death, myocardial infarction (MI), and target vessel revascularisation (TVR). Angiographic and intravascular ultrasound follow-up was at six months with clinical follow-up through five years. Mean age (N=28) was 60.9±9.3years and 17.9% of patients had medically treated diabetes. Main branch (MB) lesion length was 13.8±5.9mm with 4.4±2.5mm in the side branch (SB). TAXUS Petal was successfully implanted in 89.3% of patients (25/28). On a per device basis, 73.5% (25/34) of Petal deployments were successful. The primary endpoint occurred in onepatient (3.7%, in-hospital non–Q-wave MI). Through oneyear, TVR was 11.1%, target lesion revascularisation was 7.4%, and there were no deaths, Q-wave MIs, or stent thromboses. In-segment late loss (n=21) was 0.47±0.45mm (proximal MB), 0.41±0.57mm (distal MB), and 0.18±0.39mm (SB).
Conclusions: The requirement for rotational alignment made delivery of this first generation TAXUS Petal stent challenging and accounted for the relatively low device delivery success. Clinical and angiographic outcomes were satisfactory when successful delivery was achieved.
Introduction
Coronary bifurcation disease remains one of the outstanding challenges of treatment with percutaneous coronary intervention (PCI), and may account for up to 20% of all lesions encountered in daily practice1. Compared with simple lesions, bifurcations have been associated with lower procedural success rates, higher adverse event rates, and poorer angiographic outcomes1-3. The less favourable outcomes associated with bifurcation compared with nonbifurcation lesions may in part result from the inability of current devices and techniques to scaffold adequately and preserve the side-branch (SB) ostium, which is a common site for restenosis4-6. Compromise of the SB during stent implantation is also common as many techniques, such as provisional T-stenting, do not allow the operator to maintain a usable wire in the SB1,2. The use of drug-eluting stents (DES) has resulted in significantly improved outcomes compared with bare metal stents (BMS)3. A variety of dedicated bifurcation stents, both BMS and DES, have been studied with variable results and to date none has become widely used4. The TAXUS® Petal™ (Boston Scientific Corporation, Natick, MA, USA) paclitaxel-eluting dedicated bifurcation stent has a side aperture mid-stent with deployable struts to scaffold the SB ostium so as to prevent occlusion after main branch (MB) stenting and deliver paclitaxel to the side-branch ostium, a frequent site of bifurcation restenosis5. We describe below the results of the first-human-use (FHU) study intended to evaluate safety and feasibility of this novel drug-eluting dedicated bifurcation stent.
Methods
Device description
The characteristics of the TAXUS Petal dedicated bifurcation stent (Figure1A) have been described previously5. Briefly, this paclitaxel-eluting stent, which is an improved version of the bare metal AST Petal6, is made of platinum chromium and includes a main branch (MB) section with a side branch (SB) aperture (petal) that provides mechanical scaffolding of the SB ostium by means of outwardly deploying strut elements that extend up to 2 mm. The novel platinum chromium alloy isstronger and moreradiopaque than stainless steel alone. In this pilot study of a new device, use of an 8 Fr guide catheter was recommended in the directions-for-use (DFU). The stent delivery system is a dual-side exchange catheter with a MB wire lumen and a SB (side sheath) wire lumen (Figure 1B).

Figure 1. TAXUS Petal stent (A) and delivery system (B).
Four radiopaque markers assist with proper alignment of stent and petal (Figure 2). A cylindrical-shaped balloon deploys the stent into the MB while a secondary gumdrop-shaped balloon connected to the same inflation lumen deploys the petal into the ostium of the SB. TAXUS Petal uses the same drug and polymer as the TAXUS Express and TAXUS Liberté stents7. One stent size (3.0 mmx16 mm) with 1 µg/mm2 of paclitaxel was available for this study.
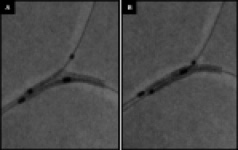
Figure 2. Correct and incorrect alignment of TAXUS Petal stent. Dual balloon and dual wire system with four marker bands ensure correct alignment (A). Figure B is 180° out of alignment.
Study design and data management
This prospective, single-arm, multicentre study (Clinicaltrials.gov Identifier NCT00497367) was designed to enrol 45-60 patients in two phases. Monitors verified all data and an independent Clinical Events Committee (CEC, Appendix 1) adjudicated events. There was no protocol-mandated roll-in phase. Interim safety and performance criteria in Phase 1 patients were assessed at 30days, including external input by an Independent Data Reviewer (Appendix2). A determination was then made as to whether/how to proceed (enrol more complex patients in Phase 2, repeat Phase1, or end the study). The study was supported by Boston Scientific Corporation.
Patient selection, procedure, and follow-up
Eligible patients (≥18years) had a de novo bifurcation lesion in a native coronary artery and clinical and angiographic indications for PCI. Key lesion inclusion criteria included a bifurcation takeoff angle ≥30° and ≤90°; main branch reference vessel diameter (RVD) ≥3.0 to ≤3.5mm with MB lesion length ≤20 mm; side branch RVD ≥2.25 to ≤3.5 mm with lesion length ≤14 mm. Placement of one additional planned MB stent and one additional planned SB stent was allowed. A maximum of one non-target lesion, located in a different vessel distribution from the target lesion, could also be treated. The non-target lesion was to be successfully treated first, either with a TAXUS stent or a bare metal stent. Kissing balloon post-dilatation was required for all procedures. Key exclusion criteria included excessive target lesion tortuosity, left main lesions, moderate or severe target lesion calcification, previous treatment of the target vessel with a BMS within nine months of the procedure or at any time with a DES or vascular brachytherapy, documented MI <72 hours before the procedure, cerebrovascular accident <6months before the procedure, and allergy or contraindication to aspirin, clopidogrel, ticlopidine, platinum, or stainless steel. Study enrolment occurred following successful predilatation of the target lesion.
Before catheterisation patients were pretreated with ≥300 mg aspirin plus ≥300 mg clopidogrel or 500mg ticlopidine. After the procedure patients received aspirin at a dose level according to the institutional standard of care for at least oneyear and 75 mg clopidogrel daily (or 250 mg ticlopidine twice a day) for sixmonths with oneyear recommended. Clinical follow-up was scheduled at 30 days, sixmonths (both office visits), and then annually through fiveyears (telephone interview allowed).
Follow-up QCA and IVUS were scheduled at six months; IVUS was to be performed in the MB and in the SB if possible. Quantitative coronary angiography (performed using software from Medis Medical Imaging Systems, Leiden, The Netherlands) of the target lesion was performed in accordance with consensus guidelines on analysis of bifurcation lesions8. Paired analyses were conducted on patients for whom both procedure and 6-month data were available. All QCA and IVUS data were analysed by independent core laboratories (Appendix 2).
Ethics Review Committees of participating institutions approved the protocol and patients provided written informed consent before enrolment. The study was conducted under Good Clinical Practice conditions and in compliance with regulations for the participating countries.
Endpoints
The primary endpoint was a composite 30-day safety endpoint of death, myocardial infarction (MI), and target vessel revascularisation ([TVR], see Appendix 3 for definitions). Secondary endpoints included device success, technical success, and clinical procedural success (Appendix 3); death, MI, revascularisation, and stent thrombosis (ST) at six months and annually through five years; and quantitative coronary angiography (QCA) and intravascular ultrasound (IVUS) analyses at six months.
Statistical methods
Patient, lesion, procedural characteristics, and event rates were analysed with SAS System Software, Version 8.2 or higher (SAS Institute, Cary, NC, USA) using descriptive statistics for continuous variables and frequency tables or proportions for discrete variables. No formal statistical testing was done in this single-arm, hypothesis-generating study.
Results
Patient, lesion, and procedural characteristics
The TAXUS Petal FHU study enrolled 28patients total at threesites in New Zealand, France, and Germany (Appendix 4). Baseline demographics and lesion characteristics are shown in Table 1 and Table 2, respectively.
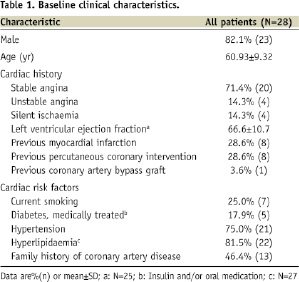
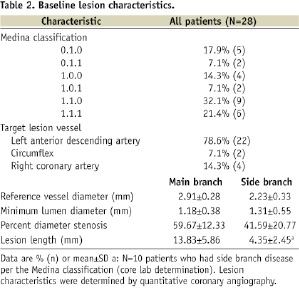
Procedural, technical, and device performance are shown in Table 3.
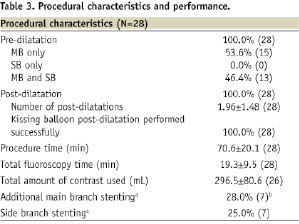
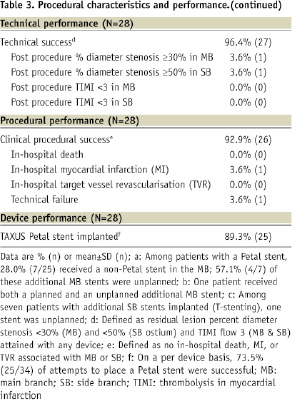
The Petal stent was successfully implanted in 25/28patients (89.3%), reflecting a success rate of 73.5% (25/34) per device attempt. Wire wrap (twisting together of the wires which prevented further stent advancement), wire bias (wire position guiding the stent SB component away from the SB), and the oval cross-sectional shape of the device made it challenging to achieve correct rotational alignment. In all successful cases, however, wire access to the side branch was maintained after deployment. Table 4 provides information on the seven cases where at least one Petal stent was not successfully implanted.
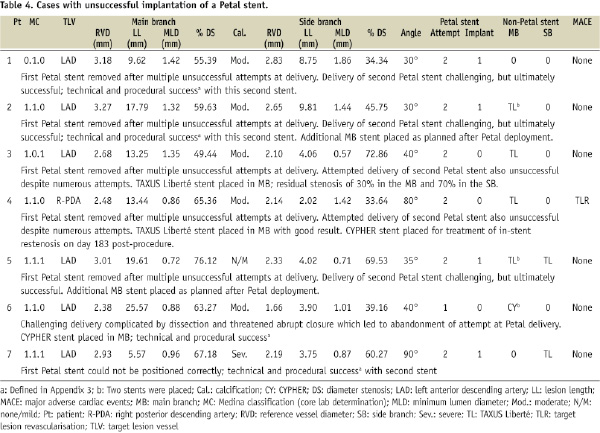
In cases with at least one unsuccessful attempt, moderate/severe calcification was more frequent than in cases where the first attempt was successful (6/7, 85.7% vs. 5/21, 23.8%; P=0.007). Depending on lesion anatomy, additional non-study stents were placed as necessary. Among patients receiving a Petal stent, 28.0% (7/25) received an additional MB stent and 28.0% (7/25) received a SB stent, all of which were TAXUS Liberté.
In Phase 1 of the study (11 July – 21 November, 2007), the Petal stent was successfully implanted in 14/16 patients (87.5%), reflecting a success rate of 14/20 (70.0%) per device attempt. Additionally, device delivery was challenging in several cases where implantation was ultimately successful. After careful consideration of this initial Phase 1 experience, 12 additional patients with lesion criteria as in Phase1 were enrolled (7 April – 30 June, 2008) to assess whether delivery difficulties improved as operators gained experience with the device. Enrolment in the study was terminated after 28patients due to shelf-life expiration of study devices.
Clinical outcomes at 30 days, 6-months, and 1-year
Clinical outcomes are shown in Table 5.
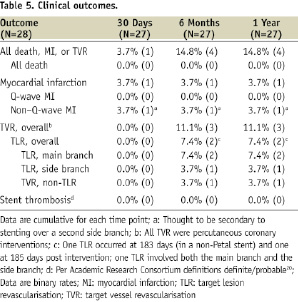
The composite primary endpoint at 30days occurred in one patient (3.7%) and consisted of an in-hospital non–Q-wave MI thought to be secondary to stenting over a second side branch. Through one year, the TLR rate was 7.4% (n=2) and there were no deaths, Q-wave MIs, or STs. One of the two TLRs occurred in a Petal stent but the other was in a main branch stent of a patient in whom Petal implantation had failed. The other two patients without Petal stents had no major events. One patient withdrew consent after the unsuccessful Petal implantation.
QCA and IVUS results
At six months, angiographic follow-up among patients who received the Petal stent was 84% (21/25); QCA results are presented in Table 6.
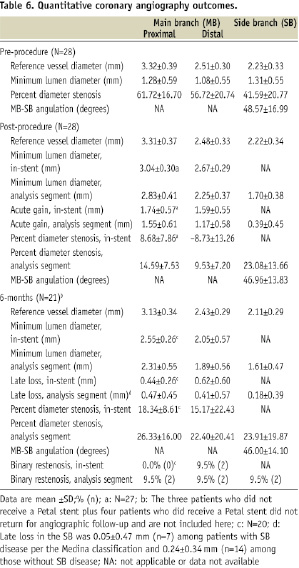
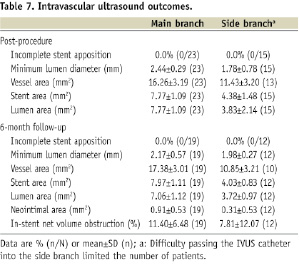
In-segment late loss was 0.47±0.45mm (proximal MB), 0.41±0.57mm (distal MB), and 0.18±0.39mm (SB). Among patients with binary restenosis, MB restenosis was located just proximal to the Petal stent (two cases) or in the body of the Petal stent distal to the SB aperture (two cases). Side branch restenosis was at the SB ostium (two cases, one of which occurred in one of the patients with proximal MB restenosis). None of these cases of restenosis were related to placement of a second nearby/ overlapping stent. As shown in Table 7, there were no cases of incomplete stent apposition observed with IVUS post-procedure or at 6-months. Mean lumen area in the SB was 3.83±2.14 (n=15) post-procedure and 3.72±0.97 (n=12) at 6-month follow-up.
Discussion
This FHU study evaluated safety and clinical performance of the TAXUS Petal bifurcation stent, which was designed to provide mechanical scaffolding and drug delivery to the SB ostium. Results showed that treatment is feasible in bifurcation lesions though the requirement for rotational alignment made delivery more challenging than with conventional stents. Through oneyear the TLR rate was 7.4% (2/28) with no deaths, Q-wave myocardial infarctions, or stent thromboses. In-segment late loss at sixmonths was 0.47mm in the proximal MB, 0.41 in the distal MB, and 0.18mm in the SB. Binary restenosis was 9.5%. Net volume obstruction was 11.4% in the MB and 7.8% in the SB. Thus, as assessed by 1-year clinical follow-up and 6-month QCA and IVUS, outcomes were satisfactory with the TAXUS Petal stent in patients where successful delivery was achieved.
There are few clinical data available with other drug-eluting dedicated bifurcation stents4. AXXESS Plus is a biolimus-eluting, self-expanding nitinol stent designed for the treatment of bifurcation lesions. In the first human use study (N=139), overall 6-month TLR was 7.5%, MI was 6.0%, and stent thrombosis was 2.2%9. In the follow-on single-arm, non-randomised DIVERGE study (N=302) at ninemonths TLR was 4.3%, MI was 4.3% and stent thrombosis was 1.0%10. Additional stenting, mostly DES, of one or both branches was performed in the majority of patients. It should be noted that the bifurcation lesions treated with Petal were less complex than in AXXESS and DIVERGE.
Dedicated bifurcation stents are not widely used in everyday practice and conventional stents remain the mainstay for PCI of bifurcation lesions. While variations in bifurcation anatomy preclude a single overarching approach, results from DES randomised controlled trials and registry data have supported the provisional strategy of stenting the MB and performing balloon angioplasty in the SB, with SB stenting reserved for cases where the initial SB result is suboptimal11-17. Routine DES implantation in both branches appears to offer no clear advantage over the provisional method with respect to restenosis in the MB or SB or repeat bifurcation revascularisation. With its projecting petal elements TAXUS Petal is designed to support the SB which may make the provisional stenting procedure safer by both maintaining wire access to the SB, and in addition, avoiding carina shift18 during the intervention. Furthermore, long term outcomes may also improve through SB ostial support and minimisation of gaps between stents if a SB stent is required. As such, the support provided by the petal may preclude the requirement for a second stent or facilitate its placement if needed.
The results described here suggest that the Petal approach has promise, though the delivery difficulties must first be overcome. It became apparent during the course of the study that meticulous attention to wire management was important to maximise the likelihood of successful delivery. Passive rotational alignment was limited by the oval cross-sectional nature of the device. A major limitation to passive rotational alignment was wire bias where the wire curving into the SB directed the SB component of the stent away from the SB. Use of more or less stiff wires also at times reduced wire bias and enabled successful delivery. If wire wrap was observed or suspected, withdrawal of either the MB or SB wire into the Petal stent and subsequent re-advancement of the wire frequently resolved wire wrap and facilitated successful delivery of the stent though it should be noted that this approach may lead to an inability to re-access the vessel in the presence of dissection. In some cases, however, this first generation of the Petal stent could not be delivered despite the best efforts of an experienced operator. An improved version of the Petal stent incorporating a torquable shaft and a modified delivery system is under development. These modifications may make it easier for the operator to successfully deliver the device.
Limitations
Limitations to this study include the small number of patients typical of a first human use study which was insufficient to provide accurate estimates of clinical event rates, relatively low lesion complexity, and the absence of a randomised control group. The single available stent size made it difficult to enrol patients and some who met the enrolment criteria had limited SB disease. Additionally, because this study did not prospectively assign a particular SB treatment method, the use of additional stents was at operator discretion, resulting in a heterogeneous study population. Limitations of dedicated bifurcation devices in general include larger crossing profiles (8 Fr guide calibre for TAXUS Petal recommended in the DFU) with less flexibility compared with conventional stents. In addition, the requirement for two wires introduced new challenges to delivery and rotational alignment such as wire twisting (wrap) and wire bias, which sometimes may be addressed by careful guide wire management19. The oval cross sectional shape of the device also limited passive rotational alignment.
Conclusions
In this FHU study the TAXUS Petal dedicated bifurcation stent was delivered with an acceptable success rate though the requirement for rotational alignment made delivery more challenging than with conventional stents. Clinical, QCA, and IVUS outcomes were satisfactory in patients where successful delivery was achieved.
Acknowledgements
The authors thank Edmund McMullen, M.Math. and Gina Garding, for statistical analyses and Ruth M. Starzyk (all of Boston Scientific Corporation) for assistance with drafting and editing the manuscript.
Appendices
Appendix 1. Clinical events committee members.
Donald E. Cutlip, MD, Chair
Beth Israel Deaconess Medical Center, Boston, MA, USA
Julian Aroesty, MD
Beth Israel Deaconess Medical Center, Boston, MA, USA
Manish Chauhan, MD
Texas Cardiovascular Consultants, PA, Austin, TX, USA
Germano DiSciascio, MD
Campus Bio-Medico University, Rome, Italy
Kalon Ho, MD
Beth Israel Deaconess Medical Center, Boston, MA, USA
Joseph Kannam, MD
Beth Israel Deaconess Medical Center, Boston, MA, USA
Michel Vandormael, MD
Tampa Bay Heart Institute, Largo, FL, USA
Appendix2. Sponsor and study personnel.
Sponsor: Boston Scientific Corporation, Natick, MA, USA
Angiography Core Lab: Beth Israel Deaconess Medical Center, Boston, MA, USA
Intravascular Ultrasound Core Lab: MedStar Research Institute,
Washington, DC, USA
Independent Data Reviewer: Adam Greenbaum, MD, Farmington Hills, MI, USA
Appendix3. Cardiac event and procedural definitions in the TAXUS Petal first human use study.
CARDIAC EVENT
Event: Myocardial infarction (MI)
Definition: Myocardial infarction was defined as follows:
1. Q-wave MI: Development of new (i.e., not present on the subject’s ECG before allocation) pathological Q-waves in two or more leads lasting ≥0.04 seconds with CK-MB/troponin levels elevated above normal.
2. Non-Q-Wave MI: Elevation of post-procedure CK levels >2x ULN with positive CK or, if the assay for CK-MB was not performed, elevation of CK levels >2x ULN with positive troponin.
Drawing a CK-MB or troponin was mandated if CK was greater than 2x ULN. If no CK-MB or troponin was drawn, CK >2x ULN was considered an MI. ULN was determined per local laboratory specifications.
For subjects undergoing bypass surgery post-procedure, a perioperative MI was defined as follows:
a) Total CK-MB >5x upper limits of local laboratory normal -OR-
b) Presence of new pathologic Q waves (as defined above)
Event: Stent thrombosis
Definition: Academic Research Consortium definitions definite/probable20
Event: Target lesion revascularisation (TLR)
Definition:
Target lesion revascularisation was defined as any ischaemia-driven repeat percutaneous intervention (to improve blood flow) of the target lesion or bypass surgery of the target lesion.
A target lesion revascularisation was considered ischaemia-driven if the target lesion diameter stenosis was ≥50% by QCA and any of the following were present:
1. The subject has a positive functional study corresponding to the area served by the target lesion.
2. The subject has ischaemic ECG changes at rest in a distribution consistent with the target lesion.
3. The subject has ischaemic symptoms referable to the target lesion.
A target lesion revascularisation for a lesion with a diameter stenosis <50% might also be considered ischaemia-driven by the CEC if there was a markedly positive functional study or if there were ECG changes corresponding to the area served by the target lesion.
A target lesion revascularisation was considered as ischaemia-driven if the lesion diameter stenosis was ≥70% even in the absence of clinical or functional ischaemia.
An ischaemia-driven TLR was considered if the angiogram and TLR would have been performed for clinical indications in the absence of this protocol.
Event: Target vessel revascularisation (TVR)
Definition: Target vessel revascularisation was defined as any ischaemia-driven repeat percutaneous intervention (to improve blood flow) of the target vessel or bypass surgery of the target vessel.
A target vessel revascularisation was considered ischaemia-driven if the target vessel diameter stenosis was ≥50% by QCA and any of the following were present:
1. The subject has a positive functional study corresponding to the area served by the target vessel.
2. The subject has ischaemic ECG changes at rest in a distribution consistent with the target vessel.
3. The subject has ischaemic symptoms referable to the target lesion.
A target vessel revascularisation for a lesion with a diameter stenosis less than 50% might also be considered ischaemia-driven by the CEC if there was a markedly positive functional study or if there were ECG changes corresponding to the area served by the target lesion.
PROCEDURAL EVENT
Event: Clinical procedural success
Definition: Technical success with no in-hospital death, MI or TVR associated with the MB or SB
Event: Device success
Definition: Successful delivery and deployment of the stent to the target lesion, including petal deployment and apposition in the SB with no petal balloon rupture or stent embolisation, and successful retrieval of the stent delivery system with <30% MB stenosis and <50% stenosis in the SB ostium
Event: Technical success
Definition: <30% MB stenosis and <50% SB ostium stenosis with TIMI flow 3 (MB & SB) attained with any device
CEC: Clinical Events Committee; CK: creatine kinase; ECG: electrocardiogram; MB: main branch; QCA: quantitative coronary angiography; SB: side branch; ULN: upper limit of normal
Appendix4. Investigators and institutions participating in the TAXUS Petal first human use study.
John Ormiston, Principal Investigator, Mercy Hospital and Auckland City Hospital, Auckland, New Zealand
Thierry Lefèvre, Institut Cardiovasculaire, Paris Sud, Massy, France
Eberhard Grube, Helios Klinikum Siegburg, Siegburg, Germany

