Abstract
Aims: The BRANCH study was a prospective, multicentre, non-randomised, single arm trial to investigate the feasibility, safety, efficacy, and performance of the bare metal Medtronic Bifurcation Stent System for the treatment of de novo bifurcation lesions.
Methods and results: Sixty patients were enrolled in the study. After a learning curve of one case at seven centres, 53 patients from six centres were prospectively treated. The primary endpoint was target vessel failure (TVF) at 30 days. Secondary endpoints included acute device, lesion, and procedure success and TVF at 12 months. Medina complex bifurcation lesions (1,1,1; 1,1,0; 1,0,1; 0,1,1) were treated in 71.7%. The stent was successfully implanted in 86.8% of cases. Acute device, lesion, and procedure success rates were 83.0%, 92.5%, and 88.7%, respectively. TVF occurred in 2/52 patients (3.8%) at 30 days. No other major adverse cardiac adverse events (MACE) occurred through 30 days follow-up. At 12 months, TVF occurred in 6/47 (12.8%) patients, and MACE occurred in 5/47 (10.6%) patients.
Conclusions: Results from the BRANCH study demonstrate that the Medtronic Bifurcation Stent System is safe and can be successfully and effectively deployed in a variety of bifurcation lesions with good clinical outcomes.
Introduction
Treatment of bifurcation coronary artery disease remains a significant challenge in interventional cardiology. The optimal treatment strategy of these lesions has been the subject of much debate; however, several randomised studies have shown that in the majority of cases, the provisional side branch strategy (i.e., treating the main branch and, if needed, the side branch as well) can produce excellent clinical results1-5.
There are situations, however, where the use of a two-stent strategy may be unavoidable, such as with long side branch lesions, or with abrupt closure of the side branch that puts downstream myocardium at risk of infarction. When two stents are needed, the optimal approach is still unresolved. A number of two-vessel stenting techniques using conventional stents have been proposed, including T-stenting, V-stenting (also called simultaneous kissing stents [SKS]), Y-stenting, crush technique, and culotte stenting6-10. However, each method suffers from one or more potential limitations, including inconsistent coverage at the lesion site (T-stenting), gapping at the ostium of the side branch (T-stenting), creation of a false carina (culotte, V-stenting, Y-stenting), and disturbed blood flow from overlapping layers of metal over the ostium and carina (crush, culotte, V-stenting, Y-stenting). In addition, all of these techniques are technically complex and are associated with higher radiation exposure and contrast loads, longer procedure times, and greater risk of periprocedural complications and side branch restenosis3,5.
The challenges surrounding the use of two-stent techniques have led to the development of stents specifically designed to treat bifurcation lesions. The Medtronic Bifurcation Stent (Medtronic Inc., Santa Rosa, CA, USA) is a bare metal, dual branch, Y-shaped, dedicated bifurcation stent that was designed to provide easy access to the side branch while maintaining main vessel and side branch patency. The BRANCH (Bare Metal BifuRcAtion SteNt Clinical Trial in Humans) study was performed to investigate the feasibility, safety, efficacy, and performance of this device for the treatment of de novo bifurcation lesions.
Methods
Device description
The Medtronic Bifurcation Stent is a bare metal stent that is made of the same cobalt-based alloy (MP35N) used in the commercially approved Driver/Micro-Driver® stent stent (Medtronic Inc.). The bifurcation stent is Y-shaped, and consists of a 12-crown, 7 mm proximal main vessel section, an 8-crown, 4 mm side branch section, and a 10-crown, 8 mm distal main vessel section (Figure 1). The three sections are fused so as to accommodate multiple bifurcation angles. The stent has thin struts (91 microns) and a small crossing profile (1.55 mm), which provide a high level of flexibility, deliverability, and conformability. Two models of the stent were available for the BRANCH study, one with a proximal main vessel diameter of 3.8 mm, a distal main vessel diameter of 3.0 mm, and a side branch diameter of 2.5 mm, and the other with a proximal main vessel diameter of 4.3 mm, a distal main vessel diameter of 3.5 mm, and a side branch diameter of 2.5 mm.
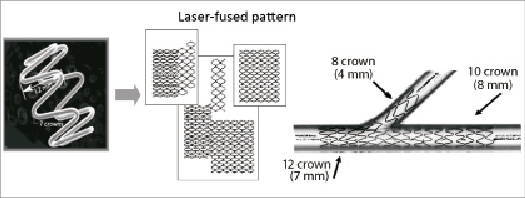
Figure 1. The Medtronic Bifurcation Stent is Y-shaped and consists of a 12-crown, 7 mm proximal main vessel section, an 8-crown, 4 mm side branch section, and a 10-crown, 8 mm distal main vessel section. The three sections are fused so as to accommodate multiple bifurcation angles.
The stent is supplied pre-mounted on a dual rapid exchange stent delivery system that is compatible with 8 Fr guide catheters. The system uses two balloons, one for main vessel stent deployment and the other for side branch stent deployment. Both balloons extend the length of the stent. The side branch balloon uses a stepped design to match the anatomy of the target vessel (Figure 2). Each balloon has a nominal inflation pressure of 9 atmospheres and a maximum inflation pressure of 16 atmospheres.
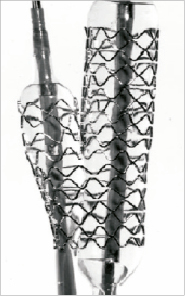
Figure 2. The Medtronic Bifurcation Stent is supplied pre-mounted on a dual rapid exchange stent delivery system that is compatible with 8 Fr guide catheters. The system uses two balloons, one for main vessel stent deployment and the other for side branch stent deployment. Both balloons extend the length of the stent. The side branch balloon uses a stepped design to match the anatomy of the target vessel.
The stent delivery system is advanced over two guidewires, with one placed in the main vessel and one in the side branch. Four radiopaque markers, one at each end of the stent and one in the carina region, aid positioning of the stent in the target lesion. Once the correct position and orientation of the stent are achieved, the balloons are inflated simultaneously through a single inflation/deflation port on the proximal end of the delivery system. This method of deployment is similar to the “kissing balloon” technique commonly used in bifurcation stenting.
Study overview and patient population
The BRANCH study was a prospective, multicentre, non-randomised, single arm trial. From February 25, 2008 to March 19, 2009, seven centres in Australia and New Zealand enrolled 60 patients with symptomatic ischaemic heart disease attributable to a bifurcation lesion amenable to percutaneous treatment with stenting.
Patients with single or multiple vessel coronary artery disease were eligible to participate, but only a single bifurcation lesion per patient could be treated. Previous stenting anywhere in the target vessel was not permitted, and the vessel could not have undergone any percutaneous intervention within 30 days of the index bifurcation procedure. Only the target bifurcation lesion could be treated during the index procedure; however, other lesions in the target vessel could be treated six months post-procedure, and any lesion in other vessels could be treated 30 days post-procedure with any approved stent. The target lesion had to be a single de novo bifurcation lesion involving a native coronary artery suitable for treatment with a bifurcation stent. The proximal main vessel had to have a reference vessel diameter (RVD) of 3.8-4.3 mm, a distal main vessel RVD of 3.0-3.5 mm, and a side branch RVD of up to 2.5 mm. Target lesion lengths could be any combination of the following: <16 mm proximally from the carina in the proximal main vessel; ≤16 mm distally from the carina in the distal main vessel; and <12 mm from the carina in the side branch. The target lesion in the main vessel had to have a stenosis of ≥50% and <100%. The target vessel had to have a thrombolysis in myocardial infarction (TIMI) flow ≥2.
Major exclusion criteria included a left ventricular ejection fraction <30%; evidence of an acute myocardial infarction (MI) within 72 hours of the procedure; percutaneous coronary intervention (PCI) of a non-target vessel within 24 hours prior to the index procedure; planned PCI of any vessel within 30 days post-procedure or of the target vessel within six months post-procedure; a bifurcation angle of >90 degrees; >50% stenosis proximal or distal to the target lesion that might require revascularisation or impede run off; excessive target vessel tortuosity; co-existence of unprotected left main coronary artery disease (>50% obstruction); and a target lesion that had been previously grafted, was severely calcified, had evidence of thrombus, was aorto-ostial, was an unprotected left main lesion, or was within 5 mm of the origin of the left anterior descending, left circumflex, or right coronary artery.
The study was conducted according to the Declaration of Helsinki, and the medical ethics committees at all sites approved the study protocol. Written informed consent was obtained from each patient.
Interventional procedure
Prior to the procedure, all study patients received aspirin (minimum of 75 mg daily, starting at least 24 hours prior to procedure and continued indefinitely) and clopidogrel (≥300 mg loading dose within 24 hours prior to the procedure, then 75 mg daily for a minimum of one month following the procedure). In cases where the patient had or developed a sensitivity to clopidogrel, ticlopidine could be substituted (250 mg twice daily for at least one month post-procedure). During the procedure, heparin or bivalirudin was administered to maintain an activated clotting time of ≤250 sec or 200-250 sec if a glycoprotein IIb/IIIa inhibitor was administered. Administration of 50-200 µg of intracoronary nitroglycerine was required prior to stenting and before post-intervention angiograms.
Pre-dilatation of each bifurcation segment was mandatory for the study. Post-dilatation of the stent segments was left to the discretion of the operator. Additional Driver® and/or MicroDriver® coronary stents of no more than 12 mm in length could be implanted in an overlapping fashion to ensure complete lesion coverage; however, only one overlapping stent per segment of the Medtronic Bifurcation Stent was allowed under these circumstances. If a patient experienced a major dissection or an occlusive complication, bailout procedures could be performed using additional stenting with any approved stent. At the end of the procedure, an intracoronary injection of nitroglycerine was administered, and quantitative angiography and intravascular ultrasound (IVUS) of the main vessel were performed to document the final vessel results.
Patient follow-up
Patient follow-up was performed at 30 days post-procedure at the same investigational site where the index procedure was performed. Additional patient contacts were performed by telephone, e-mail, and/or office visits at 6, 9, and 12 months post-procedure. At each visit, angina status, adverse events, concomitant medications, and any coronary treatment since the previous follow-up were recorded.
Study endpoints and definitions
The primary endpoint was target vessel failure (TVF), defined as a composite of cardiac death, target vessel MI, and clinically driven target vessel revascularisation (TVR) at 30 days post-procedure. Secondary end points were acute device, lesion, and procedure success, total fluoroscopy time, total contrast volume used, total index PCI procedure time, and TVF at 12 months.
For the study, a Q-wave MI was defined as chest pain or other acute symptoms consistent with myocardial ischaemia and new pathological Q-waves in two or more contiguous ECG leads in the absence of timely cardiac enzyme data, or as new pathologic Q-waves in two or more contiguous ECG leads and elevated cardiac enzymes. A non-Q-wave MI was defined as an elevated CK-MB ≥3 times the laboratory upper limit of normal in the absence of new pathological Q-waves. Target vessel MI was defined as an MI that occurred in a territory that could not be clearly attributed to a vessel other than the target vessel. Target lesion revascularisation (TLR) was defined as any percutaneous intervention or bypass surgery performed on the index target lesion at any time after the index procedure. TVR was defined as any percutaneous intervention or bypass surgery performed on the index target vessel at any time after the index procedure. Major adverse cardiac events (MACE) were defined as death, myocardial infarction (Q-wave and non-Q-wave), emergent coronary bypass surgery, or repeat TLR by percutaneous or surgical methods.
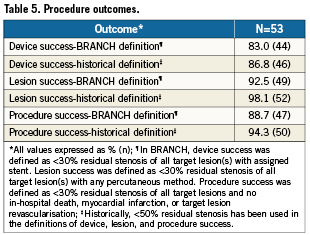
Device success was defined as <30% residual stenosis of all target lesion(s) with the assigned stent. Lesion success was defined as <30% residual stenosis of all target lesion(s) with any percutaneous method. Procedure success was defined as <30% residual stenosis of all target lesion(s) and no in-hospital death, MI, or TLR. To permit comparison with historical definitions, <50% residual stenosis of all target lesion(s) was also used in the definitions of device, lesion, and procedure success.
Sample size and statistical analysis
The BRANCH study was designed to minimise the number of subjects exposed to the Medtronic Bifurcation Stent while still providing enough information about the stent’s feasibility, safety, and efficacy. A sample size of 60 patients was deemed sufficient to meet these objectives. For the study, a lead-in phase of one patient per centre was permitted. There were seven lead-in patients; thus, 53 patients were included in the main analysis. All analyses were based on the intention-to-treat principle. Discrete variables are expressed as frequencies and percentages, and continuous variables are expressed as means and standard deviations.
Data collection and core laboratories
Conduct of the trial was monitored by a contract research organisation (Pacific Clinical Research Group, New South Wales, Australia). All data were submitted to a central data coordinating facility (Medtronic Inc., Santa Rosa, CA, USA). All major adverse events were adjudicated by an independent clinical events committee managed by the Harvard Clinical Research Institute (Boston, MA, USA). All ECGs, coronary angiograms, and IVUS images were reviewed by independent core laboratories (ECGs: Harvard Clinical Research Institute, Boston, MA, USA; Angiograms: Brigham and Women’s Angiographic Core Lab, Boston, MA, USA; IVUS: Stanford University Vascular Core Analysis Lab, Stanford, CA, USA).
Results
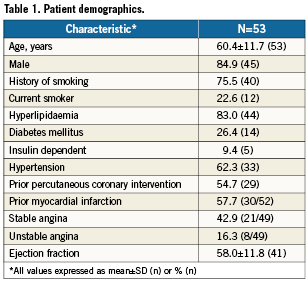
Patient demographics
Patient demographic data are summarised in Table 1. Patients were mostly male (84.9%) and had an average age of 60.4 years. Angina was present in 59.2% of patients: 42.9% had stable angina, and 16.3% had unstable angina. More than half (54.7%) of patients had a previous PCI, and 57.7% had a prior MI. Diabetes was present in 26.4%.
Lesion characteristics
Lesion characteristics are summarised in Table 2. The majority (73.6%) of lesions involved the left anterior descending coronary artery. Eighty-seven percent of the lesions were American College of Cardiology/American Heart Association class B2 or C. The most frequent lesions (71.7%) were Medina complex bifurcation lesions (1,1,1; 1,1,0; 1,0,1; 0,1,1) (Figure 3). The average overall main vessel lesion length was 13.68±6.05 mm, and the average side branch length was 6.08±3.92 mm. In the majority of cases (73.6%), the bifurcation angulation (main vessel–side branch) was between 45 and 90 degrees.
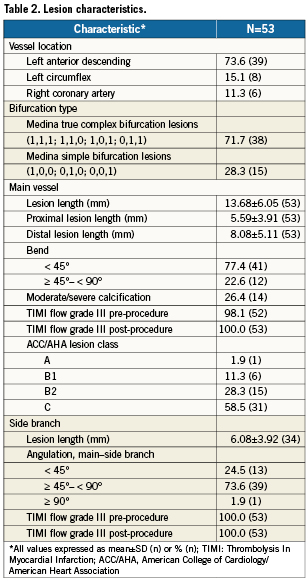
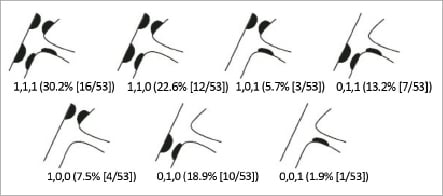
Figure 3. The Medina classification of BRANCH bifurcation lesions. Of the lesions treated with the Medtronic Bifurcation Stent, 71.7% were Medina complex bifurcation lesions (1,1,1; 1,1,0; 1,0,1; 0,1,1). (Graphic adapted from: LeGrand V, et al. EuroIntervention 2007;3:45)
Procedure outcomes
The Medtronic Bifurcation Stent was successfully implanted in 86.8% (46/53) of cases (Figure 4). Of the seven unsuccessful implantation attempts, there were three cases where the stent could not be delivered to the target lesion, two cases where the side branch portion of the stent could not be manoeuvred into position, one case where the side branch portion of the stent could not be delivered distally, and one case where a dissection occurred at the time of stent delivery or deployment. In these seven cases, patients were successfully treated with non-investigational, approved stents without sequelae. Of the seven lead-in patients, there was one case where the stent was not successfully delivered due to wire wrap requiring pullback of the main and side branch guidewires. This patient was successfully treated with non-investigational, approved stents without complications.
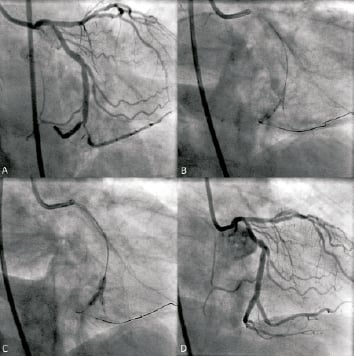
Figure 4. Deployment of the Medtronic Bifurcation Stent in a study patient with a lesion at the bifurcation of the distal right coronary artery and first marginal branch. A: lesion, pre-treatment; B: stent orientation and alignment; C: main and side branch balloon deployment; D: vessel and lesion following stent deployment.
In patients who successfully received the Medtronic Bifurcation Stent, additional stenting to cover the full lesion length was required in 26.1% (12/46) of proximal main vessel lesions, 21.7% (10/46) of distal main vessel lesions, and 39.1% (18/46) of side branch lesions. Overall, subsequent kissing balloon dilatation after stent implantation was performed in 76.1% (35/46) of cases.
For the study, average total fluoroscopy time was 22.9±10.6 min, average total volume of contrast used was 322.7±87.8 ml, and average total index procedure time was 70.9±22.8 min.
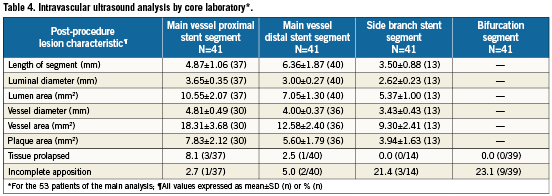
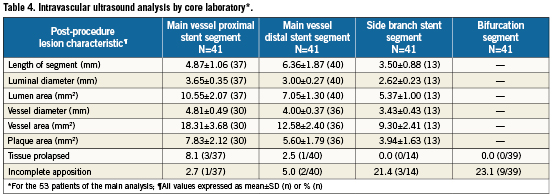
Post-procedure quantitative angiographic and IVUS results are summarised in Tables 3 and 4, respectively. The acute gain was 1.53±0.62 mm in the proximal main vessel, 1.32±0.49 mm in the distal main vessel, and 0.77±0.63 mm in the side branch. With IVUS, the post-procedure lumen area was 10.55±2.07 mm2 in the main vessel proximal stent segment, 7.05±1.30 mm2 in the main vessel distal stent segment, and 5.37±1.00 mm2 in the side branch stent segment. The vessel diameter was 4.81±0.49 mm in the main vessel proximal stent segment, 4.00±0.37 mm in the main vessel distal stent segment, and 3.43±0.43 mm in the side branch stent segment. Tissue prolapse was noted in 8.1% (3/37) of the proximal main vessel stent segment and in 2.5% (1/40) of the distal main vessel stent segment. Incomplete apposition was noted in 2.7% (1/37) of the proximal main vessel segment, 5.0% (2/40) of the distal main vessel segment, 21.4% (3/14) of the side branch stent segment, and 23.1% (9/39) of the bifurcation segment.
Overall acute device, lesion, and procedure success rates were 83.0% (44/53), 92.5% (49/53), and 88.7% (47/53), respectively (Table 5). Using the historical definition, the acute device, lesion, and procedure success rates were 86.8% (46/53), 98.1% (52/53), and 94.3% (50/53), respectively.
Clinical outcomes
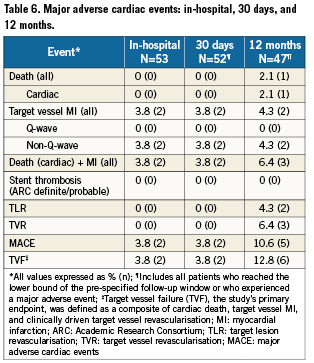
One patient was not included in the 30-day analysis because the patient received follow-up at 20 days, which did not reach the pre-specified minimum interval (25 days) for follow-up. The primary endpoint, TVF at 30 days post-procedure, was 3.8% (2/52). In both cases, the patient experienced a post-procedure, non-Q-wave MI and recovered uneventfully. No other MACE occurred through 30 days follow-up. At 12 months, TVF occurred in 6/47 (12.8%) patients, and MACE occurred in 5/47 (10.6%) patients (Table 6). None of the seven lead-in patients experienced MACE during the 12 month follow-up period.
Discussion
In this first-in-man feasibility study of the Medtronic Bifurcation Stent, we were able to successfully implant the stent in 86.8% of cases. Overall procedure success (88.7%) was quite good and was achieved despite having to treat comparatively long and complex lesions (main vessel length, 13.68 mm; side branch length, 6.08 mm; 86.8% B2/C lesions; 26.4% moderate-to severe calcification). Procedurally, we found proper stent orientation and alignment challenging at times; however, once the stent was in place, it was easily deployed. We also found that the deployed stent provided exceptional side branch access, which greatly simplified stenting of long side branch lesions.
The stent was well-sized to the vessels, and its flexibility and conformability permitted treatment of a wide range of carina angles. The stent also provided very good scaffolding and coverage of all three vessel segments. Device success was 83.0%, with post-procedure quantitative angiography and IVUS showing very good acute gain, lumen areas, and lumen diameters. The overall acute lesion success rate was high at 92.5%. Of the four lesion failures that occurred, two occurred with the Medtronic Bifurcation Stent; the other two occurred in cases where two non-investigational, approved stents were used to treat the bifurcation lesion when the BRANCH device could not be delivered.
The 30-day MACE rate of 3.8% (2/52) and TVF rate of 3.8% (2/52) with the Medtronic Bifurcation Stent were excellent. The 12-month MACE rate of 10.6% (5/47), TLR rate of 4.3% (2/47), and TVF rate of 12.8% (6/47) were good, and comparable to rates achieved using a provisional side branch treatment strategy with drug-eluting stents (DES).11
We noted several limitations while using the first generation of the Medtronic Bifurcation Stent. First, the stent has a larger profile compared to conventional stents. Thus, a relatively large 7 or 8 Fr guiding catheter must be used to deliver the device, which increases the risk of vascular complications. Second, stent delivery requires the use of two guidewires, which are subject to wire wrapping. Third, there is a learning curve associated with delivery and deployment of the device as evidenced by the longer procedure and fluoroscopy times and the amount of contrast agent used3,5. Fourth, proper stent orientation and alignment were at times difficult to achieve due to the limited torquability of the stent system. Fifth, the suitability of the stent for the treatment of more tortuous vessels remains unknown. Sixth, the stent is a bare metal stent at a time when DES has become the preferred platform for dedicated bifurcation devices. Finally, despite the stent’s ability to provide ready access to, and treatment of, the side branch, MACE and TLR rates were not substantially improved over those achieved using a provisional side branch treatment strategy. Nonetheless, this was any early feasibility study using a new technology. Greater operator experience combined with refinements in the stent’s design may result in improved procedural and clinical outcomes.
The BRANCH study design itself also had several limitations. It was a first-in-man study involving a small number of patients, a significant proportion of whom had simple, non-calcified, bifurcation lesions. The study was also a non-randomised, single-arm trial conducted at a limited number of centres. Although the study’s results demonstrate the feasibility, safety, and efficacy of the stent, larger, randomised trials with longer-term follow-up are necessary to draw more definite conclusions about the stent’s performance.
Conclusion
The BRANCH study investigated the feasibility, safety, efficacy, and performance of the Medtronic Bifurcation Stent System for the treatment of de novo bifurcation lesions.
The study results demonstrate that the stent system is safe and can be successfully and effectively deployed to treat a variety of bifurcation lesions with acceptable clinical outcomes at one year.
Acknowledgments
The authors thank Janice Hoettels, BS, PA-C and Jane Moore, MS for their assistance with the preparation of this manuscript.
Funding source
Medtronic Inc., Santa Rosa, CA, USA.
Conflict of interest statement
Dr. Meredith, has served as an advisory board member for Boston Scientific and Medtronic CardioVascular. Dr. Ormiston serves as an advisory board member for Abbott Vascular and Boston Scientific. Dr. Fitzgerald serves as an advisory board member for Medtronic and receives research grant support from Medtronic. Dr. Worthley, Dr. Whitbourn, and Dr. Webster have no conflicts of interest to report.

