Abstract
Aims: We aimed to compare healing responses with optical coherence tomography, and clinical and angiographic outcome after treatment of coronary bifurcation lesions with a dedicated stent versus a conventional culotte technique.
Methods and results: Forty patients with true and complex coronary bifurcation lesions were randomly assigned to treatment with the Axxess™ bifurcation stent in the proximal main vessel (MV) and additional BioMatrix™ stents in the branches (Biosensors Europe SA, Morges, Switzerland), versus a culotte technique using XIENCE™ stents (Abbott Vascular, Santa Clara, CA, USA). The primary endpoint of percentage of uncovered struts at nine months was similar with the dedicated strategy vs. culotte in the proximal MV (median 17.8 [IQR 3.3-24.7] vs. 6.8 [2.0-20.5]; p=0.19), bifurcation core (9.5 [5.7-19.5] vs. 4.0 [0.7-17.6]; p=0.17), distal MV (2.6 [2.3-18] vs. 2.2 [0.5-6.0]; p=0.09) and side branch (5.7 [1.5-11.5] vs. 1.9 [0-5.8]; p=0.14). As compared with culotte, a strategy using Axxess resulted in a significantly larger lumen in the proximal MV both acutely (minimum lumen diameter 3.03±0.51 vs. 2.71±0.44 mm, p=0.04) and at follow-up (mean lumen area 10.0±2.1 vs. 7.1±1.8 mm2, p<0.001), and in a lower angiographic late lumen loss (p=0.05). Both strategies resulted in good clinical outcomes at one year, and no stent thromboses.
Conclusions: As compared with a culotte strategy with XIENCE stents, complex bifurcation stenting using a dedicated strategy combining Axxess and BioMatrix stents results in similar stent strut coverage at nine-month follow-up, and a significantly larger lumen and lower angiographic late lumen loss in the proximal MV. (ClinicalTrials.gov NCT01486095)
Introduction
A provisional strategy with a single drug-eluting stent (DES) is generally considered the preferred strategy for percutaneous revascularisation of coronary bifurcation lesions1. More technically demanding dual stenting techniques may present potential advantages in some anatomical subsets, especially when atherosclerotic disease significantly involves both the main vessel (MV) and a side branch (SB) of large calibre. Most dual stenting techniques, however, present limitations, inherent to a tubular stent design not conforming to a variable bifurcation anatomy, and rely on stent deformation, neocarina formation and multiple overlapping strut layers to provide full lesion coverage2. These technical challenges affect optimal stent apposition and re-endothelialisation, and may put the patient at risk for restenosis and stent thrombosis3-5. Therefore, dedicated stents have been designed to accommodate the specific anatomy of a bifurcation, and to preserve branch access and support with a single stent layer6.
The present study compares a dual stenting culotte approach with a thin-strut DES, widely accepted as one of the preferred two-stent bifurcation techniques, with a strategy using Axxess™ (Biosensors Europe SA, Morges, Switzerland), a dedicated self-expanding conical bifurcation stent system with thicker struts, focusing on early and medium-term outcomes using multimodality imaging. The primary hypothesis of this study is that a bifurcation strategy using the Axxess stent leads to similar stent strut coverage to the culotte bifurcation technique. In parallel, this study assesses bifurcation healing after treatment with an everolimus-eluting stent with permanent polymer (PP-EES) versus a biolimus-eluting system with biodegradable polymer (BP-BES).
Methods
The COBRA (COmplex coronary Bifurcation lesions: RAndomized comparison of a strategy using a dedicated self-expanding biolimus-eluting stent versus a culotte strategy using everolimus-eluting stents) study is a prospective randomised controlled two-centre trial. The local ethics committee of both institutions involved approved the study design, and all patients provided written informed consent before the index procedure.
PATIENT POPULATION
The study population consisted of patients with documented stable or unstable angina or a positive functional study, identified for elective percutaneous coronary intervention (PCI) of a de novo and true native coronary bifurcation lesion (Medina classification [1,1,1], [1,0,1] or [0,1,1])7. To be eligible, a two-stent bifurcation treatment strategy was deemed necessary as per the operator’s judgement for optimal lesion treatment, indicating complex and diffuse disease and a large myocardial territory at risk. The required reference vessel diameter (RVD) by visual estimate was 2.75-3.75 mm in the proximal MV and >2.25 mm in the SB.
PROCEDURAL PROTOCOL, DEVICES, AND IMPLANTATION TECHNIQUE
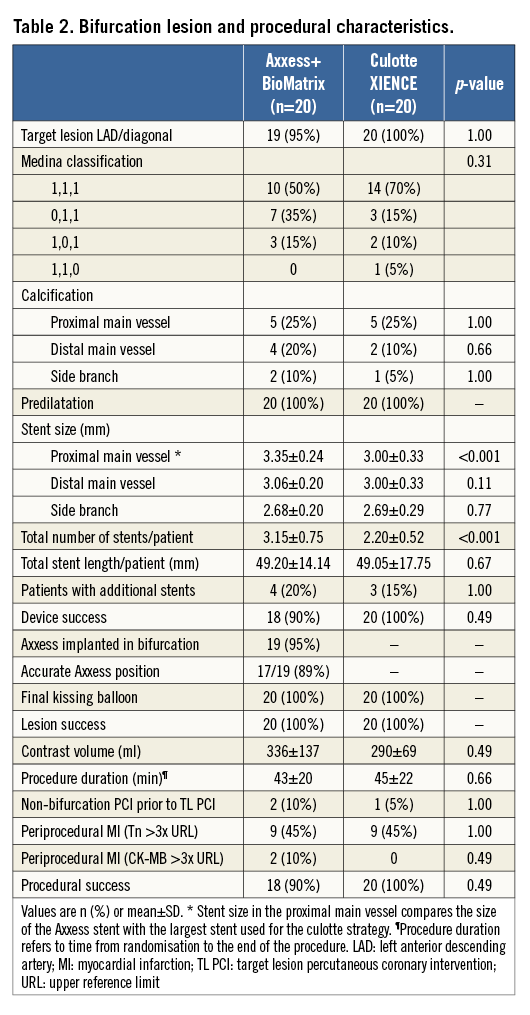
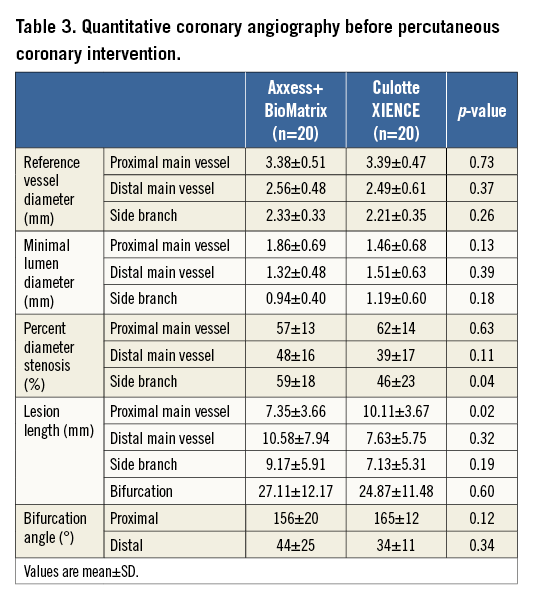
After mandatory predilatation, patients were randomly assigned to treatment with an Axxess stent in combination with two BioMatrix™ stents (Biosensors Europe) or a culotte strategy using two XIENCE PRIME stents (Abbott Vascular, Santa Clara, CA, USA) through an interactive voice response system (IVRS) via an independent entity (Leuven Coordinating Centre), and using a centralised computer-generated random sequence. Product specifications and implantation techniques have been extensively described2,8-10. All procedures were completed by sequential high-pressure, preferably non-compliant balloon inflations in both the MV and the SB, followed by kissing balloon inflations to nominal balloon pressure. A schematic comparison of strategies, devices, and their impact on bifurcation anatomy at the stent strut level is presented in Figure 1.
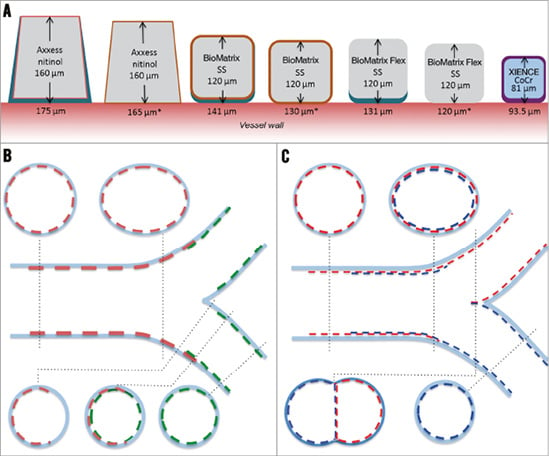
Figure 1. Schematic comparison of devices and techniques at the stent strut level. A) A drawing to scale of struts of the different stents used. Strut thickness of the metallic part (nitinol, stainless steel [SS] or cobalt-chromium [CoCr]) is indicated inside the strut. Values below each strut refer to the total strut thickness indicated by the bidirectional arrow, including primer (whenever applicable) and polymer drug. The values with an asterisk refer to struts after resorption of the polymer (only for BP-BES). BioMatrix Flex™ (not used in this trial) is included for reference purposes only and is identical to BioMatrix, except for the absence of a primer layer. B) & C) Schematic representations of longitudinal and short-axis cross-sections after optimally performed procedures in each treatment group, showing the interaction of different stents. With Axxess+BioMatrix (B), all bifurcation segments are covered with a single strut layer, except for a very short segment at the entry of the branches showing a non-circumferential double strut layer. Notably, the flow divider remains free of stent struts. With XIENCE in culotte (C), the proximal main vessel presents a relatively long segment covered with a double strut layer, and a neocarina is formed at the level of the flow divider.
ANTIPLATELET REGIMEN AND FOLLOW-UP
Aspirin (75-100 mg/day) was given daily and clopidogrel (loading dose of 600 mg and 75 mg/day thereafter) was administered for at least 12 months in all patients. Serial blood samples for creatine kinase, creatine kinase-MB and troponin were routinely obtained eight and 16-24 hrs after the intervention.
All patients were scheduled for nine-month repeat angiography, with optical coherence tomography (OCT) of the target bifurcation. Patients were evaluated clinically at one month after PCI, at eight months, immediately before follow-up angiography at nine months, and at one year.
OCT was performed after intracoronary injection of nitroglycerine, by using the C7XR Fourier domain OCT system (pullback speed 20 mm/sec) and Dragonfly™ intravascular OCT catheters (St. Jude Medical, St. Paul, MN, USA).
QUANTITATIVE CORONARY ANGIOGRAPHY (QCA) AND OCT ANALYSIS
Detailed analysis methodology for both QCA and OCT in bifurcated vessel segments has previously been reported by our group11,12. Analyses were performed by the local core lab, blinded to the assigned treatment.
ENDPOINTS
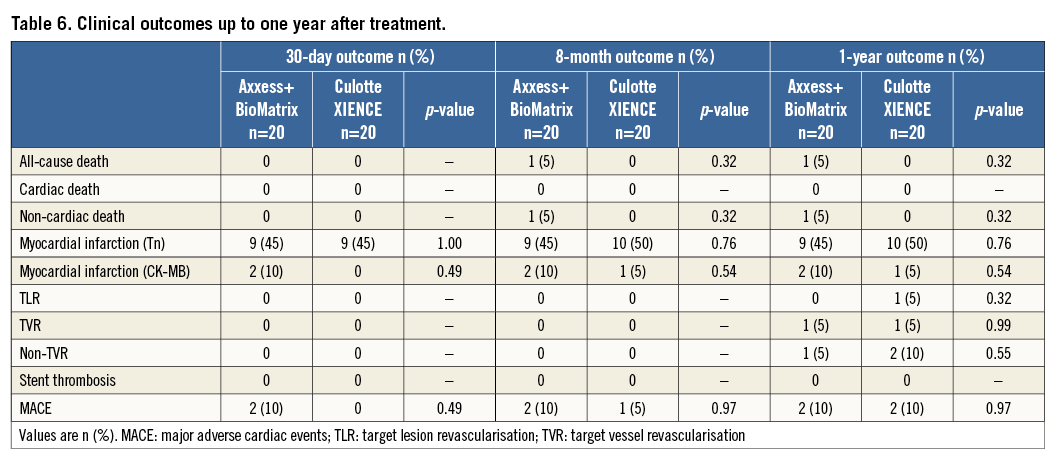
The primary endpoint of the study was the percentage of uncovered stent struts per bifurcation segment, as assessed with OCT at nine-month follow-up. Secondary endpoints included lumen and stent area, and neointimal thickness and area. Angiographic endpoints included binary restenosis (>50% diameter reduction) and late lumen loss (LLL), evaluated per sub-segment (proximal MV, distal MV and SB) and combined. The clinical endpoints of the study were reported for descriptive purposes only and included the rate of major adverse cardiac events (MACE) and their components, all-cause death, target vessel revascularisation (TVR), non-target vessel revascularisation (non-TVR), and stent thrombosis at one month, eight months and one year. MACE were defined as any of the following: cardiac death, myocardial infarction (MI) and ischaemia-driven target lesion revascularisation (TLR).
Data verification was performed by an independent and external international monitoring bureau (MedPass International, Paris, France).
STATISTICAL ANALYSIS
All analyses were performed according to the intention-to-treat principle. Summary statistics are given per randomised treatment group. For continuous measurements, the number of observations with non-missing data, means and standard deviations or medians and interquartile ranges (IQR) are presented, where appropriate. For categorical variables, the observed frequencies and percentages are reported. For baseline lesion and angiographic characteristics, treatment groups were compared using a t-test for continuous variables and a chi-square test for categorical variables. The primary endpoint and all secondary OCT and QCA endpoints were analysed by means of a Wilcoxon rank-sum test on all patients in the intention-to-treat set who had data. In the absence of OCT data on stent strut coverage with both techniques, no formal power calculation could be performed, and patient sample size was arbitrarily set at 40 (20 per group). The clinical endpoints at 30 days were assessed with a Fisher’s exact test. At eight months and one year, comparisons were made using the log-rank test (death) and the Gray’s test for competing risk data (remaining endpoints). All tests are two-sided and assessed at a significance level of 5%. The statistical analysis was performed using SAS/STAT software, Version 9.2 (TS2M3; SAS Institute, Cary, NC, USA).
More detailed descriptions of angiographic and OCT analyses, endpoint definitions, and statistical background are provided in the Online Appendix.
Results
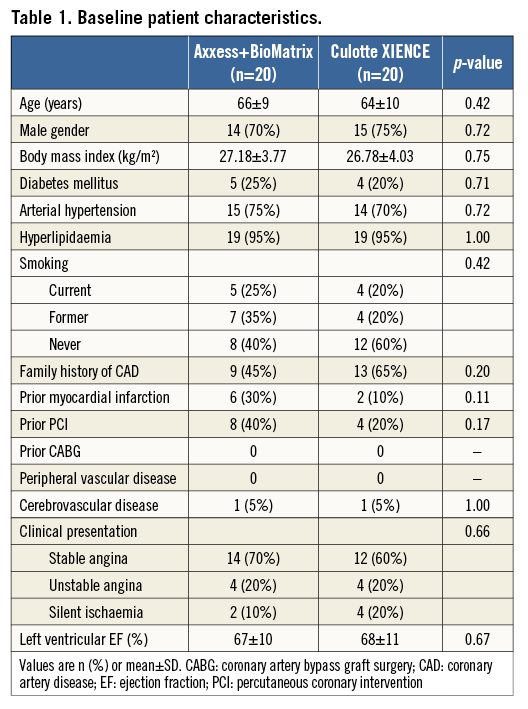
Between November 2011 and November 2012, 40 patients were included in the study at two different sites. Baseline patient characteristics are presented in Table 1. The target bifurcation was the left anterior descending/diagonal in 39 cases; one patient was treated for a lesion in the right coronary artery/posterior descending bifurcation. Bifurcation lesion and procedural characteristics are presented in Table 2. All but one patient had true bifurcation lesions: this patient had a Medina 1,1,0 lesion, but was judged by the operator to require a double stent approach and was therefore included in the study. QCA measurements before PCI are presented in Table 3 and show similar RVD and lesion length in both treatment groups.
PROCEDURAL OUTCOME
Of the 20 patients randomised to Axxess, ultimately 19 underwent successful implantation of the device (95%). In two patients, the partially deployed device was withdrawn, and finally embolised. In one of them, a consecutive attempt with another Axxess was successful; in the other, a provisional bifurcation strategy with a balloon-expandable DES was adopted. In two patients (11%), Axxess position was judged too proximal by the core lab, albeit not affecting later clinical outcome. All patients assigned to culotte underwent a successful implantation of two XIENCE stents. Procedures were completed with kissing balloon inflation in all 40 patients. Notably, stent size in the proximal MV was significantly larger in the Axxess group. Per protocol, the number of stents used per patient assigned to a strategy with Axxess was significantly higher as compared with culotte, but cumulative stent length per patient was similar in both groups.
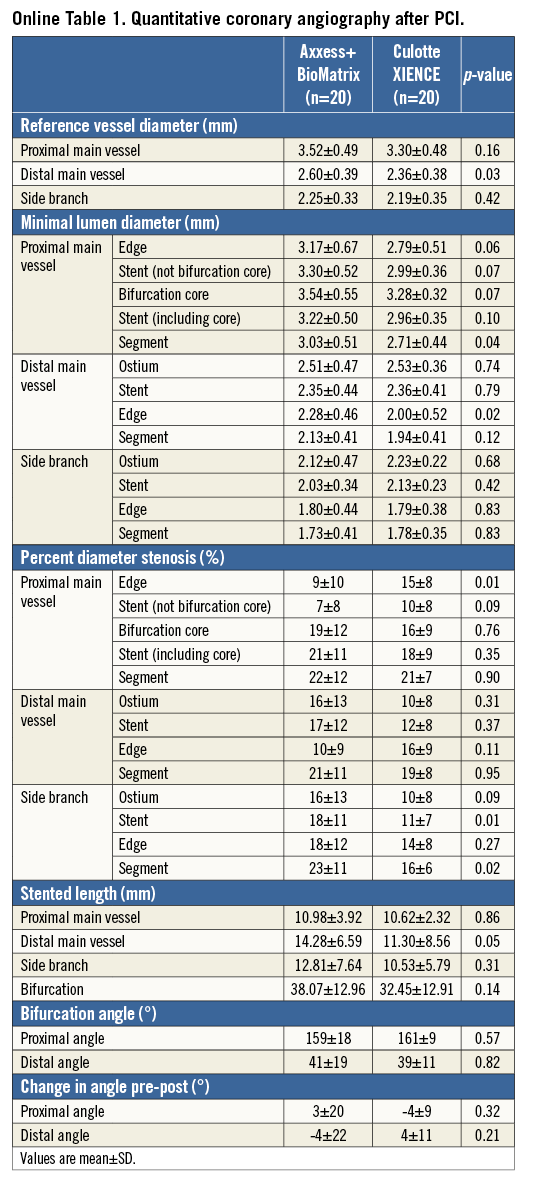
QCA measurements immediately after PCI are presented in Online Table 1. Overall, these results point towards a consistently larger lumen growth in the proximal MV segment with Axxess, whereas similar minimal lumen diameters (MLD) were achieved in both treatment arms in the distal branches. Despite identical cumulative stent length in both groups (Table 2), the stented length in the distal branches was somewhat shorter in patients treated with culotte, due to partial stent overlap in the proximal MV with this approach.
ANGIOGRAPHIC AND OCT OUTCOMES AT FOLLOW-UP
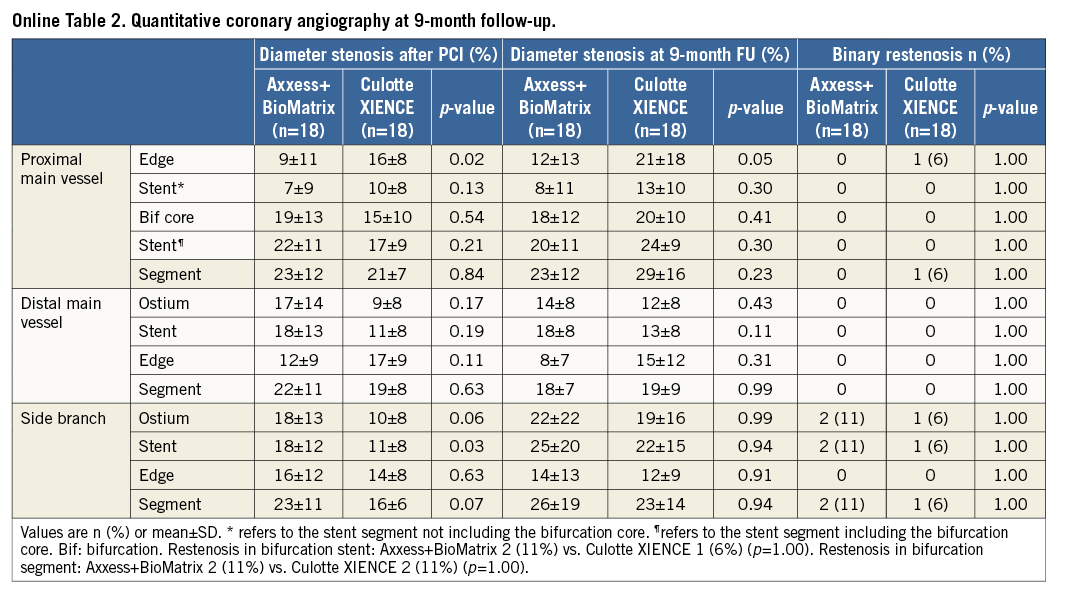
Angiographic follow-up at nine months was available in 36 patients (90%) and is presented in Table 4 and Online Table 2. Three patients refused repeat angiography, and a fourth died before follow-up. The LLL was significantly lower with Axxess in the proximal MV stent segment (0.11 mm vs. 0.35 mm for culotte, p=0.05). In contrast, we observed no significant differences in LLL between BioMatrix and XIENCE in the distal MV stent (0.15 mm vs. 0.31 mm, p=0.09) and in the SB stent (0.29 mm vs. 0.46 mm, p=0.10). Two patients in each group developed binary restenosis in the treated bifurcation segment. These were located at the ostium of the SB for both Axxess patients, whereas with culotte one occurred at the proximal MV stent edge and the other at the ostium of the SB.
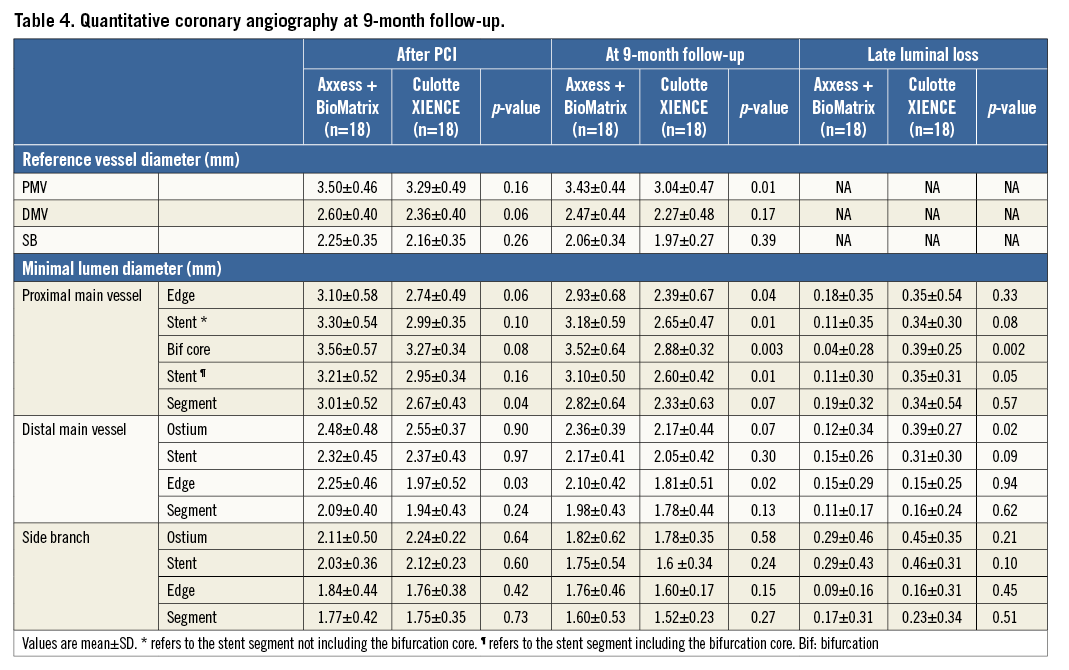
Table 5 shows a summary of the quantitative OCT analysis in 35 patients (88%). In the Axxess group, significantly more stent struts were analysed for the primary endpoint in the proximal MV, due to the specific design of the stent having more struts per cross-section as compared with XIENCE (Figure 2-Figure 4). The primary endpoint of percentage of uncovered struts nine months after bifurcation treatment was not different in the distinct bifurcation segments treated with the Axxess strategy vs. culotte, but numerically there was a tendency towards delayed healing with the dedicated stent strategy (mean 16.4, 11.6, 8.7 and 9.2% vs. 12.8, 8.3, 4.0 and 4.0% uncovered struts in the proximal MV [p=0.19], bifurcation core [p=0.17], distal MV [p=0.09] and SB [p=0.14], respectively). Notably, a gradient in the degree of healing was observed from the proximal bifurcation segment towards the distal branches. Indeed, the percentage of uncovered struts in the proximal MV was significantly higher in each treatment group when compared with the distal branches (estimate 7.44 [95% confidence interval 0.15-14.73] [p=0.045] for Axxess vs. BioMatrix segments; 8.83 [1.76-15.90] [p=0.016] for the double layer proximal XIENCE segments vs. the distal single layer XIENCE segments). With OCT, the significantly larger stent and lumen area with Axxess in the proximal MV confirmed the larger MLD observed with QCA after implantation and at follow-up, respectively (Table 4). Regarding the true bifurcation, a separate analysis on jailing and floating struts and their coverage and bridging, as well as on malapposition at the opposite side of the SB, is presented in Figure 5.
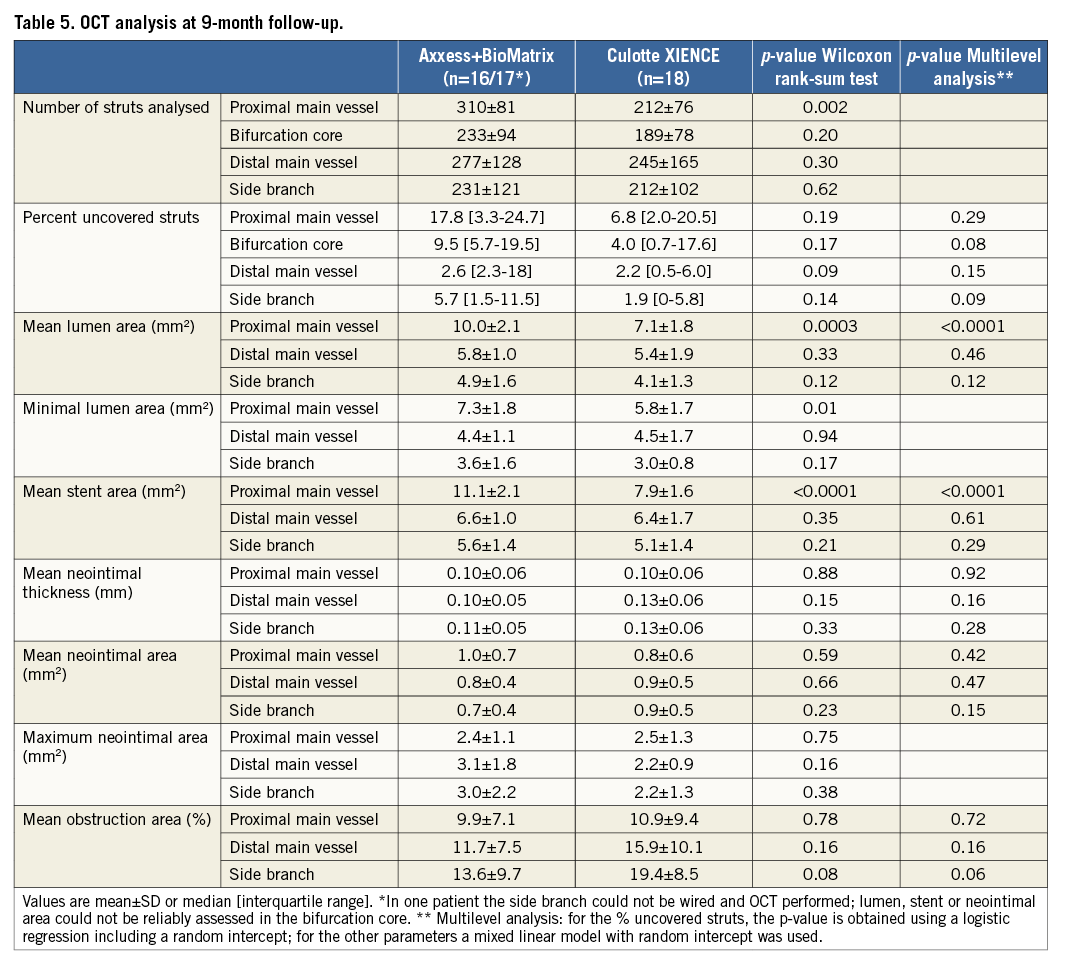
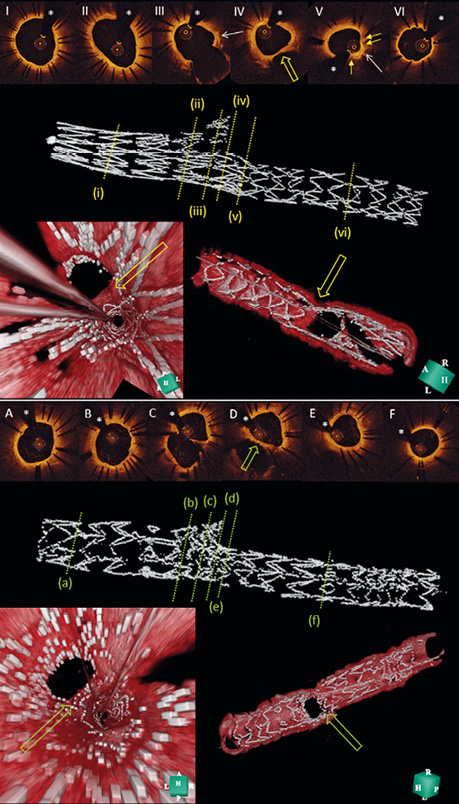
Figure 2. Representative OCT images of optimal results after treatment with Axxess+BioMatrix and culotte with XIENCE. I and II in the top image show a widely patent proximal MV nine months after treatment with Axxess. Note the typical pairwise appearance of the stent struts (I), due to the specific stent structure, and the oval shape of the lumen near the bifurcation (II). At the level of the bifurcation (III-IV), there are no struts visible at the SB ostium and the carina (yellow open arrow in IV and in 3D fly-through [left] and reconstruction [right] bottom panels). The middle panel shows a 3D reconstruction of the stented segment in the MV, with very limited overlap (V) with the BioMatrix stent (yellow arrows). Note the presence of two of the three distal Axxess markers at the level of the bifurcation (white arrows). VI shows a distal branch segment after additional BioMatrix implantation, with a thin layer of neointima covering the stent struts at nine-month follow-up. In the lesions treated with a culotte strategy (lower images), a single layer of stent struts is seen in the proximal segment of the proximal MV (A), whereas a double layer of overlapping stent struts is visible near the bifurcation (B and middle panel with 3D reconstruction of MV stent with higher strut density). At the level of the bifurcation (C-E), stent struts can be seen covering the carina (green open arrow). The distal MV and the SB are covered with a single layer of XIENCE struts (F). *indicates the guidewire artefact. MV: main vessel; OCT: optical coherence tomography; SB: side branch
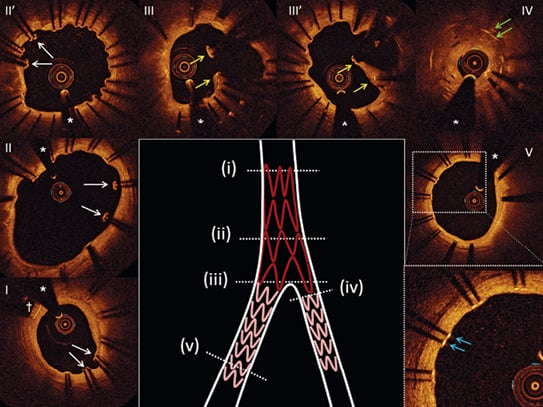
Figure 3. Representative OCT images of inappropriate healing in Axxess-treated lesions at nine-month follow-up. Uncovered and malapposed stent struts in the proximal MV (I - II - II’, white arrows). Note the proximal marker of the Axxess device (dagger); floating stent struts forming a neocarina (yellow arrows) due to a too proximal position of the BioMatrix stent overlapping the Axxess stent at the level of the bifurcation (III - III’); restenosis at the level of the SB ostium (IV); the double layer of stent struts (green arrows); uncovered struts in the distal MV (V, blue arrows). * indicates the guidewire artefact. MV: main vessel; OCT: optical coherence tomography; SB: side branch
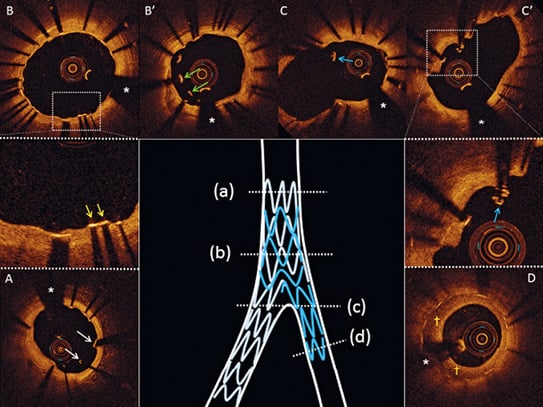
Figure 4. Representative OCT images of inappropriate healing nine months after treatment in culotte. Uncovered and malapposed stent struts in the proximal part of the MV (A, white arrows). At the level of the overlapping stents in the MV, lack of coverage (B, yellow arrows) and malapposition (B’, green arrows) of the abluminal layer of struts can be seen. At the level of the bifurcation, floating stent struts can be noted (C - C’, blue arrows). In the SB neointimal hyperplasia with a speckled pattern can be seen (D, yellow daggers). *indicates the guidewire artefact. MV: main vessel; OCT: optical coherence tomography; SB: side branch
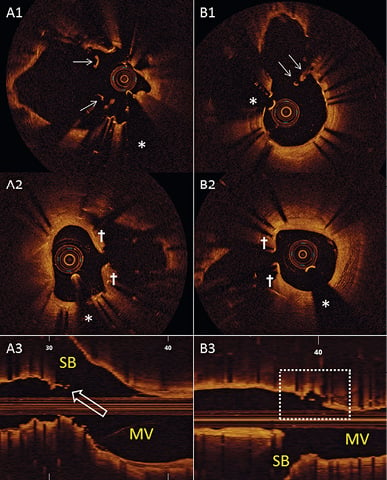
Figure 5. True bifurcation analysis. Three patients treated with Axxess (A) versus five with culotte (B) had floating (A1 and B1) and jailing (A2 and B2) struts in front of the SB ostium, half of which remained uncovered at nine-month follow-up. For Axxess, this finding was exclusively related to a less accurate Axxess position, leading to short neocarina formation with the additional BioMatrix (A3, open arrow). For culotte, this finding was related to the unpredictable interplay of both XIENCE stents at the level of the flow divider. In one additional culotte patient, we observed frank malapposition at the opposite side of the SB (dashed box in B3). *indicates the guidewire artefact. MV: main vessel; SB: side branch
CLINICAL OUTCOMES
We did not observe differences in clinical outcome at 30 days, eight months and one year, and the overall event rate was low (Table 6). One patient in the Axxess arm died three months after PCI as a consequence of an assault-induced intracranial trauma. All but one MI were periprocedural and without clinical consequences; one patient suffered a minor non-ST-elevation myocardial infarction five months after PCI in culotte. At nine months after inclusion, one patient underwent TLR after a culotte procedure due to critical restenosis in the proximal MV segment (fractional flow reserve=0.61); another patient underwent TVR after an Axxess procedure due to angina and severe de novo stenosis of the target vessel distal from the target lesion. There were no cases of stent thrombosis up to one year after the index procedure.
Discussion
The present study reports detailed nine-month angiographic, OCT, and one-year clinical results in 40 patients with true coronary bifurcation lesions, randomly assigned to percutaneous treatment with the dedicated Axxess bifurcation system in combination with two BioMatrix stents, or a conventional double stent culotte technique using XIENCE stents. We observed the following main findings. 1) The primary OCT endpoint of percentage uncovered struts at nine-month follow-up was not different with the dedicated bifurcation strategy with BP-BES vs. a culotte strategy with PP-EES. 2) In both groups, we observed delayed stent endothelialisation of the proximal bifurcation segment as compared with the distal branch stent segments. 3) As compared with culotte, a strategy using Axxess in the proximal MV resulted in significantly larger lumina both acutely and at medium-term follow-up. 4) Both strategies resulted in good clinical outcomes at one year, with no incidence of stent thrombosis.
The primary focus of this study was to explore potential differences in healing related to distinct technical approaches and coronary stent systems in a complex bifurcation setting. While completeness of strut coverage at nine months was not significantly different between the two groups, numerically more struts remained uncovered with Axxess and BioMatrix as compared with XIENCE in culotte in most bifurcation segments. These observations should be interpreted with caution, and may reflect differences in stent platform, antiproliferative drug, and polymers used to control drug release. Firstly, distinct differences in strut thickness are known to affect complete re-endothelialisation, especially when carrying potent antiproliferative drugs or in regions with stent overlap13,14. These differences in thickness of the struts to be re-endothelialised may explain why at nine months coverage tends to be more complete in the culotte patients, and why with both approaches a significant gradient in completeness of coverage was observed from the proximal vessel to the distal branches. Secondly, the simultaneous elution of the highly lipophilic drug and resorption of the polymer over six to nine months in BP-BES may have generated different inflammatory responses in the vessel wall, when compared with PP-EES, which releases the drug over a period of 90 days. Higher tissue drug concentrations and a protracted polymer resorption process with the BP-BES may impact favourably on antirestenotic efficacy, while adversely affecting strut coverage and healing, as previously reported by our group in non-bifurcation lesions15. Thirdly, healing characteristics at nine-month follow-up do not necessarily represent the final stage of re-endothelialisation. While little change in strut coverage was observed in long-term OCT follow-up studies of BioMatrix16,17, no data are available on the long-term fate of uncovered struts with Axxess, or in overlapping stent segments in a coronary bifurcation. Finally, and most importantly, there is no evidence that the slightly less complete stent strut coverage with the BP-BES approach in this study would translate into a higher risk for stent thrombosis. Indeed, large-scale direct comparisons of BP-BES with the thinner-strut PP-EES have shown similar safety and efficacy at one year10,18, and the further increment of definite stent thrombosis from one to five years in the LEADERS trial was limited to 0.7%, suggesting a very low risk of stent thrombosis after the first year9. Similarly, the definite stent thrombosis rate three years after treatment of coronary bifurcation lesions with Axxess in combination with first-generation sirolimus-eluting stents (SES) in the DIVERGE trial was as low as 2.0%, with most events limited to the SES and not involving Axxess19. Of note, the definite stent thrombosis rate three years after culotte stenting with SES in the NORDIC trial was 4.7%20.
The patients in this study had long lesions in large calibre coronary bifurcations, and can therefore truly be considered as showing complex and true bifurcation lesions. All patients were successfully treated with the assigned technique, except for one who in retrospect technically did not qualify for either strategy. Procedure duration was similar for both approaches, and time spent in careful positioning of the Axxess system at the level of the bifurcation carina was compensated by avoiding double wire recrossing to allow for kissing balloon inflation, an often time-consuming step when using a culotte technique. Procedures were clinically uneventful, and all patients obtained a satisfactory angiographic result. Notably, MLD in the proximal MV segment was significantly larger immediately after treatment with the Axxess vs. culotte strategy, a finding that was further supported by the significantly larger stent area in the proximal MV at OCT follow-up. This difference can most likely be attributed to a more correct stent sizing with Axxess, according to the true RVD of the proximal segment, as opposed to a more conservative sizing according to the distal branches when performing a culotte procedure. Despite proximal optimisation, high-pressure inflations, and kissing balloon inflation, these manoeuvres with the culotte approach appear not to allow the double-layered undersized stent cage to conform fully to the true proximal RVD. Improved stent conformability with the conical Axxess is further supported by its self-expanding properties, a quality that is especially beneficial at the level of the flow divider and appears to be further accentuated over time21,22.
Both QCA and OCT at nine months reveal sustained benefit of Axxess vs. a culotte approach in the proximal MV in terms of lumen diameter and cross-sectional area, resulting in a significantly lower LLL with Axxess, particularly in the bifurcation core. In contrast, no volumetric differences were seen in the distal branches. These findings can predominantly be attributed to the specific design and persistent outward radial force of Axxess as opposed to a fixed double-layered XIENCE segment, while the balloon-expandable DES in the branches are known to present similar LLL15,18.
Limitations
The main limitation of our study is the small number of patients included, not allowing conclusions to be drawn on the clinical impact of different bifurcation stenting techniques. This limitation is, however, largely compensated for by the analysis of more than 32,000 struts for the primary endpoint, despite five patients not being available for OCT follow-up. OCT and QCA analyses were not performed in independent core labs, but with blinding to the assigned treatment.
Conclusion
In conclusion, as compared with a culotte strategy with the XIENCE PP-EES, complex coronary bifurcation lesion stenting using a dedicated strategy combining the Axxess and BioMatrix BP-BES results in similar stent strut coverage at nine-month follow-up, and a significantly larger lumen in the proximal MV.
| Impact on daily practice The present study adds to the available evidence that modular reconstruction of complex bifurcation anatomy with the dedicated Axxess bifurcation drug-eluting stent (DES) in the proximal main vessel and additonal DES in the branches is safe and highly effective. More than with the popular culotte technique with balloon-expandable DES, the conical shape of Axxess and its self-expanding properties restore bifurcation anatomy and vessel patency in a predictable fashion, while preserving the vessel healing, clinical performance and safety of latest-generation DES. |
Funding
This study was an investigator-initiated trial, supported with a grant from Biosensors Europe SA (Morges, Switzerland). Investigators equally benefited from unrestricted research grants from Abbott Vascular (Santa Clara, CA, USA).
Conflict of interest statement
The authors have no conflicts of interest to declare.
SUPPLEMENTARY DATA
Online Appendix. Supplement to the Methods section
QUANTITATIVE CORONARY ANGIOGRAPHY (QCA) AND OPTICAL COHERENCE TOMOGRAPHY (OCT) ANALYSIS
Digital coronary angiograms were analysed offline by the local core lab blinded to the assigned treatment, using a validated automated edge detection system (QAngioXA version 7.2; Medis, Leiden, The Netherlands). Matched views were selected for angiograms recorded before and immediately after the intervention and at follow-up. Measurements were performed and results reported according to the consensus statement of the European Bifurcation Group, including two-dimensional angulations between MV and SB, segmental analysis and combined lesion results23.
OCT was performed after intracoronary injection of nitroglycerine, by using the C7XR Fourier domain OCT system (pullback speed 20 mm/sec) and Dragonfly™ intravascular OCT catheters (St. Jude Medical, St. Paul, MN, USA). Pullbacks were started at least 5 mm distal from the most distal stent struts in both MV and SB as soon as optimal blood clearance was obtained.
Quantitative and qualitative OCT analyses were performed offline by the local core lab blinded to the assigned treatment. The validated OCT Detecting Instrument for StEnt Reendothelialization aNd Apposition (ODIERNA, Leuven, Belgium) was used for automatic detection of struts and their coverage, neointimal thickness, lumen area, and stent area12,24. In brief, OCT analysis was performed separately in four bifurcation segments (proximal MV, bifurcation core, distal MV, and SB). In the proximal MV, distal MV and SB, each third frame was analysed (0.6 mm interval); in the bifurcation core each frame was analysed (0.2 mm interval). In each analysed frame, the centre of the luminal surface of the strut was determined for each strut detected, and its distance to the lumen contour was calculated automatically to determine strut-level intimal thickness. For the primary endpoint, a strut was considered uncovered if any part of the strut was visibly exposed to the lumen (strut-level intimal thickness=0). The results of the automatic analysis were visually inspected and manually corrected by an expert OCT image reader whenever needed.
ENDPOINT DEFINITIONS
All deaths were considered cardiac unless an unequivocal non-cardiac cause could be demonstrated. MI was defined as evidence of myocardial necrosis in a clinical setting consistent with myocardial ischaemia. Typically, this included detection of rise in cardiac biomarkers with at least one value above the 99th percentile of the upper reference limit (URL) together with symptoms of ischaemia, or pathognomonic electrocardiographic or imaging evidence of ischaemia. By convention, increases of cardiac biomarkers above 3x the URL were used to define PCI-related MI. We report separately MI based on troponin25 and CK-MB26. For MACE, only MI based on CK-MB elevation was taken into consideration.
Repeat revascularisation for restenosis of the index bifurcation lesion (TLR) was only performed if there was significant angiographic restenosis (70% diameter stenosis anywhere within the target lesion) in combination with clear angina or a fractional flow reserve <0.80 in the MV or a SB subtending a large myocardial territory. Stent thrombosis was defined according to the Academic Research Consortium criteria27. In addition, several procedure parameters were assessed. Primary device success was defined as deployment of the assigned stents without system failure or device-related complication. Lesion success was defined as attainment of <50% residual stenosis of the target lesion using the assigned device or any percutaneous method. Procedure success was defined as attainment of a final lesion (angiographic) success in the absence of any in-hospital MACE.
STATISTICAL ANALYSIS
In the first instance, the primary endpoint and all secondary OCT and QCA endpoints were analysed by means of a Wilcoxon rank-sum test on all patients in the intention-to-treat set who had data, using patient/bifurcation segment-level data. In brief, with regard to the primary endpoint, for each bifurcation segment the patient-level percentage of uncovered struts was first obtained separately for each patient, and then the median of these percentages was reported as the overall percentage estimator. In addition, mixed linear or logistic regression models were used to analyse the strut-specific data, whereby a random intercept was included to account for clustering of the data within a patient (multi-level model).