Abstract
Aims: The aim of this IVUS substudy was to assess the efficacy of the Y-shaped Medtronic bifurcation-dedicated stent (BDS) for the treatment of de novo coronary bifurcated lesions.
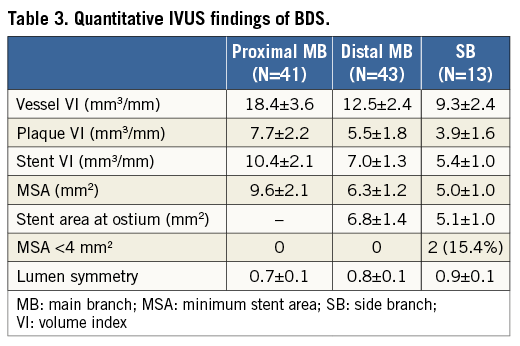
Methods and results: In the BRANCH trial, post-procedure IVUS was performed in 45 patients. IVUS was available in both branches in 19 lesions and only the main branch (MB) in 26 lesions. IVUS analysis included four distinct locations: proximal MB, bifurcation site, distal MB, and side branch (SB). Lumen symmetry was calculated as minimum/maximum lumen diameters. The quantity of isolated stent struts across the SB ostium was used to assess inadequate strut apposition to the carina resulting in partial jailing of the SB orifice. A minimum stent area (MSA) <4 mm2 was found in 0% of proximal and distal MB, and in 15.4% of SB. In SB, MSA was located mainly at mid or distal segments (84.6%), rather than at the SB ostium. Eccentric stent expansion and edge dissection were seen primarily at proximal MB. Isolated struts were seen in only 20.9% of SB ostia with a minimum length of 0.7±0.4 mm.
Conclusions: Implantation of BDS resulted in adequate stent dimensions and strut apposition at the carina and SB ostium. ClinicalTrials.gov Identifier: NCT0060732
Introduction
Compared to non-bifurcation lesions, percutaneous coronary intervention (PCI) of bifurcation lesions is complex and challenging with lower procedural success rates and higher restenosis rates1-3. Although drug-eluting stents (DES) have reduced neointimal proliferation, high rates of restenosis are still observed, especially in the side branch (SB) ostium. Multiple randomised studies1,4-8 and meta-analyses9-11 indicate that a provisional main branch (MB) stenting strategy is as efficacious as a two-stent strategy. Despite the simplicity of the provisional approach, since this technique frequently leaves a significant residual stenosis at the SB ostium, it may not be suitable for extensive disease at the SB. The development of a dedicated bifurcation stent system to provide adequate mechanical scaffolding at the SB ostium and carina of coronary bifurcation lesions may provide an important alternative. The Medtronic Y-shaped bifurcation-dedicated stent (BDS) (Medtronic Inc., Santa Rosa, CA, USA) is a novel dual balloon-deployed, thin-strut, Y-shaped, cobalt-based bare metal stent designed to provide easy SB access and reduce residual stenosis in the MB and SB. The BRANCH trial (Bare metal bifuRcAtion steNt Clinical trial in Humans) is a first-in-man, prospective, multicentre, non-randomised, single-arm trial to assess the safety and feasibility of the BDS for the treatment of single de novo bifurcation lesions in native coronary arteries12. The aim of this intravascular ultrasound (IVUS) substudy of the BRANCH trial was to describe quantitative and qualitative IVUS findings in the proximal/distal MB and SB following BDS treatment. A secondary aim was to perform detailed analyses of stent dimensions and strut apposition at the SB ostium and carina, and to compare these results with historical IVUS data from bifurcation lesions treated with MB stenting and SB angioplasty.
Methods
TRIAL DESIGN
Details of patient selection criteria for the BRANCH trial have been described previously12. Briefly, eligible patients had single or multivessel coronary artery disease, but only one bifurcation lesion per patient was treated. Lesion criteria for BDS implantation in this trial were as follows: (a) a bifurcation lesion with ≥50% stenosis in the MB; (b) reference diameters (RD) for the proximal MB of 3.8-4.3 mm, distal MB of 3.0-3.5 mm, and SB of ≤2.5 mm; and (c) total lesion lengths could be any combination of ≤16 mm proximal from the carina in the MB, or ≤16 mm distal from the carina in the MB, or ≤12 mm from the carina in the SB. Patients with acute myocardial infarction, left main disease, or with additional lesions in the target vessels were excluded. The current analysis includes IVUS results from all of the lead-in patients enrolled in the BRANCH trial which are not reported in the primary trial publication12.
DEVICE AND PROCEDURE DESCRIPTION
The BDS is a novel dual balloon-deployed, thin-strut (91 microns), Y-shaped, cobalt-chromium-based bare metal stent designed for the treatment of bifurcation lesions. The rationale for use of a dual-branch stent is to simplify the complexity of treatment strategy, such as rewiring of the SB through the body of the MB stent, as well as a number of additional complicated procedural steps needed in conventional bifurcation techniques with cylinder-shaped workhorse stents. Compared with other dedicated bifurcation stents which may require a second stent implantation in the SB with residual stenosis, the BDS has a longer stent length in the SB to achieve complete scaffolding of the SB lesion while avoiding additional stenting and minimising excessive stent struts at the SB ostium and carina, particularly in complex lesions with acute bifurcation angles. The dual balloon has a stepped-balloon design to avoid proximal overexpansion and a dual-wire delivery system with simultaneous inflation (Figure 1). This method of deployment is similar to the “kissing balloon” technique commonly used in bifurcation stenting. Two models of the stent were available for the BRANCH study (3.8/3.0/2.5, 4.3/3.5/2.5, proximal MB/distal MB/SB, mm). Per protocol, all target lesions were predilated prior to deployment of the BDS. After BDS implantation, additional stent(s) could be placed in order to achieve an optimal angiographic result, using bare metal stents (Driver; Medtronic Inc., Santa Rosa, CA, USA). Post-dilation, including kissing balloon inflation, was allowed but not mandatory.
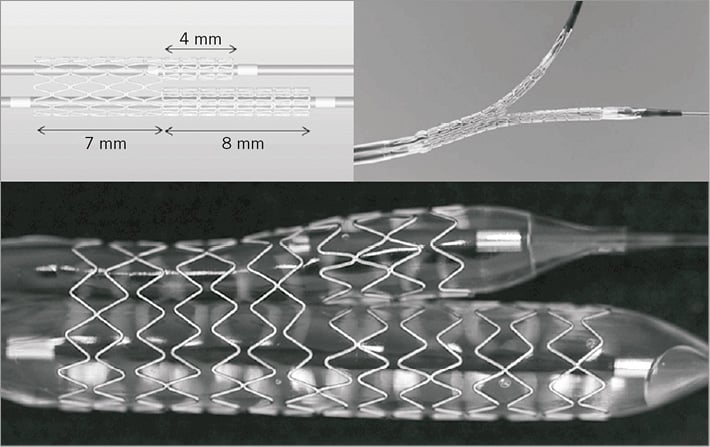
Figure 1. Medtronic Y-shaped bifurcation-dedicated stent. The BDS is a novel dual balloon-deployed, thin-strut, cobalt-chromium-based bare metal stent with dual-branch shape. The dual balloon has a stepped-balloon design to avoid proximal overexpansion and a dual-wire delivery system with simultaneous inflation.
The study was conducted according to the Declaration of Helsinki, and the medical ethics committees at all sites approved the study protocol. Written informed consent was obtained from each patient.
IVUS analysis
IVUS was performed in a standard fashion at post-procedure, using 0.5 mm/s automated motorised pullback with commercially available imaging systems (40-MHz IVUS catheter; Boston Scientific Corp., Natick, MA, USA, or 20-MHz IVUS catheter; Volcano Corp., San Diego, CA, USA). Standard IVUS analyses were performed by an independent core laboratory (Cardiovascular Core Analysis Laboratory, Stanford University, Stanford, CA, USA). Quantitative and qualitative analyses were performed using PC-based software (echoPlaque; Indec Medical Systems Inc., Santa Clara, CA, USA), as previously described13. In addition, for detailed IVUS analysis, bifurcation lesions were divided into four distinct locations: proximal MB, bifurcation site, distal MB, and SB (Figure 2A). The analyses of these MB and SB segments were performed using the images obtained from IVUS pullback of the corresponding vessel segment. In volumetric analysis performed at proximal and distal MB, and SB stent segments, lumen, stent, vessel, and plaque (vessel minus lumen) volumes were calculated using Simpson’s method, and each volume was divided by measurement length to adjust for different measurement lengths (volume index: VI, mm3/mm). 2D analysis was performed at the borders between the bifurcation site and each branch. Lumen symmetry was calculated as minimum lumen diameter divided by maximum lumen diameter. Evaluation of stent struts jailing the SB orifice was based on the extent of isolated struts found in this region using images obtained from the MB IVUS. Isolated struts were defined as one or more stent struts across the SB ostium with evidence of blood speckle between the struts (Figure 2B). In the presence of isolated struts, their longitudinal length was measured to evaluate the severity of this observation (Figure 2C). Tissue prolapse, stent edge dissection and incomplete stent apposition (ISA) were assessed by qualitative IVUS analysis. ISA was identified as one or more struts clearly separated from the vessel wall with evidence of blood speckles behind the strut14.
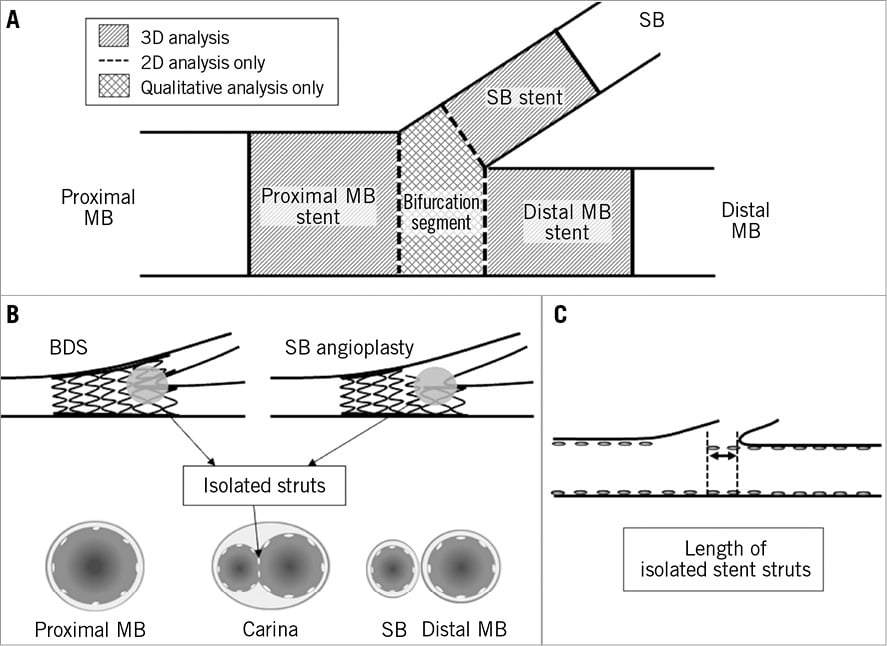
Figure 2. Method of IVUS analysis. A) A schematic diagram of IVUS analysis for bifurcation lesion. Bifurcation lesions were divided into four distinct locations: proximal main branch (MB), bifurcation site, distal MB, and side branch (SB). Volumetric analysis was performed at proximal and distal MB, and SB stent, and 2D analysis was performed at the borders between the bifurcation site and each branch. B) Schema of isolated struts at carina. Isolated struts were defined as one or more stent struts with evidence of communication of blood speckle between the stent struts across the SB ostium. BDS: dual-branch shape bifurcation-dedicated stent. C) Axial segment length with isolated struts.
To evaluate the influence of the BDS on the SB ostium, we performed a post hoc comparison of IVUS parameters of lumen dimensions and jailing stent struts at the SB with data obtained from the core laboratory database (the PRESSURE trial: a prospective, multicentre, non-randomised, single-arm trial to assess the predictor and mechanism of an SB jail after MB stent implantation in bifurcation lesions)15.
Statistical analysis
Continuous variables are expressed as mean±standard deviation or frequencies. Continuous variables were analysed by the unpaired t-test. Categorical data were compared by chi-squared or Fisher’s exact test. A p-value <0.05 was considered statistically significant. StatView 5.0 (SAS Institute, Cary, NC, USA) was used for data analyses.
Results
STUDY POPULATION AND BASELINE CHARACTERISTICS
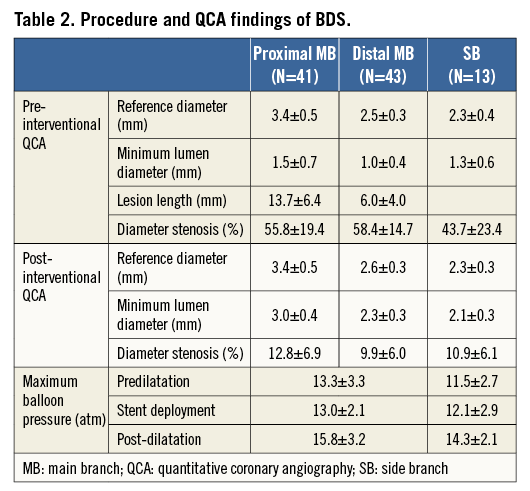
Among the 60 patients enrolled in the BRANCH trial, IVUS was performed in 45 cases post-procedure. Baseline clinical characteristics of this IVUS cohort are shown in Table 1. Procedure and quantitative coronary angiography (QCA) are shown in Table 2.
IVUS ANALYSIS IN BDS
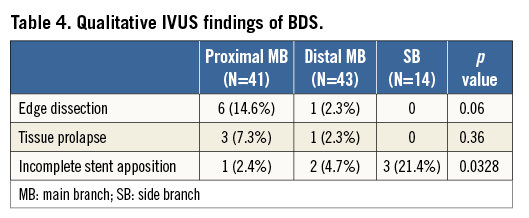
IVUS findings of BDS are shown in Table 3 and Table 4. IVUS was performed in the main branch in 45 lesions and the side branch in 19 lesions. Of these lesions, four lesions of proximal MB, two lesions of distal MB, and five lesions of SB were excluded from the qualitative IVUS analysis because of additional stent implantation or poor IVUS image quality. Also, one lesion of the SB was excluded from the volumetric IVUS analysis because of inconsistent IVUS pullback. Finally, qualitative IVUS analysis was available in 41 with proximal MB, 43 with distal MB, and 14 with SB, and volumetric IVUS analysis was available in 41 with proximal MB, 43 with distal MB, and 13 with SB. The vessel, plaque, and stent VI, and minimum stent area (MSA) were smaller in SB than in proximal and distal MB. An MSA <4 mm2 was found in 0% of proximal and distal MB, and in 15.4% of SB. Stent area at the SB ostium was smaller than stent area at the distal MB ostium. In SB, the MSA was located mainly at mid or distal segments (84.6%), rather than at the SB ostium. Less lumen symmetry and more edge dissection was seen at the proximal MB (p<0.0001 and p=0.06). Isolated struts across the SB ostium were seen in 20.9% of cases. The axial segment length with isolated struts was 0.7±0.4 mm.
COMPARISON OF IVUS FINDINGS BETWEEN BDS VS. CONVENTIONAL TECHNIQUE
When a comparison was made with a “control group” representing cases successfully treated with MB stent implantation followed by SB angioplasty (MB: N=30, SB: N=8), BDS cases showed: 1) a larger MLA in SB while pre-intervention reference diameter was similar between the two groups; 2) the incidence of MLA <4 mm2 in SB was less frequent; 3) larger lumen area and more symmetric expansion at the SB ostium; and 4) a significantly lower incidence of isolated struts compared with the SB angioplasty groups. Moreover, axial segment length with isolated struts was significantly shorter in BDS compared with the SB angioplasty group (Table 5). Representative IVUS images of the BDS and SB angioplasty groups are shown in Figure 3.
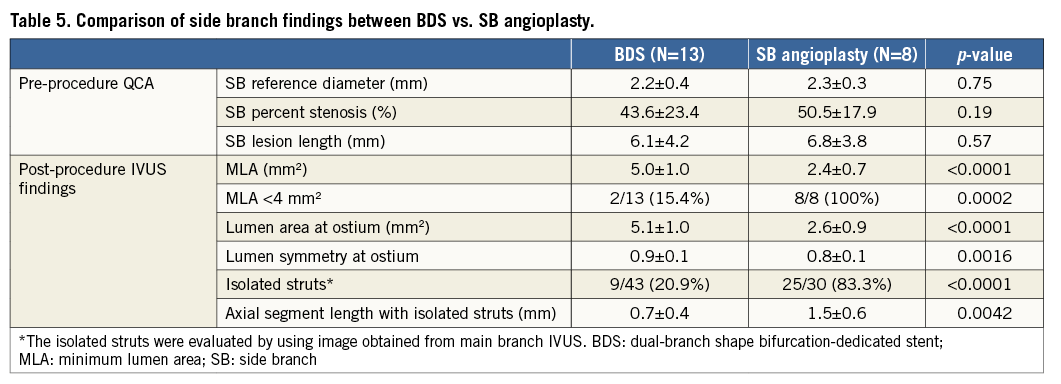
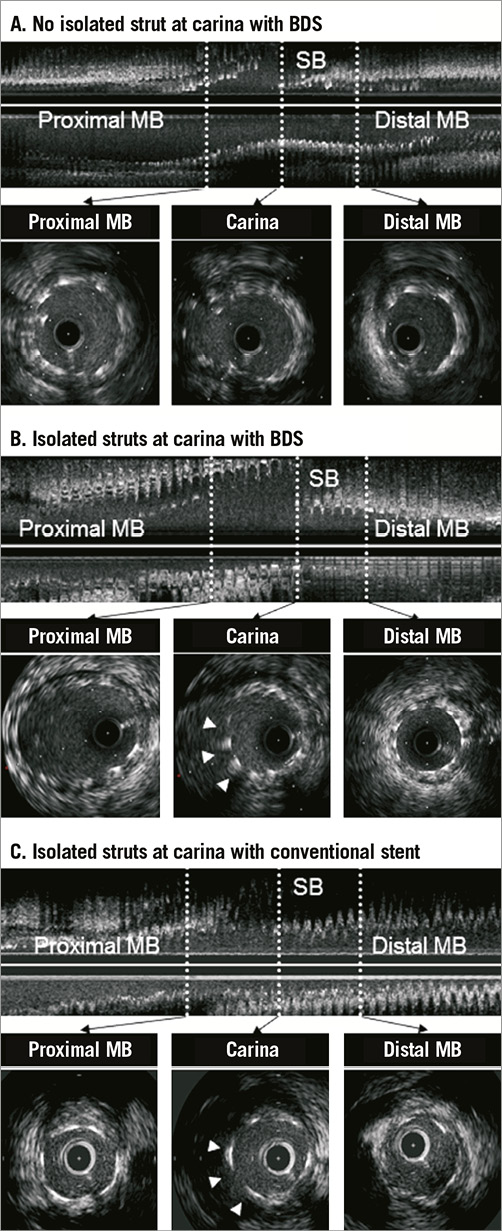
Figure 3. Representative cases. A) Case of no isolated struts at carina with BDS. Stent was well apposed against the vessel wall and isolated struts were not observed at bifurcation. B) Case of ISA at carina with BDS. Isolated stent struts (arrowheads) were observed (length=1.3 mm). C) Case of isolated struts at carina with conventional stent. Isolated stent struts (arrowheads) were observed (length=2.1 mm).
Clinical outcomes
Major adverse cardiac events (MACE) at 30 days included two non-Q MI (4.4%) with no deaths, stent thrombosis, or target lesion revascularisation occurring in this IVUS cohort (n=45).
Discussion
This IVUS analysis of bifurcation lesions treated with BDS showed: 1) frequency of stent underexpansion (MSA <4 mm2) was 0% in proximal and distal MB, and only 15.4% in the SB; 2) less symmetric stent expansion and more frequent edge dissections were seen mostly at the proximal MB; 3) the SB ostium was larger and more symmetric with BDS treatment than in the SB angioplasty group; and 4) isolated struts at the SB ostium were detected less frequently in BDS treated lesions, suggesting fewer SB-jailing stent struts with BDS than in the SB angioplasty group.
STENT UNDEREXPANSION AT SB OSTIUM
Bifurcation lesions remain a challenging lesion subset for PCI because rates of restenosis at the SB ostium are high despite the use of DES and various stent techniques. Recent IVUS studies have suggested that inadequate post-procedural MSA appeared at the SB ostium in the bifurcation lesion treated with crush technique16, and the post-procedure MSA at the SB ostium was an important predictor of restenosis after T-stent technique on bifurcation lesions17. In this study, the SB angioplasty group without an additional stent could achieve only 2.6 mm2 of lumen area at the SB ostium, and stent underexpansion at the SB ostium was seen in 100% of the SB angioplasty group at post-procedure. On the basis of these IVUS results, ostial SB lumen stenosis using conventional stenting techniques with standard workhorse tube stent(s) may be primarily determined by mechanical factors, such as stent strut underexpansion at post-procedure. A previous study reported that crush stenting can achieve 4.2 mm2 of stent area at SB ostium at post-procedure1. Nevertheless, this technique can potentially obstruct the SB ostium with multiple layers of stent mesh. In contrast, the BDS can achieve 5.1 mm2 of lumen area at SB ostium at post-procedure without multiple layers of additional stent, indicating the successful achievement of adequate luminal dimension without an excessive burden of metal at the SB ostium.
ISOLATED STENT STRUTS JAILING THE SB OSTIUM
Previous studies have suggested that a large amount of metal at the ostium and increased drug concentration at the layered strut site may cause exaggerated neointimal hyperplasia and delayed endothelialisation on the stent surface, leading to stent thrombosis after DES implantation18-20. An autopsy study demonstrated that stent thrombosis occurred at the site of non-endothelialisation of struts at the bifurcation carina area and protruding stent struts (isolated struts) at the proximal main vessel21. In this study, isolated struts at the SB ostium were less frequent in BDS treated lesions compared with SB angioplasty. Although this finding was not completely eliminated in BDS (presumably due to suboptimal stent positioning at the carina in some cases), the length of isolated struts was shorter in the BDS treated lesions than in the SB angioplasty group. The smaller amount of metal at the SB ostium compared with the SB angioplasty group may in part account for the low incidence of stent thrombosis at 30 days and 12 months in the BRANCH trial12. Further studies with drug-eluting BDS may clarify the possible association between stent thrombosis and isolated struts at SB ostium.
SYMMETRIC LUMEN EXPANSION IN MB AND SB SEGMENTS
We previously reported that the degree of luminal narrowing of SB after MB stenting was a result of carina and plaque shift, which affects stenosis eccentricity. Angiographic minimum lumen diameter of an eccentric SB ostial lesion usually underestimates the true lumen area based on limitations inherent in angiographic assessment of the SB narrowing after MB stenting15. In this IVUS study, a dual-branch, Y-shaped BDS resulted in greater lumen area and more symmetric lumen dimension at the SB ostium than the SB angioplasty group, reflecting that the BDS appears to scaffold the SB ostium more completely and evenly with less potential residual stenosis. On the other hand, a less symmetric lumen with larger stent expansion and edge dissection was seen mostly at the proximal MB in BDS. These findings may in part be due to the simultaneous dual-balloon inflation during the BDS deployment, despite its stepped-balloon design to avoid proximal overexpansion, combined with limited device size selections in the current study. Further improvement of balloon design as well as future availability of various device sizes may lead to optimal lumen expansion of both MB and SB segments while minimising vessel injury at the stent edges.
Clinical implications
The BDS was specifically designed to provide a technological solution for PCI in challenging bifurcation lesions. When compared with post-procedural IVUS results of conventional stenting techniques, BDS use appeared to overcome some consistent mechanical limitations at the SB ostium. In addition, the BDS can maintain direct access to the SB due to its unique stent design, which may be important during the index procedure, as well as if access to the SB is required in the future. Moreover, this IVUS study showed that the BDS enables greater SB ostium expansion with fewer isolated stent struts than conventional stenting techniques. While these acute results seem promising, a larger trial with long-term follow-up will be required to confirm the true clinical applicability and benefit in these complex lesions.
Limitations
Several limitations should be noted. First, this was a first-in-man study based on a relatively small sample size with full IVUS evaluations of both MB and SB not performed in all patients. Also, the SB IVUS data of the PRESSURE trial were available in a subgroup of patients, making the comparison of these two studies only hypothesis-generating. Second, the original trial was designed to test the safety and feasibility of the BDS, and therefore follow-up IVUS was not mandated by protocol. Third, IVUS analysis has some intrinsic limitations, including image reconstruction and image interpretation, as previously reported13.
Conclusion
In this first-in-man study, implantation of the bifurcation-dedicated stent appears to achieve adequate stent dimensions and strut apposition at the side branch ostium and carina.
| Impact on daily practice Bifurcation lesions remain a challenging lesion subset for PCI because rates of restenosis at the side branch (SB) ostium are high despite the development of various stenting techniques. The Y-shaped bifurcation-dedicated stent (BDS), specifically designed to achieve adequate stent dimensions and strut apposition at the SB ostium and carina, may help overcome the mechanical limitations of conventional tubular stents. In daily practice, the BDS can also maintain direct access to the SB due to its unique stent design, which may be important when operators need to rewire the SB during the index procedure or at future interventions. |
Acknowledgements
The authors thank H. N. Bonneau, RN, MS, CCA, for her review of this manuscript.
Conflict of interest statement
I. Meredith has served as an advisory board member for Medtronic Vascular. P. Fitzgerald received institutional grant support from Medtronic Vascular. The other authors have no conflicts of interest to declare.

