Abstract
Aims: Percutaneous coronary intervention (PCI) of bifurcation lesions is complex and is technically very demanding. Coronary angiography is considered the gold standard method to guide PCI but has several limitations. The purpose of this study was to determine the utility of stent enhancement with StentBoost® (StB), a novel fluoroscopic imaging technique, and its potential role during bifurcation PCI.
Methods and results: This prospective study included 97 patients who underwent bifurcation PCI (98 bifurcations), using StB. Bifurcation lesions were classified according to the modified Medina classification. StB was performed in all patients to obtain improved stent visualisation and to detect optimal release and deployment. Therefore, three groups were formed, according to the quality of image: optimal visualisation, suboptimal visualisation and poor visualisation. Most of the bifurcation disease involved the main vessel (99%) and in 80 patients (81.6%) there was side branch involvement. Most bifurcations had both main vessel and side branch lesions (Medina 1,1,1) (70 patients, 71.4%). StB image quality was good in 79.6% of the cases (optimal visualisation of the stent and guidewire), was suboptimal in 19.4%, and poor in 1% (overlapping of structures or devices). In three cases, StB enabled the identification of the guidewire and angioplasty balloon passing outside stent borders during rewiring of the side branch.
Conclusions: Imaging techniques have a primary role during bifurcation PCI. StentBoost is a simple and quick method that offers several advantages, enabling improved stent visualisation, appropriate rewiring of the side branch, adequate stent expansion and optimal apposition of the struts to the wall.
Introduction
Coronary artery bifurcations are prone to the development of atherosclerotic plaques, mainly due to haemodynamic alterations that can lead to focal or more diffuse disease1. In the era of drug-eluting stents, these lesions account for 15 to 20% of all coronary artery disease treated with percutaneous coronary intervention (PCI)2,3 and are considered highly complex and technically demanding. The most suitable strategy for treating bifurcation disease is still a matter of debate and the decision regarding main vessel stenting alone versus the employment of two stents is controversial. Several randomised trials which address this issue have been published in recent years4,5. The recently published European Bifurcation Club consensus comprised a review of clinical trials from the year 2009 and concluded that provisional T stenting remains the appropriate technique for most bifurcations, but large side branches, long side branch lesions or complex anatomy may require a two-stent strategy6,7. Despite advances in equipment and stent implantation strategies, both acute and long-term complications such as thrombosis and in-stent restenosis still occur. Coronary angiography is considered the gold standard method for the evaluation and classification of coronary stenosis, and also to guide PCI. However, this method has several limitations such as vessel overlap, foreshortening and underestimation of lesion severity. During PCI, stent structure visualisation is difficult after implantation, and rewiring the side branch during bifurcation angioplasty can become challenging. StentBoost® (Philips Medical Systems, Best, The Netherlands) is a recently developed imaging technique that enhances stent fluoroscopic visibility. Through motion-corrected acquisition frames, an enhanced picture of the stent and its relation with the vessel wall is obtained. The purpose of this study was to evaluate the improvement of stent visibility obtained with StentBoost (StB) and to summarise the potential role of this new imaging tool during bifurcation PCI.
Methods
This single-centre prospective study included ninety-seven consecutive patients who underwent complex bifurcation angioplasty with the use of StB. Inclusion criteria were patients with angiographic evidence of a significant bifurcation lesion (stenosis of at least 70% diameter at one, two or both branches) and clinical indication for PCI with stent implantation. Exclusion criteria included any contraindication for PCI or PCI without stent implantation. This study complied with the Helsinki Declaration and all patients signed informed consent.
Baseline characteristics, symptomatic status, number of diseased vessels per patient and bifurcation disease were assessed. Bifurcation lesions were classified according to the modified Medina classification8,9. The procedure was performed by one of four experienced interventionalists. All decisions regarding material, device selection or the appropriate treatment strategy were left to the treating physician.
IMAGING TECHNIQUES
Angiographic images were obtained using an Allura Xper FD 20/10 system (Philips Medical Systems, Best, The Netherlands). StB images were obtained with the deflated balloon still in place, after stent implantation. The balloon markers were used as reference points. After stent deployment and intracoronary nitroglycerine administration, 3- to 4-second motion-corrected runs were acquired. The use of a standard StB acquisition or a contrast-filled subtraction image was at the discretion of the operator. Post-acquisition, stent location was verified and balloon marks were manually corrected if necessary. Angiographic projections were the same as those used to guide bifurcation PCI.
StB image acquisition was performed when the fluoroscopic images were not sufficient to guide the bifurcation PCI, according to the operator’s criteria. The main uses of the StB during PCI were: stent expansion ratio after stent deployment, guidance during side branch rewiring, stent positioning in a two-stent strategy and positioning the balloons for post-dilation and kissing balloon technique. Stent and proper guidewire visualisation with StB were evaluated and three groups were formed, according to the quality of image:
1) Optimal visualisation: enhanced struts and guidewire resolution.
2) Suboptimal visualisation: blurred image of struts and guidewire but still allowing for a reasonable visualisation.
3) Poor visualisation: overlapping of structures or devices, not allowing a correct visualisation of the struts or guidewire.
We analysed stent deployment of 128 stents in the 97 patients, measuring minimum luminal diameter and cross-sectional area pre and post stent implantation using StB images and quantitative coronary angiography (QCA).
Continuous variables are presented as mean±standard deviation; categorical variables are presented as percentages. Statistical analyses were carried out with SPSS version 16 (SPSS Inc., Chicago, IL, USA).
Results
The studied population included 97 patients. Baseline patient and bifurcation characteristics are described in Table 1. They had a mean age of 64.4±12 years, and 17.5% were female. Clinical presentation was an ST-elevation acute myocardial infarction in 28 patients (28.8%), non-ST-elevation acute coronary syndrome in 38 patients (39.2%) and stable angina in 31 patients (32%). Ninety-eight bifurcations were treated with stent implantation and were evaluated with StB during the procedure. Most of the bifurcation disease involved the main vessel (99%), and in 80 patients (81.6%) there was side branch involvement. Most bifurcations had both main vessel (proximal and distal segments) and side branch lesions (Medina 1,1,1) (70 patients, 71.4%).
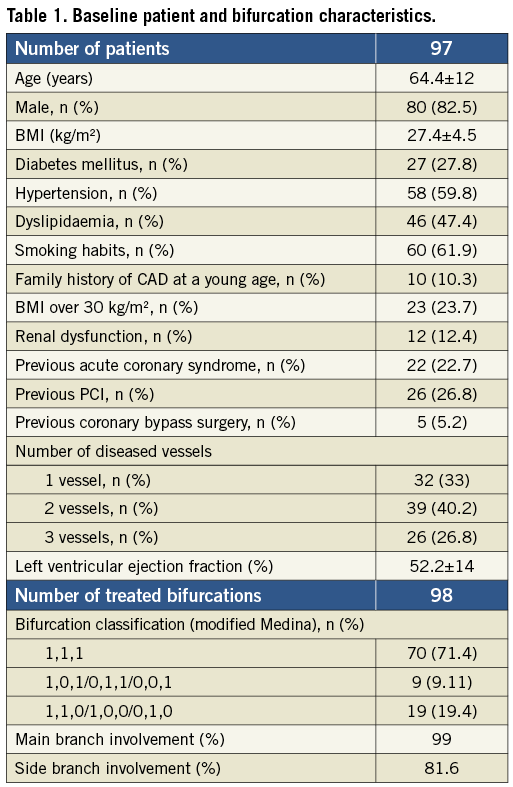
The main vessel of the bifurcation was the left anterior descending artery in 54.1% of patients, left main in 24.5%, circumflex artery/obtuse marginal branch in 14.3% and right coronary artery in 7.1%. Table 2 summarises the selected treatment strategy for each bifurcation and the QCA results pre and post PCI using StB. The provisional side branch technique was the selected initial approach in 84.7% of the patients, with a need to cross over to a two-stent implantation in 14.3% of the patients. Crush was the preferred two-stent technique and was employed in 9.2% of the patients, followed by T-stent in 8.2%. According to our results, the minimum lumen diameter pre and post PCI (after StB-guided optimisation) correlated with the reference vessel diameter, indicating a good final result.
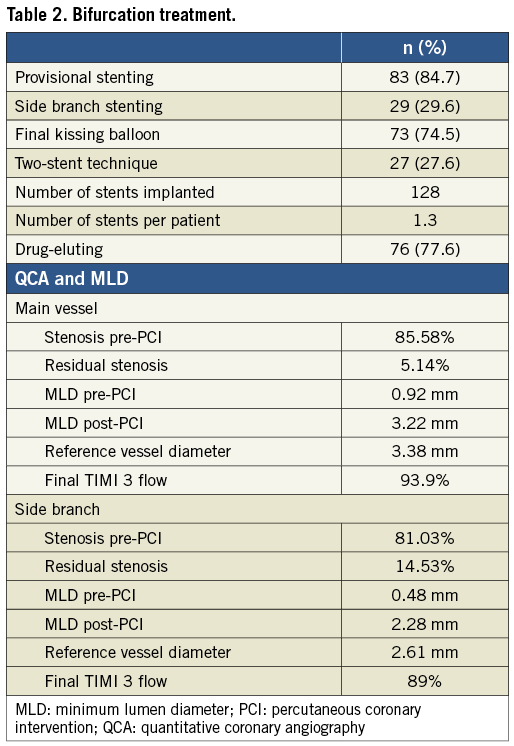
STENTBOOST IMAGES
StB imaging was performed during PCI of the 98 treated bifurcations. A total of five hundred and twenty images (mean 5.3±3.5) were obtained and analysed. Image quality was generally good. In 79.6% of the cases an optimal visualisation of the stent struts and guidewire was obtained; 19.4% had a suboptimal visualisation, and 1% had a poor visualisation. Table 3 summarises the main applications of StB in the studied cohort. The most frequent use of StB imaging was during stent deployment, rewiring and when assessing the need for post-dilation.
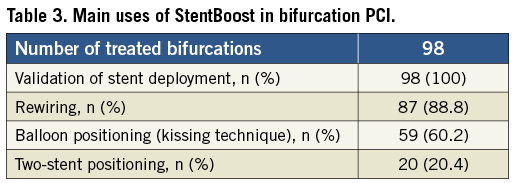
Stent enhancement enabled the identification of the guidewire and angioplasty balloon passing outside stent borders during rewiring of the side branch in three cases, allowing for their repositioning and trajectory reconfirmation. Figure 1-Figure 3 illustrate a case report and the different uses of StB. The patient was a male, 45 years of age, with circumflex artery chronic total occlusion, in which StB had an important role to guide bifurcation PCI.
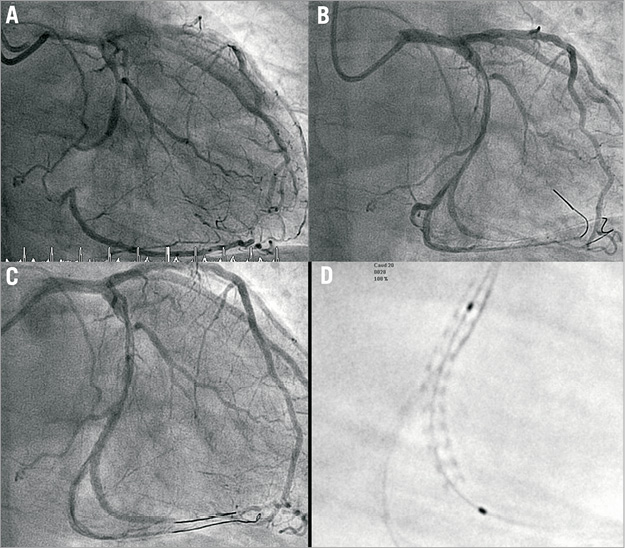
Figure 1. A) CTO of circumflex artery (LCX) with a 1,1,1 (Medina classification) bifurcation lesion (distal LCX and posterolateral branch [PL]). B) After predilation with a 2.75×12 mm angioplasty balloon. C) Two-stent technique (culotte). First stent (2.75×28 mm CYPHER®; Cordis, Johnson & Johnson, Miami, FL, USA) is implanted in the LCX. D) StentBoost image with optimal stent visualisation.
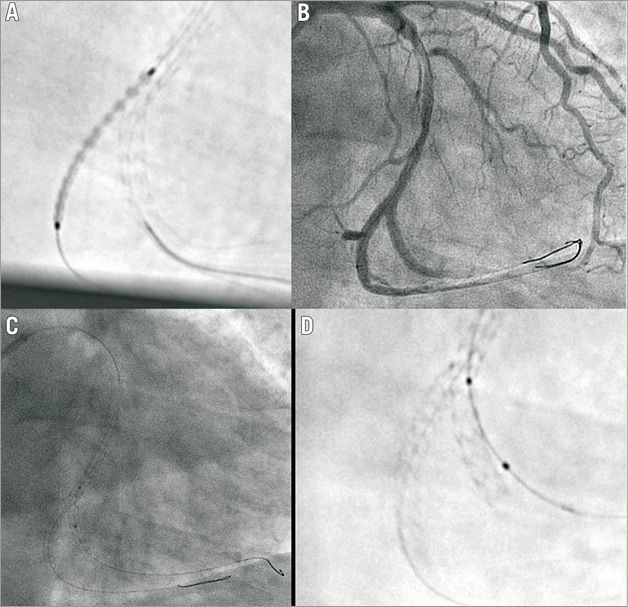
Figure 2. A) StentBoost image of stent positioning at the PL (2.5×28 mm CYPHER®; Cordis, Johnson & Johnson, Miami, FL, USA). B) After second stent deployment. C) After rewiring the LCX, during positioning of the post-dilation balloon. D) StentBoost image of the previous angiography acquisition. Both guidewire and angioplasty balloon are outside the stent (not visible in the angiography).
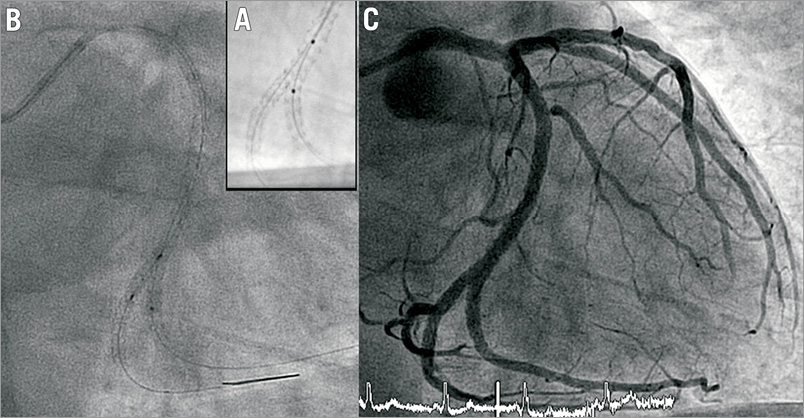
Figure 3. A) StentBoost acquisition after correct repositioning of guidewire and balloon. B) Final kissing balloon inflation. C) Final result.
Discussion
Percutaneous treatment of coronary artery bifurcations is a challenging procedure and a matter of constant debate, in which the decision as to the most suitable strategy is not always easy. The restenosis rate (more frequent when a two-stent strategy is applied) and the acute closure of the side branch are two feared complications. The employment of drug-eluting stents has reduced restenosis and repeat revascularisation rates in several reports, but restenosis of the side branch ostium remains an issue in 10-20% of cases10-12. The recent advances in the bifurcation technique such as a preferred provisional T-stent approach, final kissing balloon inflation (strut opening at the side branch ostium) and enhanced execution in a two-stent strategy13 have substantially improved the outcomes of bifurcation treatment, with some studies reporting similar results to non-bifurcation PCI14. The most significant independent predictor of clinical success during bifurcation PCI is the angiographic result in the main branch, and this is the reason why provisional stenting is preferred15. In the era of dedicated stents, an optimal long-term outcome, low rate of restenosis and stent thrombosis are expected improvements, and trials are ongoing to assess the feasibility of these stents to treat all kinds of bifurcation16.
Imaging techniques have a primary role during PCI and are under constant development. Angiography, considered the standard for the evaluation of coronary lesions, does not permit proper lumen assessment or visualisation of stent struts (especially at the side branch ostium) after deployment. Intravascular ultrasound (IVUS) is a valuable tool. This technology permits the evaluation of vessel size, lumen diameter (lesion severity), plaque composition, quantification of calcium content and assessment of complications post intervention17-19. However, there is a learning curve (requiring trained operators and laboratory staff), and its routine use would significantly increase procedural duration and costs.
StentBoost is a recently introduced method that permits the visualisation of a high quality image of the deployed stent. It does not require the insertion of additional devices in the coronary arteries, does not need specific training of the operator or staff (images are automatically transferred to a workstation and become immediately available in the catheterisation laboratory), and does not significantly increase radiation exposure or procedural time (3 to 4-second runs). It has been employed more frequently in the evaluation of stent positioning and adequate expansion20,21. Mishell et al conducted a study in which they hypothesised that StB use would result in improved identification of underexpanded stent segments relative to standard quantitative angiography (QCA) and IVUS22. They concluded that the three techniques had good correlation regarding minimum stent diameter measurements, with a higher correlation between StB and IVUS. Regarding bifurcation PCI, StB has been useful in the evaluation of novel dedicated bifurcation stents23,24. In the present study, we sought to assess the importance of StB to guide bifurcation PCI. Our results indicate that this technique can provide valuable information through several stages of the procedure (visualisation of guidewire, balloon and stent, strut opening at the side branch ostium, balloon and stent positioning, need for post-dilatation) and avoid periprocedural complications. Frequently, angiographic images alone do not permit adequate visualisation of stent deformation or incomplete stent expansion at the ostium of the side branch. StB allows for an adequate ostium visualisation and is particularly sensitive to detect severe stent distortion. The visibility of the stent(s) was optimal in most patients. In a provisional stent technique, StB was useful during rewiring of the side branch, visualisation of adequate strut dilation and during balloon positioning for the final kissing. In a two-stent strategy, an enhanced visualisation of stent positioning and deployment is an additional utility. Figure 4-Figure 8 are examples illustrating the utility of StB in various situations. No real improvement of visibility was detected in only one case and this was due to overlapping of devices and movement of the guidewire tips relative to the stent.
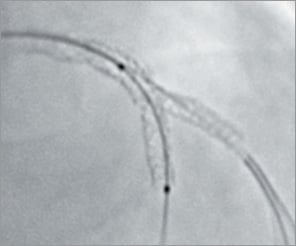
Figure 4. Culotte technique: the need for post-dilation.
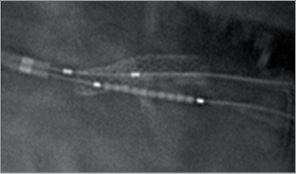
Figure 6. Inverse crush technique: stent positioning.
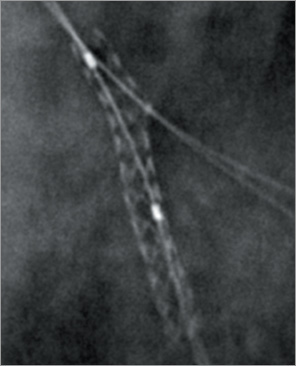
Figure 7. Provisional stenting approach: side branch rewiring.
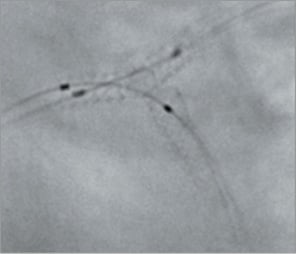
Figure 8. T-stent technique: final kissing.
Limitations
This is a single-centre study and, although the sample size was relatively large, these results need to be validated with further studies. StB has several potential disadvantages: although it results in enhanced image quality, the signal-to-noise ratio is reduced in heavily calcified vessels and segments with multiple stents and, especially in bifurcations, there is the problem of overlap of radiopaque structures (partially solved with a proper view selection). Other potential disadvantages are the inability to describe the lumen and the necessity for additional x-ray exposure to patients and personnel, although the amount of added radiation is small and not likely to be of clinical significance.
Conclusion
Percutaneous treatment of bifurcation disease remains a challenging procedure in interventional cardiology and imaging techniques have a primary role, permitting proper execution of the selected strategy. The integration of technologies is the key to achieving procedural success. StentBoost is a simple and quick method which offers several advantages during bifurcation PCI and which can compensate for the shortcomings of angiography. It allows for adequate stent and balloon positioning and improves stent visualisation and deployment, without the use of other imaging modalities such as intravascular ultrasound.
Conflict of interest statement
The authors have no conflicts of interest to declare.

