Abstract
Coronary angiography is unable to visualise the atherosclerotic involvement of the arterial wall. Bifurcation lesions are particularly difficult to assess by angiography because overlapping mother and daughter vessels often obscure the lesion and carina. On the contrary, IVUS imaging allows for precise, real-time, cross-sectional assessment of all bifurcation lesion segments, enabling measurements of luminal and vessel areas. Moreover, IVUS evaluation of stent expansion, apposition and edge problems is also superior to angiographic assessment. In spite of the lack of adequately powered randomised trials, there is growing evidence from large registries and meta-analyses showing better acute and long-term outcomes of DES implantation guided by IVUS, in comparison to angiography-guided procedures. In this review, we summarise current scientific evidence, the European Society of Cardiology recommendations and the European Bifurcation Club consensus for the use of IVUS in bifurcation stenting.
Introduction
Determining anatomic configuration, selecting strategy and assessing the final result are the key factors in bifurcation lesion treatment that may have a significant impact on both acute and long-term outcomes1. In the bare metal stent (BMS) era, intravascular ultrasound (IVUS)-guided PCI was mainly associated with a lower risk of target vessel revascularisation (TVR). In the drug-eluting stent (DES) era, however, there is growing evidence for the reduction of death, stent thrombosis (ST), myocardial infarction (MI) and TVR by IVUS-guided DES implantation. The 2014 ESC/EACTS Guidelines on myocardial revascularisation recommend the use of IVUS: 1) in selected patients to optimise stent implantation, 2) to assess severity and optimise treatment of unprotected left main lesions, and 3) to assess mechanisms of stent failure2.
Pre-procedural IVUS assessment of the bifurcation lesion
Pre-procedural IVUS imaging of the bifurcation lesion allows for precise: 1) measurement of a true lumen and vessel (external elastic membrane [EEM]) dimensions in the mother and daughter vessel, 2) analysis of atherosclerotic plaque burden (PB), morphology and longitudinal distribution, 3) stent landing zone and reference analysis, 4) stenosis severity and negative remodelling assessment (mainly at ostial location), 5) measurement of the lesion length (only during automatic pullback), and 6) detection of angiographically silent disease (LM stenosis, diffuse disease in reference segments).
Plaque distribution
A study which analysed LM bifurcation reported that plaques were continuously distributed from the left anterior descending artery (LAD) to the LM, and the carina was always spared3. In addition, studies which used greyscale or virtual histology IVUS reported plaque accumulation and vulnerability more frequently in the proximal main vessel (MV) than in the distal MV4,5. These anatomic characteristics of coronary bifurcation appear to suggest that: 1) plaque rupture is more likely to occur in the proximal MV than in the other segments, 2) overinflating the hugging balloon during final kissing inflation (FKI) carries a risk of inducing longitudinal plaque redistribution (shift) and a risk of inducing distal embolism and no/slow flow, especially in an acute coronary syndrome, and 3) stent edge restenosis most likely occurs due to PB in the proximal stent landing zone. In the CROSS trial, angiographic restenosis was frequently observed eight months after PCI at the proximal stent edge in one stent strategy (FKI vs. non-FKI: 5.7% vs. 0.9%, p=0.06) (European Bifurcation Club 2014, unpublished data). In the previous study of IVUS for non-bifurcated lesions, a PB <50% was recommended as the appropriate landing zone to reduce stent edge restenosis after DES implantation6. Although no evidence is available regarding the MV-PB to avoid edge restenosis in coronary bifurcation, plaque observation on IVUS in the proximal MV provides us with valuable information before the procedure.
How to anticipate side branch compromise
Side branch (SB) ostial stenosis can be aggravated after crossover stent deployment in the MV. A recently published IVUS study which directly observed the jailed SB ostium reported that the main mechanism for the SB compromise was carina shift, and that pre-procedural PB and luminal expansion after stenting of the distal MV were associated with the carina shift7. In addition, a sub-analysis of the J-REVERSE registry revealed that negative remodelling in the distal MV was a predictor of SB residual stenosis after FKI (European Bifurcation Club 2014, unpublished data). These findings demonstrate that carina shift is enhanced by stretching of the distal MV and a shift in the flow divider outward towards the SB ostium. Therefore, selection of an appropriately sized stent (according to the precise lumen diameter at the MV reference and at the negative remodelling carina site) via pre-procedural IVUS is an important technical step for avoiding unnecessary SB occlusion after MV stenting. Subsequent proximal optimisation technique (POT) can correct any proximal stent malapposition that is due to the luminal discrepancy between the proximal and distal MV.
Plaque accumulation at the SB ostium can also be detected via pre-procedural IVUS, and this can predict SB occlusion or compromise that may induce physiological myocardial ischaemia8,9. In the provisional SB approach after MV stenting, SB intervention (predilatation or FKI) might be needed if plaque accumulation is found in the SB. Unfortunately, IVUS assessment of the SB ostium from the MV is not precise, and direct imaging is needed for an accurate evaluation of the SB ostium10.
Interestingly, previous greyscale IVUS studies with pullback from the SB demonstrated that negative remodelling is frequently encountered at the SB ostial location, even in SB with extensive and severe disease. Therefore, it may play a substantial role in lumen compromise as assessed by the angiographic “luminogram”11. In general, negative remodelling is not associated with functional ischaemia, and such observation with IVUS may be helpful in decision making before approaching bifurcation PCI with more complex techniques.
With respect to the LM lesion, a pre-procedural minimal lumen area of <3.7 mm2 and PB >56% at the left circumflex (LCX) ostium predicted a post-stenting FFR <0.80 after LM bifurcation stenting with the crossover technique12. In LM bifurcation lesions with mild LCX ostial disease, the use of a single-stent technique rarely resulted in functional LCX compromise13.
Cut-off for minimum lumen area to predict myocardial ischaemia in LM bifurcation
Appropriate decision making for revascularisation in LM disease is a clinically important issue, as it can help avoid unnecessary stenting or surgery in patients with non-significant stenosis and undertreatment of patients with significant LM disease ambiguous by angiography. Fractional flow reserve (FFR) is the gold standard for identifying myocardial ischaemia (FFR ≤0.75-0.80) in the cathlab14. To predict myocardial ischaemia in LM disease, the cut-off value for the IVUS-derived minimum lumen cross-sectional area (IVUS-MLA) has been recognised as 4.5-6.0 mm2 15-17. The IVUS-MLA in LM seems to be population-dependent. The average IVUS-MLA in the patients included in two different studies was strikingly different (7.6 mm2 in the US study and 4.8 mm2 in the Korean study)18. Another recent study compared coronary LM lesions among 99 white North American and 99 Asian patients. Again, Asian patients had a significantly smaller LM-MLA (5.2±1.8 vs. 6.2±1.4 mm2; p<0.0001)19.
In a prospective multicentre LITRO study, investigators evaluated the safety of an IVUS-MLA threshold of 6 mm2 to guide decision making on revascularisation in 354 patients with LM disease. In 179 patients with an LM segment IVUS-MLA of ≥6 mm2 revascularisation was deferred, whereas the remaining 152 patients with an IVUS-MLA <6 mm2 were revascularised. Importantly, the two-year event-free survival from cardiac death and MI was 97.7% in those patients who had no LM revascularisation, compared with 94.5% in the revascularised group (p=0.5)15. IVUS is potentially limited to identifying functional ischaemia (via the lumen area), as ischaemia also depends on myocardial territory, lesion length, collateral supply, and microvascular injury in the distal myocardium. Thus, McDaniel et al proposed that LM patients with an IVUS-MLA of <6 mm2 should be evaluated via FFR or non-invasive testing, whereas an IVUS-MLA of ≥6 mm2 should indicate deferred revascularisation14.
IVUS assessment of bifurcation during the procedure
Intravascular ultrasound imaging during the procedure allows for precise: 1) direct control of wire re-crossing through the jailed SB, 2) assessment and optimisation of stent apposition (stent strut adherence to the vessel wall), 3) measurement and optimisation of stent expansion (ratio between minimum stent area and lumen area in adjacent reference segment of the vessel), 4) assessment of full lesion coverage by the stent (especially at the SB ostial location when using some double-stenting techniques such as T-stenting), and 5) diagnosis and treatment of stent edge problems (geographic miss, secondary lesions, large plaque burden, dissection, etc.).
During the provisional SB approach for coronary bifurcation, coronary wire re-crossing through the jailed SB (at an appropriate point near to the carina) is an important step to perform FKI, as it can provide optimal SB scaffolding without stent malapposition at the carina20. For this purpose, IVUS can manually and repeatedly track the SB wire between the SB and proximal MV, and proximal stent malapposition can be detected immediately after the MV stenting (Figure 1). This phenomenon is rarely associated with wire twisting outside of the strut when re-wiring is performed.
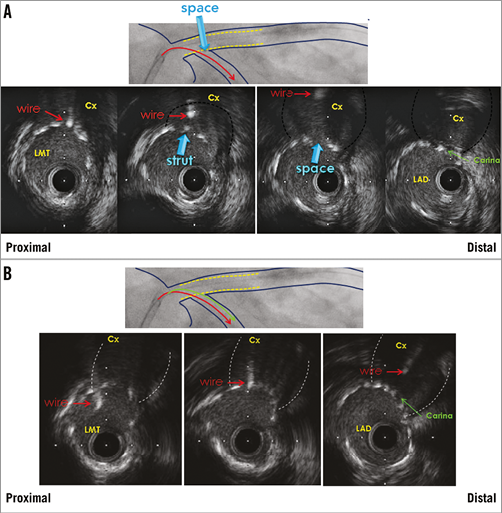
Figure 1. IVUS-guided left main (LMT) PCI with provisional side branch stenting. Wire re-crossing through the jailed left circumflex coronary artery (Cx) before final kissing inflation. A) Wire position is too proximal. B) Optimal wire position near carina. LAD: left anterior descending coronary artery
When a single-stent strategy is used, wiring of the jailed SB with imaging wires should be avoided due to the risk of distorting the stent. On the contrary, pullbacks in both SB and MV are recommended in evaluation of two-stent techniques if intracoronary imaging is used21.
Definitive guidelines for IVUS-guided stent optimisation are not available, and it is still performed at the operator’s discretion. Small (<5.0-5.5 mm2) IVUS-derived minimum stent area (IVUS-MSA) or stent underexpansion and edge problems are the most important IVUS predictors of DES thrombosis and restenosis22,23. Non-compliant (NC) balloon post-dilatation with a maximum balloon to EEM diameter ratio 0.9-1.0 and inflation pressure >16 atm is encouraged to achieve complete stent strut apposition to the vessel wall and IVUS-MSA >5.0-5.5 mm2 or IVUS-MSA >90-100% of the lumen area at the adjacent reference segment24,25. In LM lesions, optimal IVUS-MSA to prevent TVR was reported to be >8.7 mm2 26. Kang et al evaluated IVUS predictors of in-stent restenosis (ISR) after LM bifurcation stenting. Post-stenting IVUS-MSA cut-offs that best predicted ISR on a segmental basis were 5.0 mm2 (ostial left circumflex [LCX] ISR), 6.3 mm2 (ostial left anterior descending [LAD] ISR), 7.2 mm2 (ISR within polygon of confluence [POC, confluence zone of LAD and LCX]), and 8.2 mm2 (ISR within the LM above the POC) (Figure 2). A smaller IVUS-MSA within any one of these segments was responsible for a higher rate of angiographic ISR and clinical major adverse cardiovascular events (MACE)27. Thus, correcting underexpansion with these optimal IVUS criteria may reduce cardiac events after DES treatment for unprotected LM disease.
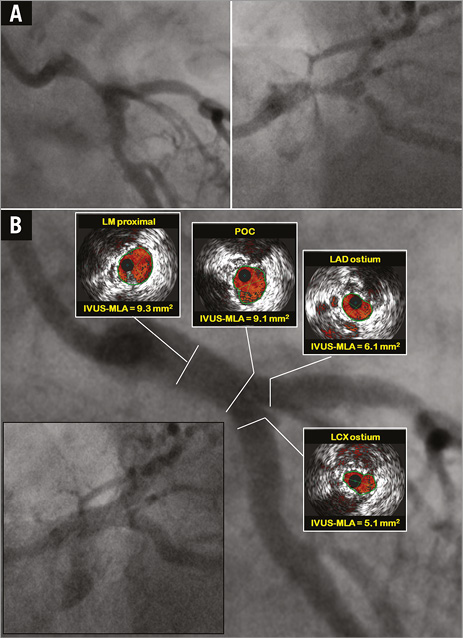
Figure 2. IVUS-guided left main (LM) PCI with DK crush technique. A) Baseline angiography. B) Final IVUS assessment of the mother and daughter vessel confirmed optimal stent apposition and expansion with minimum lumen areas (IVUS-MLA) above optimal cut-offs in all segments of LM bifurcation27. LAD: left anterior descending coronary artery; LCX: left circumflex coronary artery; POC: polygon of confluence
Edge dissection, which is complicated by lumen narrowing <4.0 mm2 or dissection angle ≥60° has been associated with an increased incidence of acute stent thrombosis23. Thus, additional stent implantation should be considered in such situations to lower the risk of stent thrombosis.
Clinical outcome of IVUS-guided bifurcation PCI
NON-LM BIFURCATION STENTING
The long-term effect of IVUS-guided non-LM bifurcation stenting was assessed for the first time by Kim SH et al. In patients receiving DES, but not BMS, IVUS-guided bifurcation stenting significantly reduced four-year mortality compared to conventional angiographically guided stenting. In addition, IVUS guidance reduced the development of very late ST in patients receiving DES28. Kim JS et al evaluated 1,668 patients with non-LM bifurcation lesions who underwent DES implantation with or without IVUS guidance. The two-stent technique and FKI were more frequently performed in the IVUS-guided group. Maximal stent diameters at both the MV and the SB were larger in the IVUS-guided group. Periprocedural creatine kinase-MB elevation (>3 times upper normal limits) was more frequently observed in the angiography-guided group. The incidence of death or MI was significantly lower in the IVUS-guided group compared to the angiography-guided group (3.8% vs. 7.8%; p=0.04)29. Chen SL et al analysed the difference in one-year outcomes following two-stent techniques involving implantation of DES for coronary bifurcation lesions between IVUS-guided and angiography-guided groups. The late ST at 12-month follow-up was 0% in the IVUS-guided group versus 4.9% in the angiography-guided group (p=0.029), resulting in significant differences in ST-elevation MI between the two groups (2.4% vs. 9.8%; p=0.030)30.
LEFT MAIN BIFURCATION STENTING
In the MAIN-COMPARE registry, there was a tendency of lower risk of three-year mortality with IVUS guidance compared to angiography guidance (6.0% vs. 13.6%, p=0.061). In particular, in patients receiving DES, the three-year incidence of mortality was lower with IVUS guidance as compared to angiography guidance (4.7% vs. 16.0%; p=0.055). In contrast, the use of IVUS guidance did not reduce the risk of mortality of patients receiving BMS26. However, data regarding total mortality favouring IVUS guidance are difficult to interpret in the absence of comparative data on cardiac mortality and stent thrombosis. In a recently published study, de la Torre Hernandez et al evaluated the clinical impact of IVUS guidance on DES implantation for unprotected LM disease based on patient-level pooled analysis of four registries31. Survival free of cardiac death, myocardial infarction, and target lesion revascularisation at three years was 88.7% in the IVUS group and 83.6% in the no-IVUS group (p=0.04) for the overall population, 90% and 80.7%, respectively (p=0.03), for the subgroup with distal LM lesions, and 83.3% and 59%, respectively (p=0.02), for the distal LM subgroup treated with two stents. The incidence of definite and probable ST was significantly lower in the IVUS group (0.6% vs. 2.2%; p=0.04). Finally, IVUS-guided revascularisation was identified as an independent predictor of major adverse events in the overall population (hazard ratio: 0.70, 95% CI: 0.52 to 0.99; p=0.04), and to a greater extent in the subgroup with distal lesions (hazard ratio: 0.54, 95% CI: 0.34 to 0.90; p=0.02).
META-ANALYSIS OF IVUS-GUIDED PCI
Notable limitations of all of the above-mentioned studies were the lack of randomisation and lack of pre-specified guidelines for stent optimisation by IVUS. Moreover, the sample size of those studies was not large enough to evaluate precisely the low incidence of MACE at follow-up. A recently published comprehensive meta-analysis evaluated the clinical impact of IVUS-guided PCI with DES compared with conventional angiography-guided PCI. This meta-analysis included 26,503 patients from three randomised and 14 observational studies (including two studies dedicated to LM stenosis and three studies dedicated to bifurcation stenosis). Intravascular ultrasound-guided PCI was significantly associated with more, longer and larger stents. Regarding clinical outcomes, IVUS-guided PCI was associated with a significantly lower risk of death, MI, ST and TLR32.
Conflict of interest statement
The authors have no conflicts of interest to declare.