Abstract
Aims: The optimal treatment strategy for coronary bifurcation lesions is still unknown. The BiOSS Lim stents (Bifurcation Optimisation Stent System) is a novel dedicated bifurcation stent introduced over a single wire in to the main vessel, covered with biodegradable polymer and sirolimus. It has wider proximal and narrower distal parts. The aim of the study was to assess applicability of the the BiOSS Lim stent in a porcine coronary model.
Methods and results: A total of 14 BiOSS Lims were implanted in normal non-atherosclerotic porcine coronary bifurcations of 14 animals (six stents for 28 days, eight stents for 90 days) using 1.1:1.0 stent-to-artery ratio. Stent geometry and morphology were evaluated by Faxitron imagery (Faxitron Bioptics, LLC, IL, USA). Vascular effects were assessed based on angiographic and histological analysis. Analysis of Faxitron images revealed no major abnormalities except two struts fractures at the place of connection between the mid-portion and proximal wider part of the stent. Histomorphometry showed decreased area stenosis and intimal thickness at 90 days compared with the 28 days cohort. The inflammatory scores were low (<1) at both time points and struts endothelialisation was completed at 28 days.
Conclusions: The novel BiOSS Lim stent demonstrates good short- and mid-term vascular effects in a porcine coronary bifurcation model.
Introduction
Coronary bifurcations constitute the most challenging subset of lesions in percutaneous coronary interventions being related to lower procedural success rates and worse clinical outcomes than non-bifurcation lesions. The main issues related to their optimal treatment pertain to their complex anatomy and pathophysiology, what makes the use of conventional stents challenging. In order to optimise procedural effects of bifurcation treatment and to further improve its long-term effects dedicated bifurcation stents have been proposed.
The aim of this study was to assess 28- and 90- day safety, biocompatibility, feasibility and mechanical integrity of the novel Bifurcation Optimisation Stent System (BiOSS Lim stent), with sirolimus elution implanted in normal non-atherosclerotic porcine coronary arteries. This stent represents technology which retains conformity of the target bifurcation anatomy, yet allowed for easy access to the side branch in the case of balloon post-dilatation or another stent implantation.
Methods
Device description
The BiOSS Lim is a coronary bifurcation balloon expanding stent made of 316L stainless steel releasing sirolimus from the surface of biodegradable coating comprised of a copolymer of lactic and glycolic acid. In vitro evaluations showed that the coating degrades entirely and releases sirolimus in a time-controlled process lasting around eight weeks. All tested BiOSS stents were available in two different sizes including 2.5 (distal part)×3.25 (proximal part) ×15 mm (stent length) and 3.0×3.75×15 mm. The profile of the device pre-mounted on the balloon catheter is 1.06 mm with the strut thickness of 0.115 mm. In terms of bifurcation design, the BiOSS Lim stent consists of two main separate parts with different diameters: wider proximally, and distally smaller. The ratio of proximal to distal parts varies between 1.15 to 1.3, ensuring physiological compatibility and optimal flow conditions. There is a 1.5 mm intermittent zone with two connection struts which join the parts together (Figure 1). This zone ensures “self–positioning” of a stent after balloon deflation, as well as opening to the side branch. After BiOSS Lim implantation (during which the main vessel segment is straightened), the proximal and distal main vessel segments return to their initial position / angulation, opening the side of the stent to the branch. The wider proximal part of the stent ensures lateral stretching of the side branch lateral wall, counterbalancing the carina displacement from the distal part of the stent. Moreover, this stent provokes much less carina displacement, because it copies the exact bifurcation construction with proximal – distal main vessel diameter mismatch. In order to safely and effectively deliver the BiOSS stent to the target lesion, and to optimise effects of bifurcation treatment, the BiOSS utilises a specially designed “Bottle” bifurcation balloon catheter. The “Bottle” (Balton, Warsaw, Poland) is a dual-diameter balloon with a step-down zone which minimises carina displacement, keeping the physiological geometry of the bifurcation whilst at the same time optimising diameter ratios. The “Bottle” has a mid-marker, which further improves the exact stent placement. This marker must be positioned exactly at the level of the bifurcation carina tip. The BiOSS Lim stent is introduced over a single guidewire, the opposite of many other dedicated systems which are guided with two guidewires, eliminating the risk of wire wrap (twisting) or other complications which could occur with double guidewire driven systems.
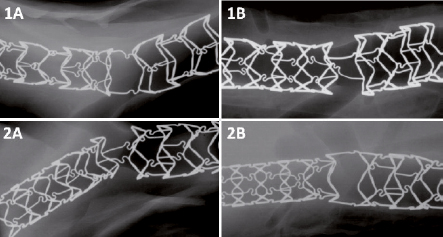
Figure 1. Geometry and structural integrity of the BiOSS Lim stents based on representative high resolution radiography images (Faxitron MX-20) at 28 days (1A and 1B) and at 90 days (2A and 2B).
Study design
The study protocol was reviewed and approved by the AccelLAB’s Institutional Animal Care and Use Committee. The review insured compliance with Canadian Council on Animal Care regulations. A total of 14 BiOSS Lim stents were implanted in 14 normal non-atherosclerotic porcine coronary bifurcations (six stents designated for 28 days and eight stents designated for 90 days). All animals received dual antiplatelet therapy which consisted of oral acetylsalicylic acid (325 mg) and clopidogrel (300 mg initial dose, 75 mg subsequently) starting three days prior to intervention and continuing until sacrifice. After anaesthesia induction with propofol, the animals were intubated and supported using mechanical ventilation. Isoflurane in oxygen was administered to maintain a surgical plane of anaesthesia. Subsequently, an arterial sheath was introduced to the left or right femoral artery through an inguinal skin incision. An initial bolus of heparin (~ 400 U/kg) was administered and ACT was measured every 30 minutes to maintain ACT time of at least 300 seconds. Coronary angiograms were performed after administration of intracoronary nitroglycerine (200 μg). The selection of the target bifurcation site was made based on visual assessment of anatomy and quantitative coronary angiography (QCA) analysis. The balloon was then inflated at a steady rate to a pressure sufficient to achieve a stent to artery ratio of 1.1:1 (acceptable range of 1.05:1 to 1.15:1). The procedural success was defined as the complete delivery of the stents and the absence of any major or sub-acute complications during the procedures. After the final angiogram was obtained, the delivery system was removed and then the femoral artery was ligated and inguinal incision layers were sutured. All animals were injected with an antibiotic. At termination, all animals were anaesthetised as described above, and final angiography was performed. The animals were then euthanised by a lethal injection of saturated potassium chloride (KCl, rapid IV bolus). Subsequently, a comprehensive necropsy, defined as the gross examination of the heart, stented vessels, thoracic and abdominal cavities was performed. The hearts were then excised and perfused with lactated Ringer’s solution, followed by neutral-buffered formalin, and finally immersed in neutral-buffered formalin for further histological analysis.
Angiographic analysis
All treated arteries were qualitatively evaluated for stent migration, dissection and aneurysms. The Medis QCA-CMS 6.0 system was used for quantitative coronary analysis (QCA). Of two angles available for analysis, the image with a minimal foreshortening that showed maximal stenosis was chosen. Balloon diameter was measured at baseline. Minimal lumen diameter (MLD) and reference vessel diameter (RVD) were measured at baseline and at terminal angiogram. From these measurements the following parameters were calculated: balloon to artery ratio (defined as balloon/pre-stent mean luminal diameter), stent to artery ratio (defined as post-stent/pre-stent mean luminal diameter) and late lumen loss (LL: defined as post-stent MLD – final MLD). Diameter stenosis was calculated based on the following formula (1– [MLD/RVD])×100%. All these parameters were evaluated for both proximal and distal segments of the BiOSS Lim stent.
Stent radiography
High resolution radiographs of fixed whole hearts and explanted stented arteries were obtained. Radiographs of explanted stented vessels were evaluated to identify and document stent expansion, morphology, stent continuity and/or other abnormalities. Radiography was performed at two perpendicular angles using a Faxitron MX-20 (Faxitron Bioptics, LLC, IL, USA).
Histology evaluation
All stented segments were embedded in methyl methacrylate and cut to obtain approximately 8 µm sections. Subsequently, these sections were stained with hematoxylin and eosin (H&E) and Verhoeff-van Gieson (VVG) stains. All sections were examined by experienced study pathologist for semi-quantitative and descriptive histopathology. In addition, a comprehensive histomorphometry analysis was performed.
Histomorphometry analysis
VVG-stained sections of stented arteries were examined using light microscopy and quantitative morphometric computer-assisted methods with Image Pro Plus 6.1.0.346 software (Media Cybernetics, Inc., Bethesda, MD, USA). For each section, the cross-sectional areas (external elastic lamina [EEL area], internal elastic lamina [IEL area] and lumen area) were measured and then the following parameters were calculated: medial area (defined as: EEL area– IEL area), intimal area (defined as IEL area – luminal area), area stenosis (calculated as: {1 – (luminal area/IEL area)} ×100), and mean intimal thickness (calculated as follows:
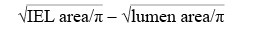
Semi-quantitative and descriptive histopathology
All sections were evaluated using semi-quantitative scoring criteria. To evaluate the amount of injury, criteria defined by Schwartz et al were used: 0=IEL intact; 1=IEL lacerated; 2=media completely lacerated; 3=EEL lacerated1. Each strut in the section was scored and the mean injury score for each section was calculated and reported. To evaluate the extent of inflammatory reaction the following grade was used: 0=no or very few (≤3) inflammatory cells around strut; 1=few (~4-10) inflammatory cells around strut; 2=many (>10) inflammatory cells around strut, can extend into but do not efface surrounding tissue; 3=many (>10) inflammatory cells, effacing surrounding tissue. Each strut in the section was scored, and the mean inflammation score for each section was calculated and reported. The predominant inflammatory cell type(s) was described, per stent section. In addition, when peri-strut granulomas were present, their incidence was graded (as a percentage of affected struts), per section and per stent segment. The extent of fibrin deposits was assessed as follows: 0=absent or rare minimal spotting around strut; 1=fibrin in small amounts, localised only around strut; 2=fibrin moderately abundant or denser, extending beyond strut; 3=abundant, dense fibrin, bridging between strut. Each strut in the section was scored; the mean fibrin score for each section was calculated and reported. The endothelialisation score was based on the following criteria: 0=<25% of artery circumference covered by endothelium; 1=25-75% of artery circumference covered by endothelium; 2=76-99% of artery circumference covered by endothelium; 3=complete endothelial coverage. The neointimal immaturity, which estimates the proportion of neointimal areas containing a few to no myofibroblasts, a high proportion of mucinous matrix, oedema or fibrin, and undifferentiated mesenchymal cells or inflammatory cells (areas are interpreted as less mature) was defined as: 0=no immature areas; 1=<25% of neointima containing immature areas; 2=25-75% of neointima containing immature areas; 3=>75% of neointima containing immature areas.
Results
Procedural results
A total of 15 BiOSS stent were successfully implanted (six for 28 days and nine for 90 days). One animal died at day 19, because of bacterial endocarditis. The cause of the endocarditis did not appear to be associated with the stents themselves, and therefore this pre-terminal death was not considered a treatment-related adverse event. As there was lack of plaque at the target sites, the exact stent implantation was difficult due to excessive heart/artery movements. In two cases in the 28-day group and in three cases in 90-day group, implantations were done slightly too proximal or too distal from the exact location. However, in all these cases, these stents kept their dedicated shape and fitted well to the vessel anatomy. There were no cases of periprocedural artery dissections, thrombus formation, slow-flow or any other coronary or access site complications. The stent was easy to track and implant with quick deflation time with excellent angiographic visibility. The procedural success was 100%.
High resolution radiography
High resolution radiography (Faxitron) analysis revealed no major abnormalities except two struts fractures of two stents designated for 90-day follow-up, both at the place of the proximal connection of the mid-portion with the proximal wider part of the stents. Both these stents were implanted in unintended positions, with proximal displacement of the stents.
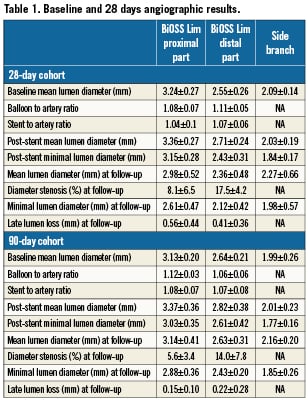
Angiographic results
The angiographic results are summarised in Table 1. The data for proximal and distal segments are presented separately. The baseline size of artery segments where stents were implanted varied from 2.24-3.55 mm for 28-day samples, and 2.32-3.36 mm for 90-day samples. There were no dissections, aneurysm formations, filling defects, stent migration, abnormal vessel patency or malapposition at terminal angiography in any cohort. The mean diameter stenosis at 28-day follow-up was 8.1% and 17.5% at proximal and distal parts, respectively; and 5.6% and 14% at 90-day follow-up. The value of late lumen loss decreased over time in both distal and proximal parts of the stent. Of interest, at 90 days, three side branches jailed because of inaccurate stent positioning (stents implanted too proximal, with the distal part of the stent jailing the side branch ostium). The QCA analysis of the side branches, however, demonstrated that vessel diameters were generally similar before and after stenting, as well as at final angiography in both 28- and 90-day cohorts.
Histomorphometry
The histomorphometry data are summarised in Table 2. Similar to the QCA analysis, there was a decrease in area stenosis and mean intimal thickness at 90 days as compared to the 28-day cohort (Figure 2). There was no late catch-up effect in any of the parameters analysed.
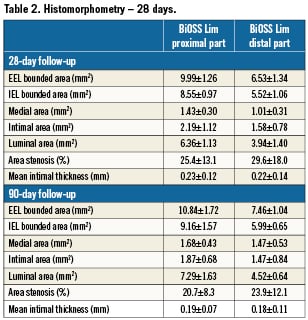
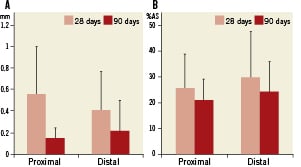
Figure 2. Late lumen loss (A) and percent area stenosis (B) in proximal and distal stent segments of the BiOSS Lim stent at 28- and 90-day follow-up. Error bars represent standard deviation (SD).
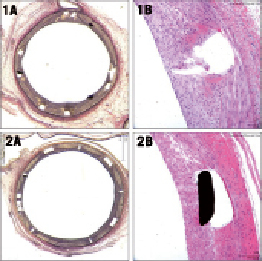
Figure 3. Representative typical histological appearance of the treated bifurcation segments with BiOSS Lim stent at 28-days (1A and 1B) and at 90-days (2A and 2B).
Histopathology
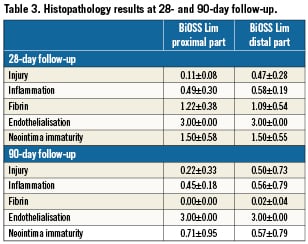
Summary of pathological changes at 28 and 90 days are presented in Table 3. At 28 days, injury and inflammation scores were similarly low in both distal and proximal parts of the stent (mean <1). The inflammatory infiltrates were composed mainly of macrophages and, occasionally, multi-nuclear giant cells. Also, some larger fibrin deposition was present. The endothelialisation was completed in all tested stents. Immaturity scores were at mid-range. In one studied stent, the adventitial inflammation was present (infiltration of inflammatory cells beyond the EEL, associated with peri-strut inflammation). At 90-day follow-up, injury and inflammatory scores were low; however, in two stents, peri-strut granulomas reflecting the presence of continuous inflammation were present. In those two stents, subendothelial leucocytes –mainly at distal parts of the stents– have also been found. At this time point, there was practically no fibrin deposition, and endothelialisation was complete in all stents. Neointima immaturity scores were noticeably lower than at 28 days.
Discussion
The future of the effective treatment of coronary artery disease must rely on dedicated technologies specifically and purposefully selected for each lesion. Coronary bifurcation dedicated stents are an emerging technology, holding out the chance of improving procedural and long-term clinical outcomes in the treatment of these lesions. The aim of this present study was to assess the 28- and 90-day safety, biocompatibility, feasibility and mechanical integrity of the novel Bifurcation Optimisation Stent System (BiOSS Lim stent) implanted in normal non-atherosclerotic porcine coronary arteries. At 28 days, the overall histological parameters of neointimal proliferation were generally low, and comparable with the data found in the literature for the similar drug-eluting stent technology1-4,7,8. The mean diameter stenosis at the proximal part of the stent tended to be lower in comparison with the distal part. This finding could be related to the differences in the vessel sizes at proximal and distal parts of the stent. At 90 days, all stenosis parameters were numerically lower as compared to the 28-day cohort. The angiographic measurements were generally in accordance with the histomorphometric analysis, with very low percent diameter stenosis and late lumen loss at 28 days, and with further reduction of stenosis severity at 90-day follow-up. This finding shows that the pattern of neointima formation between 28 and 90 days is more similar to bare metal stents tested in porcine coronary arteries, rather than to previously described first generation drug-eluting stent technology. The pattern of neointimal formation after BMS implantation into porcine coronary arteries was described previously by Virmani et al9. In this paper it was shown that after initial peak of neointimal formation at 28 days there is a regression of around 25%, taking place over the following three to six months. Over this period, the extracellular matrix becomes enriched in collagen type I, with neointimal shrinkage and remodelling.
Contrary to this, the animal studies of first generation DES technology showed that after initial inhibition of neointimal hyperplasia there is a late increase in neointimal formation (the “catch-up” phenomenon)8,10. It is assumed that this phenomenon may be related to the toxic effect of a drug and/or polymer combination, as well as persistent vascular inflammation. In our study, the polymer used for drug delivery fully degrades within the period of eight weeks, leaving behind only the bare metal stent. Hypothetically, after this period, the behaviour of the neointima may become very similar to BMS.
In our study as well, both inflammation and injury scores were very low at both time points, reflecting excellent biocompatibility of the tested device. This may explain the lack of “late catch-up phenomenon” in the BiOSS stents. In addition, speculatively, it is also possible that a specific stent shape (with a step-down diameter) and stent cell configuration could create conditions for optimal flow conditions and higher shear stress, thus inhibiting and changing the pattern of neointima proliferation. Similarly to other drug-eluting stents, there were some unresorbed fibrin deposits at 28 days, suggesting the effects of sirolimus present at the treated site.
The analysis of histomorphometrical parameters of lumen and vessel size including luminal area, EEL, IEL and medial area were numerically larger in the 90-day follow-up cohort as compared to the 28-day group. This finding could be explained by a combination of vessel growth and positive remodelling caused by a drug and/or polymer. From a practical point of view, a “dedicated coronary bifurcation stent” has to prevent complications such as periprocedural myocardial infarction and/or ischaemia being generated from the ostial stenosis of a side branch, as well as to ensure sustained results in long-term follow-up, meaning lack of restenosis in both branches. These adverse events are mainly related to carina displacement from stent struts, compromising and eventually closing the side branch ostium11. At the same time the device must be easy to use and compatible with modern small size equipment, shortening the procedural and fluoroscopy time and decreasing contrast volume12. None of the currently available devices satisfy all these requirements13-20. The elimination of carina displacement (as a main mechanism of side branch compromise) by the BiOSS stent keeps the side branches patent, and does not require further treatment. In accordance with this concept, there was no side branch compromise in our study (appearance of ostial stenosis or side branch closure after stenting) in all correctly positioned devices. In diseased vessels, with atherosclerotic plaque presence, these could be translated into less periprocedural myocardial infarctions and less late stent thromboses. The last assumption is plausible for the stent placed correctly against the side branch ostium with no prolapsing struts, which could remain un-endothelialised. From a technical point-of-view, the BiOSS stent implantation is as easy as the implantation of regular stents in straight segments. The stent is tracked over a single wire, eliminating problems previously observed with dedicated bifurcation stents tracked over two wires, such as wire entanglements and wire related device bias (improper orientation of dedicated stent inside the bifurcation region)21. The problems with an exact positioning of the mid-marker against the carina tip seen during the present study should be not relevant in real world use, as atherosclerotic plaque presence eliminates the excessive movements observed here.
Limitations of the study
Although the porcine model of neointimal hyperplasia is well established to evaluate the safety of new devices, its value to predict efficacy is limited. In this regard, we must be cautious in drawing any conclusions about the efficacy of BiOSS Lim stent while trying to predict clinical outcomes related to restenosis and revascularisation rates. In addition, it needs to be understood that the use of healthy coronary arteries without atherosclerotic disease does not allow us to test the potential influence of the intermittent zone (gap) in the BiOSS stent design on the possibility of plaque prolapse. The two cases with strut fractures found in this study require further analysis, as well as improvement in the stent design, especially in its mid-portion.
The lack of a control bifurcation stent may also be seen as a limitation of the study. Concerns could also be raised about proper stent positioning, taking into account some problems during implantation in our animal model. We underline again that this is a model without any plaque occurrence, and free movement inside the vessel during cardiac cycle is without any restriction. In diseased arteries –with different amounts of plaque, calcium and lumen restrictions– proper positioning seems not to be a problem. From the initial data in our first-in-man study in 50 patients, we did not observe any problems with system positioning precisely in place – with the mid-marker against the carina tip22.
Conclusions
The BiOSS Lim – a dedicated coronary bifurcation stent – demonstrates very good short- and mid-term angiographic and histological results. At 28- and 90- day follow-up, all parameters of vessel wall healing indicate its excellent safety and biocompatibility. The parameters describing neointimal hyperplasia were low at both studied time points, suggesting the efficacy of the device tested. This novel approach in the treatment of bifurcation lesions may facilitate interventional procedures, and improve their long-term effects; however, further clinical investigation is necessary.
Funding
This study was financially supported by the Balton Company, Warsaw, Poland.
Conflict of interest statement
The authors have no conflicts of interest to declare.

