Abstract
Bioresorbable scaffolds (BRS) provide a new tool for percutaneous treatment of coronary stenosis. Initially, relatively simple coronary artery lesions were treated with this novel technology; nowadays, we have gained more experience with a wide variety of lesions, including bifurcation lesions. Data are limited in terms of the use of BRS in coronary bifurcation lesions, although it has been demonstrated that over time the bioresorbable struts are replaced by a tissue bridge resembling a “neo-carina”. Furthermore, data are emerging about the endothelial shear stress (ESS) alterations and blood flow patterns after scaffold implantation. It is likely that ESS and blood flow determinations will guide correct future bifurcation techniques. In the future, BRS with thinner struts and more resistant to fracture are likely to improve bifurcation treatment with BRS.
Introduction
Bioresorbable technology will most likely change the landscape of interventional cardiology. Besides the widely used Absorb® bioresorbable vascular scaffold (BVS) (Abbott Vascular, Santa Clara, CA, USA), numerous other BRS, including poly-lactic acid and metallic BRS types, are being evaluated in clinical trials1. Since bifurcation lesions with side branch diameters >2 mm were excluded from previous trials2-6, data on BRS use in bifurcations are sparse. Since the introduction of Absorb BVS in daily clinical practice, data have emerged on bifurcations with larger side branches. The recent GHOST-EU registry, for example, included patients with bifurcation lesions in 23.1% of cases7. As a result, current recommendations8 are based on expert opinion, case reports9-12, or on insights from bench studies performed on the Absorb BVS13,14.
There are potential benefits of BRS in bifurcations compared to permanent metallic stents. Biodegradation of struts jailing the side branch (SB), for example, may lead to increased blood flow and improved shear stress patterns in the SB, whereas jailed metallic struts will permanently obstruct the SB, potentially impairing late outcomes15. More data are needed to define the role of BRS for the treatment of bifurcations. In the current paper we will discuss data on BRS use in bifurcations, and we will speculate on the possibilities of creating a dedicated bifurcation BRS.
The fate of non-apposed bioabsorbable side branch struts
Complete strut resorption and restoration of the vascular wall was observed five years post scaffold implantation with multi-imaging modalities in the ABSORB cohort A population evaluating the first-generation Absorb scaffold16. However, the fate of the so-called non-apposed side branch (NASB) struts is still not completely clear. It was demonstrated in one patient from cohort A that after two years NASB BVS struts had been replaced by tissue bridge formation resembling a “neo-carina” (Figure 1)17. Tissue bridge formation was also observed at one-year follow-up in a patient treated with the DREAMS absorbable metallic scaffold (Biotronik Inc., Lake Oswego, OR, USA), which is made from a magnesium alloy and designed to be absorbed nine to 12 months after implantation18. A recent five-year follow-up paper using three-dimensional (3D) optical coherence tomography (OCT) in ABSORB cohort A patients showed side branch patency with different intimal bridge patterns, including tissue bridges crossing the SB ostium. These tissue bridges were not necessarily located at the ostial rim (Figure 2)19. Serial assessment of patients included in ABSORB cohort B, evaluating the second-generation Absorb BVS, which resorbs more slowly than the first-generation Absorb, has shown that the number of compartments seen post procedure first decreases after 12 months. This is due to the occlusion of some compartments by a “membrane” of neointimal tissue like bridges in-between scaffold struts (Figure 3). However, a secondary increase of orifice area was noted between the first and the third year. A five-year follow-up study on cohort B patients will further evaluate the fate of these SB ostia and is expected soon. It is hypothesised that the remaining, patent compartments will enlarge after degradation and absorption of the jailed struts.
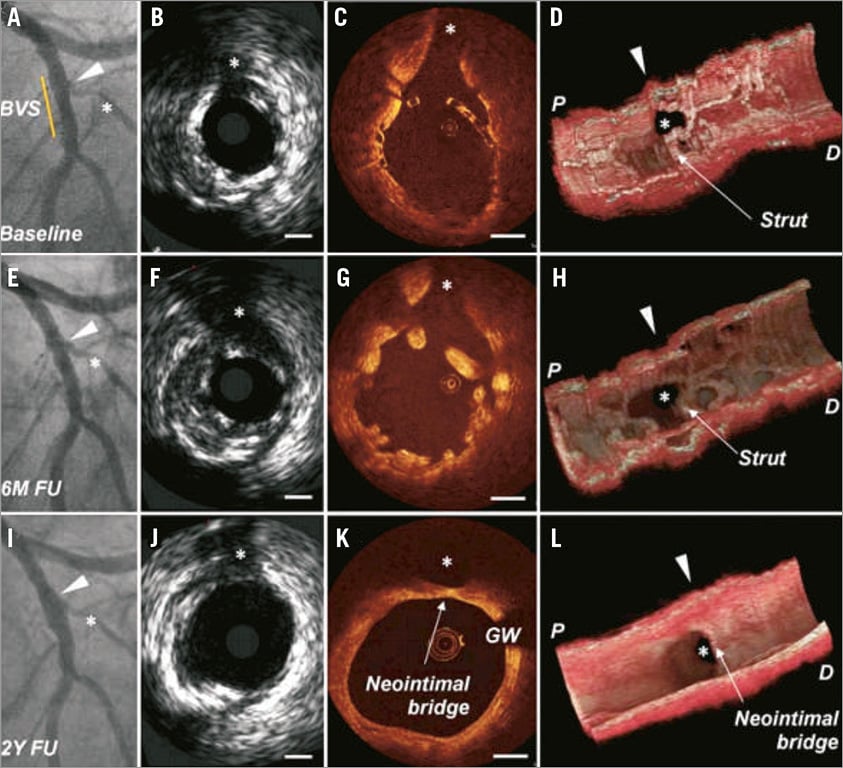
Figure 1. Case example from ABSORB cohort A of a patient who underwent serial intravascular ultrasound and optical coherence tomography after scaffold deployment at baseline, at six-month and at two-year follow-up. At baseline, the diagonal branch was jailed by NASB struts (A-D). At six months, OCT demonstrated a good lumen at the SB region with structural changes of the individual struts overhanging the SB (E-H). At two years, the scaffold was fully resorbed; however, at the ostium of the diagonal branch the scaffold was replaced by a neointimal “neo-carina” (I-L)17.
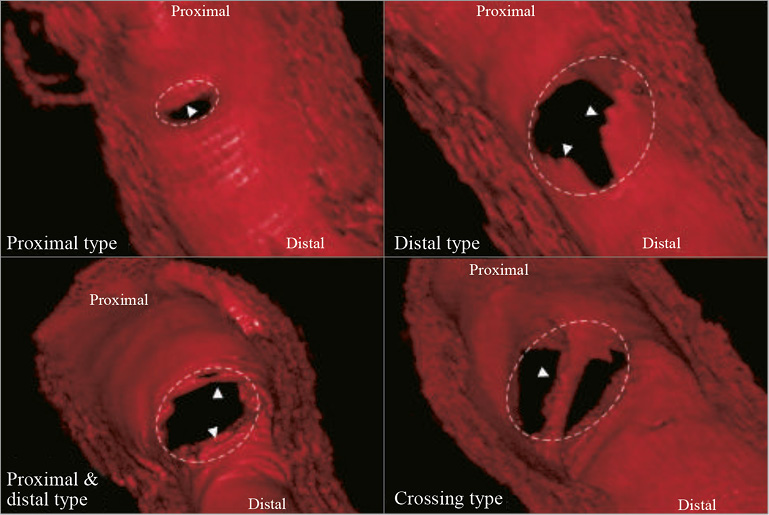
Figure 2. Three-dimensional OCT reconstruction in ABSORB cohort A patients showed different intimal bridge patterns at five-year follow-up. Four different neointimal bridge types were classified according to their location in relation to the SB ostium: proximal, distal, combined proximal and distal, or crossing19.
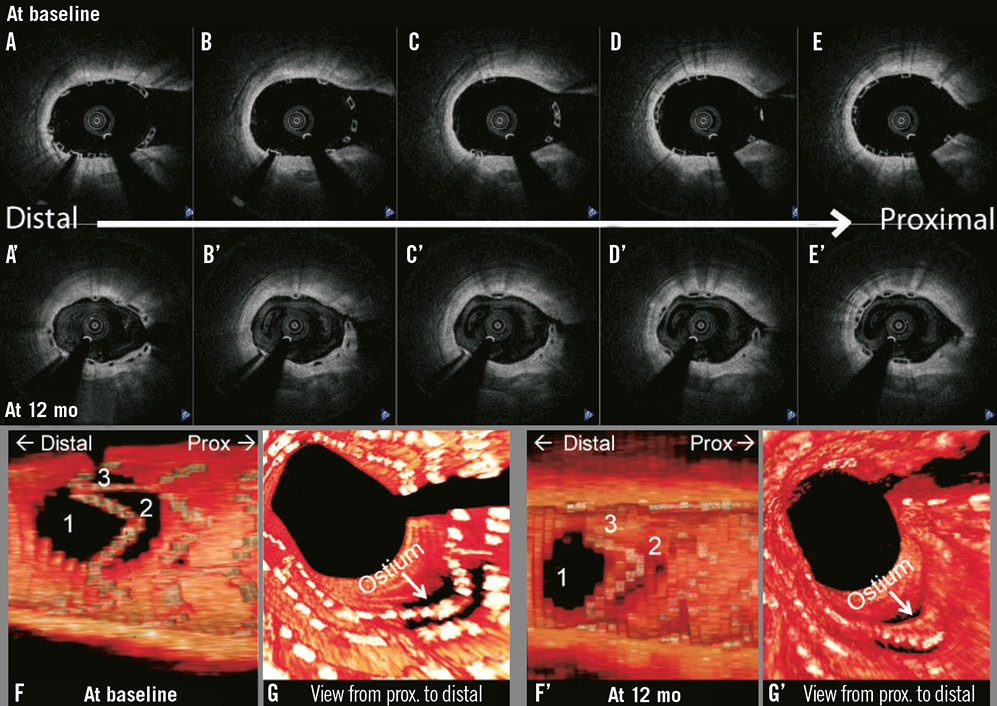
Figure 3. Three-dimensional optical coherence tomography reconstruction of a patient treated with the second-generation Absorb BVS showing SB ostium obstruction by jailed BVS struts dividing the SB ostium in three compartments directly after scaffold implantation. At 12-month follow-up, some of these compartments are replaced by a “membrane” of neointimal tissue, while one compartment remained open.
With permanent metallic stents, NASB struts are known often to be uncovered and thus a potential nidus for stent thrombosis20,21. The formation of neointima bridges combined with the absence of NASB struts after absorption of the jailed scaffolds might also lead to a lower risk of late stent thrombosis, which might, besides the improved ostial patency, be another advantage of BRS in bifurcation lesions.
Because previous trials did not allow inclusion of side branches >2.0 mm in diameter, more studies are needed in bifurcations with larger SBs to assess the absorption and vascular healing process in these lesions. It has been shown that more NASB struts, with more complex compartmentalisation, are present directly after scaffold implantation in bifurcation lesions involving a large (i.e., ≥2 mm) side branch compared with SBs of <2.0 mm22,23. It is likely that neointima bridging will also occur between these multiple overhanging side branch struts. Whether this leads to a reduced blood flow or altered shear stress patterns in the SB remains unknown. It is conceivable that, after main branch (MB) scaffolding, post-dilatation and fenestration of the scaffold in the direction of the side branch might be beneficial, creating a larger opening to the SB and limiting the formation of a membrane overhanging the ostium of the SB. BVS are prone to fractures if post-dilated through the scaffold struts too aggressively with too large balloons14. To explore this issue further, a substudy of the ongoing AIDA all-comers randomised trial (comparing Absorb BVS with XIENCE DES)24 additionally randomises between SB dilatation and no SB dilatation in bifurcation lesions with an SB >2.0 mm. To obtain more knowledge on the role of NASB struts in large SB, we would recommend performance of OCT imaging in bifurcation lesions treated with BRS. Furthermore, the use of OCT imaging could guide crossing the guidewire towards the side branch during BRS bifurcation procedures. It has been demonstrated in metallic stents that recrossing through the distal stent compartment leads to a reduction of incomplete stent apposition in the bifurcation region25.
Shear stress patterns and flow guidance: are they different with BRS?
Local haemodynamic factors, in particular low endothelial shear stress (ESS), are associated with increased neointimal formation and altered blood flow patterns after stent implantation26,27. BRS, which have thicker struts compared with metallic stents, could potentially modify blood flow patterns, neointimal formation and plaque formation. Particularly in bifurcation lesions, in which ESS and blood flow patterns are complex after stent/scaffold implantation, thick BRS struts could lead to disturbed flow in both SB and MB, even in lesions with a good final angiographic result.
Figure 4 shows an example of a three-dimensional reconstruction of an Absorb BVS implanted in a coronary bifurcation lesion. The protrusion of the SB stent into the MB changed the local haemodynamic environment at the bifurcated region, resulting in disturbed flow and low ESS. This phenomenon might promote plaque formation at the region distal to the jailed scaffold struts and tissue coverage on the scaffold struts. It was recently demonstrated that the bifurcation angle can also be an important factor that modulates this process, leading to formation of the so-called neo-carina and restenosis of the segment distal to the carina28.
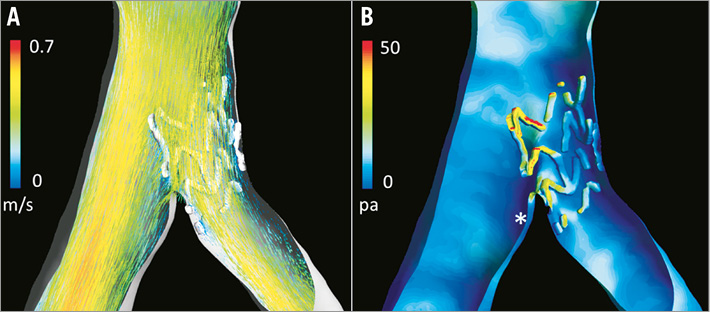
Figure 4. In vivo stent reconstruction and flow simulation of a coronary bifurcation lesion treated with ostial stenting using a BVS, partially jailing the distal main branch. A) Flow pattern, which is altered at the bifurcation region as a result of the jailed BVS struts. B) Shear stress distribution (red indicates the highest shear stress, while dark blue indicates the lowest shear stress). It is clearly shown that the highest shear stress is observed on the jailed side branch struts. Also note that low shear stress is observed in the main branch at the region distal to the jailed struts (indicated by *) due to the change in local flow pattern.
Shear stress patterns and flow reconstructions will provide important insights into the selection of the correct bifurcation technique for implantation of BRS in bifurcations, and more data are eagerly anticipated.
A dedicated bifurcation absorbable scaffold
BRS technology has some technical limitations to its use in bifurcation lesions, such as the risk of strut fracture14,29. So, which adjustments to BRS technology are needed to make it more “bifurcation-friendly”? First, the scaffold should be constructed from a backbone, either polymer or magnesium, which allows overstretching in the proximal main branch so that final kissing balloon inflation and POT have a lower risk of causing scaffold damage. Thinner struts could lead to a lower crossing profile with subsequent easier delivery. Furthermore, it will diminish the adverse effect on ESS and blood flow patterns and reduce the risk of both small side branch occlusions and excessive neointima tissue formation at the side branch ostium. Most likely, thinner and stronger BRS struts will allow scaffold behaviour that is similar to metallic DES behaviour in bifurcations and will allow more complex two-stent bifurcation techniques. However, it is doubtful whether this more complex approach will be clinically useful in most cases. Perhaps newer BRS will give us an opportunity to validate an even less aggressive strategy for bifurcations.
Besides improving the overall design of the BRS we might also think of the development of a dedicated bifurcation BRS in the hope of improving outcomes. Instead of overall thinner struts, the struts might be made thinner only at the side branch region, for instance. The opening between the struts at the SB region might also be enlarged, resembling the BiOSS design30. This will further reduce the number of NASB struts and the nidus for tissue bridging and tissue growth over the SB ostium. This special SB region of the scaffold might be made radiopaque, for example by binding iodine atoms directly to the polymer, to allow accurate positioning and implantation. Additionally, though probably technically challenging, the scaffold might have different diameters proximal and distal to account for the natural tapering of the coronary bifurcation (as is taken into account by the BiOSS and Tryton stents)31,32. Also, the development of self-expanding BRS could provide a treatment option which takes into account the natural tapering of bifurcations.
In extensive disease of a large side branch which needs treatment, a Y-shaped BRS could be beneficial, allowing mechanical scaffolding of the SB ostium, temporary support of the carina and the absence of jailed side branch struts. Such a dedicated Y-shaped BRS has already been designed and evaluated33,34. It showed superior flow behaviour to metallic stents implanted ex vivo using the T-stenting technique, suggesting that dedicated bifurcation BRS may provide a promising strategy. However, at present, dedicated bifurcation metallic DES have a limited role despite more than 10 years of development and clinical research, and Y-shaped metallic stents have been very difficult to deliver35. In addition, the current BVS is already considered to be bulky, so, before such Y-shaped BRS can be developed, the scaffold struts need to be thinner, although for the treatment of left main bifurcation lesions this may be less essential.
Conclusion
Despite the current technical limitations of BRS, implantation in coronary bifurcation lesions with the provisional technique seems to be feasible and safe. The potential theoretical advantages of BRS, better access to the SB due to the disappearance over time of struts overhanging the SB and reduced very late scaffold thrombosis risk, need to be confirmed in future observational and randomised trials. In addition, more insight into the clinical impact of shear stress patterns, neointima formation and flow distortion after scaffold implantation is essential. Ideally, in the coming years, new BRS with thinner and stronger struts will be developed to allow improved scaffold implantation in bifurcation lesions and the use of more complex implantation techniques.
Conflict of interest statement
References