Bioresorbable scaffolds are the next upcoming development in interventional cardiology. Fully bioresorbable stents have been tested for more than 20 years but only in the last few months do we finally have devices with biocompatibility and mechanical performance in in vitro, animal, and early clinical trials to justify commercial release.
Most of the experience is with the Abbott Absorb scaffold (Abbott Vascular, Santa Clara, CA, USA). As expected from first-in-man trials of revolutionary new devices, the initial experience with bioresorbable scaffolds started in short simple lesions. The time has come for practitioners to develop strategies to apply these devices in the complex lesions that represent the majority of the daily activity of busy catheterisation laboratories, including coronary bifurcations. In the ABSORB trial, no lesions covering a side branch (SB) >2.5 mm could be included, but intravascular imaging provided insights into the fate of the strut covering the SB ostium when scaffolds were implanted in the main vessel (MV)1,2. The general attitude has been to avoid instrumentation of these small SBs but in case of abrupt SB closure there have been anecdotal reports of successful dilatation through the struts1. Neointimal bridges on BVS struts across the SB ostium were also shown, possibly leading to focal lumen reduction at the SB ostium3. Therefore, it is tempting to liberate the ostium from jailing BVS struts which potentially impede flow to the side branch, especially when critical disease of the SB ostium requires implantation of a second stent.
In this issue of EuroIntervention, Džavík et al4 report a strategy indistinguishable from a classical T and protrusion technique, including final kissing, that was successfully applied in a bioresorbable vascular scaffold. The result appeared excellent not only angiographically but also using three-dimensional reconstruction of high resolution optical coherence tomography (OCT).
While praising their technical skills, the readers of EuroIntervention should be discouraged from blindly following this example.
Groundbreaking though the technology is, industry has warned us that bioresorbable scaffolds cannot be handled in the same way as conventional stents, and the margin for stretching them without breaking struts is much narrower than for metallic stents. Bioresorbable polymer-based materials have inherent mechanical limitations as compared with metal alloys used in conventional stents. Their tensile strength and elastic modulus are several times lower than metal alloys3, which can affect acute recoil and radial strength. In the second iteration of BVS, the design of the scaffold was modified but the strut thickness was maintained at 150 microns to offer a radial force comparable to the cobalt-chromium XIENCE PRIME™ platform (Abbott Vascular). Poly L-lactide (PLLA), Poly D, L-lactide (PDLA) and Polyglycolide (PLGA) are characterised by a low ductility and an elongation at break typically less than 5%3, almost ten times lower than the elongation capacity of stainless steel and other metallic alloys.
A few specific caveats must be considered when implanting BVS in bifurcations:
1. Based on Murray’s law, the difference between the diameter of the main vessel proximal and distal to a bifurcation can be as high as 1.0 mm for two daughter branches of 3.0 mm each. Stretching a 3.0 mm BVS, adequate for the distal vessel, to 4.0 mm likely causes permanent damage to the device integrity in the proximal vessel. Probably a better compromise in the case described was the deployment of a 3.5 mm BVS at low pressure to avoid damaging the distal vessel, allowing post-expansion of the proximal segment to 4.0 mm.
2. Ostial lesions tend to be fibrotic and calcific and require aggressive post-dilatation with short high-pressure balloons. BVS are estimated to have similar radial force to modern alloy-based DES. Still the wide rectangular shaped struts are unlikely to work well as wedges focusing the expanding force of a post-dilatation balloon to overcome the resistance of the vessel wall. The general experience is that these devices expand as much as you manage to expand the lesion during predilatation, suggesting the need for a more aggressive dilatation strategy before stenting, including liberal use of cutting balloons or Rotablator.
3. The same considerations apply to stretching individual cells such as during SB dilatation after wire recrossing (Figure 1A). A 3.0 mm balloon dilatation will challenge the cells of even the largest currently available BVS (3.5 mm) and often cause strut rupture.
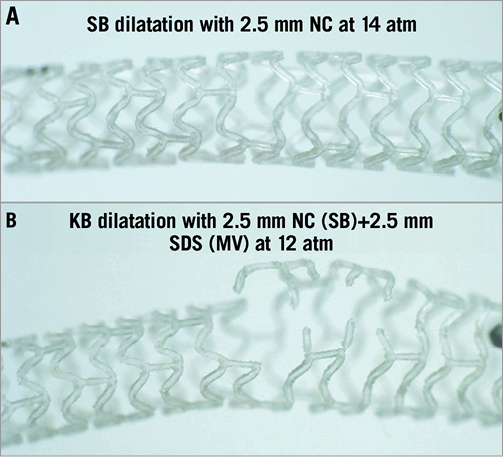
Figure 1. Sequential (A) and kissing balloon dilatation (B) strategies applied in an ABSORB BVS 2.5×18 mm deployed at 16 atm (slow inflation). A) A good result is obtained with the sequential strategy of opening a cell with a 2.5 mm non-compliant balloon (14 atm, slow inflation) and using proximal optimisation (POT) with a larger proximal 3.0 mm balloon (8 atm, slow inflation). B) After kissing inflation with a 2.5 mm NC balloon in the SB and a 2.5 mm stent delivery balloon in the MV inflated simultaneously at 12 atm gross irreversible disruption to the scaffold rings (stent unzipping) is observed.
4. Another important caveat, not sufficiently stressed by the authors, is that balloon inflation towards the side branch needs to be performed slowly with the same technique utilised to deploy a BVS.
5. As suggested by the Džavík report, when there is the need to stent the side branch following deployment of a BVS on the main branch, the most appropriate technique is to utilise a metal drug-eluting stent4. Crossing towards the side branch with another relatively high-profile BVS might be difficult to achieve consistently. Of course there are favourable anatomies where a BVS across a BVS can be advanced. Even if applicable, this approach (BVS on main branch and crossover BVS on side branch) should be considered an exception rather than the rule.
6. The authors reported that the BVS deployed in the main branch did not sustain any fracture after kissing at 4 atm. They used a 2.5 and 3.0 mm balloon inflated only at 4 atm, sufficiently low to prevent major damage even to the relatively small 3.0 mm BVS selected. Based on the reconstructions, it may be argued that some rupture of a longitudinal link may have occurred during kissing. A fracture of a longitudinal link should not be considered an unacceptable complication in stenting bifurcation lesions. We should not forget that the circular rings of the scaffold give the vessel support, while the horizontal connectors are important but not individually fundamental. A completely different and more serious problem may develop when kissing dilatation is performed as in normal metallic stents, dilating at high pressure balloons matching the diameters of the SB and distal MV. The severe strain applied by the overlapping balloon can create disconnection of the circular rings extended to the entire length where the two balloons overlap, resulting in “unzipping” of that section of the scaffold (Figure 1B). It is clear that such loss of scaffold structure integrity completely disrupts the mechanical properties of the BVS and may cause collapse of the scaffold with strut malapposition due to superimposition of the scaffold layers.
7. A more predictable and probably equally or more efficient strategy for BVS in bifurcations, unlikely to result in irreversible scaffold damage, can be the use of sequential SB dilatation followed by proximal balloon dilatation with a balloon diameter adequate to appose fully and expand optimally the proximal main vessel but still within the maximal labelled range of BVS expansion.
A final consideration comes from the fact that scaffolds degrade completely between 18 and 36 months, with variations depending on the polymer type and strut thickness. Does it make sense to risk a permanent damage of the scaffold and complicate the procedure when the free opening to the SB will be restored once the jailing struts resorb? The large flat struts of BVS are not as easy as thin alloy struts to cross with wires, balloons and stents, and all these potentially risky manoeuvres are pointless if they only achieve a transient aesthetic improvement.
Conflict of interest statement
The authors declare receiving research support from Medtronic, Abbott and Boston Scientific but none in relation to this editorial.

