
Abstract
Aims: The aim of this study was to assess prospectively the effectiveness and safety of a new version of the dedicated bifurcation BiOSS stent, the sirolimus-eluting BiOSS LIM, for the treatment of distal left main (LM) stenosis.
Methods and results: This was a prospective international registry which enrolled patients with NSTE-ACS or stable angina. Provisional T-stenting was the mandated strategy. The primary endpoint was the cumulative rate of cardiac death, myocardial infarction (MI) and target lesion revascularisation (TLR) at 12 months. Twelve-month quantitative coronary angiography endpoints included late lumen loss and percent diameter stenosis. A total of 74 patients with distal LM stenosis were enrolled. Seventy-three of the 74 patients (aged 67±9 years, 23% women, 20.3% NSTE-ACS, SYNTAX score 22.4±4.4) were successfully treated with the BiOSS LIM stent, with additional side branch placement of regular DES in 11 patients (14.9%). Periprocedural MI occurred in one (1.4%) patient. The 12-month MACE rate was 9.5% without cardiac death or definite stent thrombosis. TLR and MI rates were 6.8% (n=5) and 2.7% (n=2), respectively.
Conclusions: The use of the BiOSS LIM dedicated bifurcation stent for the treatment of distal LM stenosis was feasible and safe, with promising long-term clinical effectiveness.
Introduction
Left main (LM) coronary artery disease with >50% narrowing is found in 4-6% of all patients undergoing coronary angiography and is associated with multivessel disease in about 70% of cases1.
The introduction of newer-generation drug-eluting stents (DES) and lessons learned from intravascular ultrasound (IVUS) guidance have significantly improved the results of percutaneous coronary intervention (PCI) treatment of LM disease. Recently, two meta-analyses showed that the primary endpoint of one-year major adverse cardiovascular events (MACE) was non-significantly different in the PCI group compared to the coronary artery bypass graft (CABG) group (14.5% vs. 11.8%, respectively, p=0.11)2,3.
Previous studies have shown that PCI for ostial/mid-shaft lesions was associated with better clinical outcomes than for lesions located in the distal LM, mainly due to a lower need for repeat revascularisations4. However, in most cases atherosclerosis develops in the distal part of the LM, including the bifurcation or trifurcation5. Therefore, the optimal treatment is still the subject of debate. Dedicated bifurcation stents (DBS) are one of the proposed solutions. Devices such as the Tryton® (Tryton Medical Inc., Durham, NC, USA) and Axxess™ (Biosensors International Ltd, Singapore, Singapore) stents have already been used in LM treatment6,7.
Previously, we have published clinical data on the paclitaxel-eluting version of the BiOSS® dedicated bifurcation stent (BiOSS Expert®; Balton, Warsaw, Poland) for the treatment of distal LM disease8. The BiOSS stent was further developed and recently a new version eluting sirolimus became available: the BiOSS LIM® (Balton). The main aim of the present study was to assess prospectively the effectiveness and safety of the new sirolimus-eluting BiOSS LIM for the treatment of distal LM disease.
Methods
STUDY POPULATION AND STUDY DESIGN
Between July and December 2013, patients with distal LM disease who were considered suitable by the Heart Team to undergo PCI were eligible to be enrolled in this registry. Implantations were performed in four centres in Bulgaria, Poland and Spain. The inclusion criteria were: patients with stable coronary artery disease (CAD) or non-ST-segment elevation acute coronary syndrome (NSTE-ACS), aged ≥18 years who had a de novo coronary distal LM stenosis. The main exclusion criteria were: ST-elevation myocardial infarction (STEMI), Medina 0,0,1 bifurcations, baseline eGFR <30 ml/min/1.73 m2, inability to take dual antiplatelet therapy for 12 months, or a left ventricular ejection fraction ≤30%. Written informed consent was obtained from all patients before cardiac catheterisation. The institutional review board of each participating centre approved the study protocol.
INTERVENTIONAL PROCEDURE, DEVICE DESCRIPTION AND CONCOMITANT MEDICATIONS
The BiOSS LIM is a coronary, balloon-expandable, dedicated bifurcation stent. The platform is made of 316L stainless steel (strut thickness 120 μm) and is coated with a biodegradable polymer that elutes sirolimus (drug concentration: 1.4 µg/mm2). The rapid exchange delivery system is compatible with 0.014” guidewires and 5 Fr guiding catheters. The BiOSS LIM consists of a proximal and a distal part joined together with two connection struts at the middle zone (the length of the connection struts varies from 0.9 to 1.5 mm, depending on the stent size, when the stent is crimped on the balloon) (Figure 1).
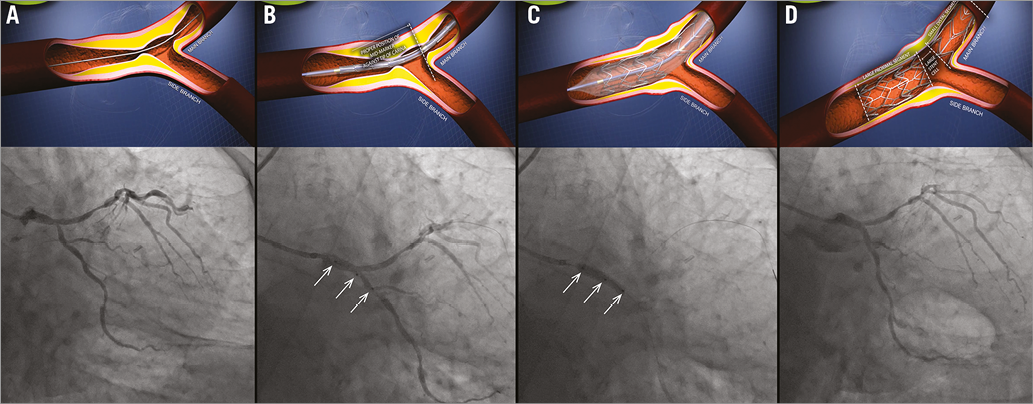
Figure 1. BiOSS LIM deployment. A) Distal left main stenosis – pre-procedure view. B) BiOSS LIM positioning with proper location of three markers. C) BiOSS LIM implantation. D) Post-procedure view.
The default strategy within this registry consisted of a single stent implantation in the main vessel (MV) - main branch (MB) crossing the side branch (SB). Bifurcation lesions were assessed according to the Medina classification (QCA assessment)9. There was no restriction regarding lesion length. MV predilatation and/or SB predilatation were performed according to the operator’s decision. The stent was then implanted in the MV-MB. Proximal optimisation technique (POT) was left to the operator’s decision. SB rewiring and SB post-dilatation/stent placement were at the discretion of the operator. A stent in the SB was implanted only if there was a proximal residual stenosis greater than 70% after balloon dilatation, a significant flow impairment after MV-MB stenting or a flow-limiting dissection (according to the provisional T-stenting strategy). Optionally, the procedure was finished with final kissing balloon (FKB) dilatation.
Procedures were performed in a standard way via radial or femoral access using 6 or 7 Fr guiding catheters. Pharmacological treatment was according to the most recent guidelines.
Troponin I (TnI), creatine kinase (CK) and creatine kinase-myocardial band (CK-MB) were measured pre-procedurally and after six and 24 hours post procedure in all patients. Periprocedural myocardial infarction (type 4a) was defined according to the third universal definition10.
FOLLOW-UP
Clinical follow-up was performed with office visits or by telephone at one and 12 months after the index procedure. Adverse events were monitored throughout the study period. Follow-up coronary angiography was performed at 12 months unless it was clinically indicated earlier.
ENDPOINTS
The primary endpoint was the cumulative rate of MACE, consisting of cardiac death, myocardial infarction (MI) and repeat revascularisation of the target lesion (TLR). Secondary endpoints included cardiac death, all-cause death, MI, TLR, target vessel revascularisation (TVR), stent thrombosis (ST), late lumen loss (LLL), device success and angiographic success. Cardiac death included death resulting from an acute MI, sudden cardiac death, death due to heart failure and death due to cardiac procedures. All deaths were deemed cardiac unless proven otherwise. MI was defined according to the third universal definition10. TLR and TVR were defined according to the Academic Research Consortium definitions.
Device success was defined as successful deployment of the BiOSS LIM stent at the intended site of the target lesion. Angiographic success was defined as an end-procedural MV-MB diameter stenosis less than 20% and SB ostial stenosis less than 70% without significant dissection and flow impairment.
ANGIOGRAPHIC ANALYSIS
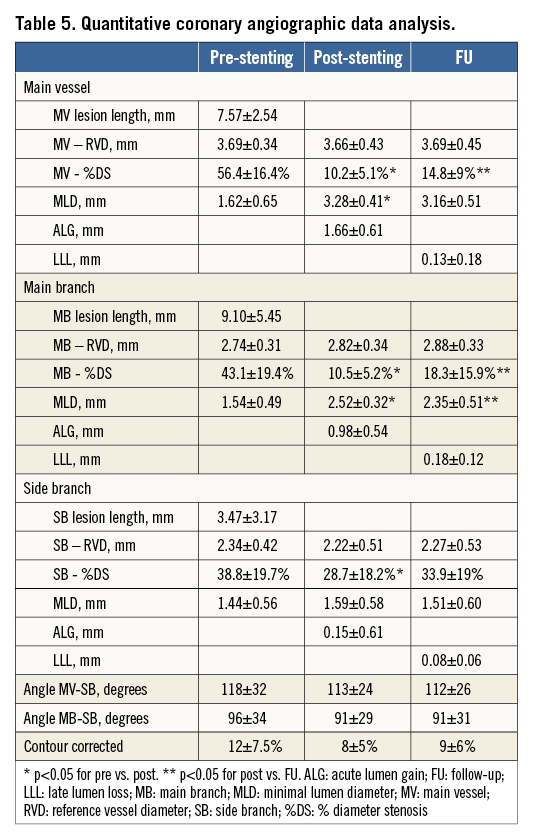
Two orthogonal projections were chosen to visualise the treated bifurcation. All recordings were performed after intracoronary administration of nitroglycerine (200 μg). Quantitative coronary angiographic (QCA) analysis was performed using the dedicated bifurcation software CAAS 5.11 (Pie Medical Imaging BV, Maastricht, The Netherlands). Calibration was performed using the guiding catheter in all cases. The three bifurcation segments (MV, MB, SB) were analysed separately according to the European Bifurcation Club (EBC) consensus11. The following parameters were reported: lesion length, reference vessel diameter (RVD), minimal lumen diameter (MLD). Percentage diameter stenosis (%DS), acute lumen gain (ALG) and LLL were calculated as described previously8. The point of bifurcation (POB, the middle point at the carina level) was determined automatically by the software.
STATISTICAL ANALYSIS
Continuous variables were presented as mean±standard deviation. Categorical data were presented as numbers (%). Continuous variables were compared using an unpaired two-sided Student’s t-test, and categorical data using the χ2 test or Fisher’s exact test, where appropriate. If distribution was not normal (verified with the Shapiro-Wilk test), Wilcoxon signed-rank tests and Mann-Whitney U tests were used. P-values of <0.05 were considered statistically significant. Statistical analyses were performed using R 3.0.2 for OS (R Foundation, Vienna, Austria).
Results
BASELINE CLINICAL CHARACTERISTICS
Between July and December 2013, 74 patients were enrolled. The baseline characteristics are presented in detail in Table 1. Mean age was 67±9 years. Multivessel disease was diagnosed in 61 patients (82.4%), and there were eight (10.8%) cases of protected LM (i.e., patent grafts on LAD/LCX). The study cohort included was a relatively diseased population with 26 (35.1%) patients being diabetic and 41 (55.4%) with a previous myocardial infarction. Fifty-nine patients (79.7%) underwent elective PCI for stable coronary artery disease, while 15 patients presented with unstable angina (n=10, 13.5%) or NSTEMI (n=5, 6.8%).
ANGIOGRAPHIC AND PROCEDURAL CHARACTERISTICS
Mean SYNTAX score was 22.4±4.4. Lesion characteristics are also presented in Table 1 and Medina classification (on QCA) is presented in Table 2. “True” bifurcation lesions (i.e., Medina 0,1,1; 1,0,1 or 1,1,1) accounted for 56.8% (n=42). The BiOSS LIM stent was implanted from the LM towards the LAD in the majority of cases (90.5%). The main procedural variables are presented in Table 3. Device success rate was 98.6%. There was only one delivery failure in which the stent was deformed due to heavy calcification, but did not fall from the balloon. After safe retrieval of this stent and further predilatation, the patient was successfully treated with a new BiOSS LIM stent. The MV was predilated in half of the cases. In four patients (5.4%) it was necessary to implant a regular DES as second stent in the MB because of a diffuse lesion not completely covered by the BiOSS stent (n=1) or because of a distal dissection (n=3). Additional balloon dilatations of the SB were performed in one third of the cases while a consequent implantation of a regular DES in the SB was performed in 14.9% of cases (n=11). The rate of POT use was 24.3%, whereas the rate of FKB use was 47.3%.
CLINICAL OUTCOMES
There was one (1.4%) periprocedural MI due to a transient SB occlusion. Additionally, five (6.7%) cases of in-hospital increase of TnI level (max 1.2 ng/ml) were registered. These were asymptomatic, without ECG changes and did not require a repeat coronary angiography (i.e., did not meet the criteria of the third universal definition).
Clinical follow-up at 12 months was available in all patients (Table 4). The cumulative incidence of MACE was 9.5% (n=7). There were no (cardiac) deaths, no strokes and no stent thromboses. One patient with diffuse in-stent restenosis experienced an MI (12-month MI rate: 2.7%). TLR was performed in five patients (6.8%), two of which were with POBA and three with another DES. Four (5.4%) of them were clinically driven.
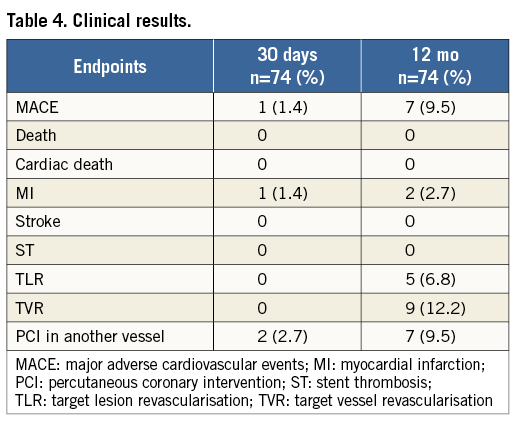
QUANTITATIVE CORONARY ANGIOGRAPHY ANALYSIS
QCA data using bifurcation software are presented in Table 5. The immediate angiographic success rate was 100%. QCA analysis revealed that the BiOSS LIM implantation caused a significant increase of MLD and decrease of %DS in the MV as well as in the MB. Both the distal bifurcation angle (between MB and SB) and the proximal MV to SB angle were not affected by the procedure. Acute lumen gain was 1.66±0.61 mm in the MV, 0.98±0.54 mm in the MB, and 0.15±0.61 mm in the SB.
Twelve-month follow-up angiography was available in 70 patients (94.6%). Follow-up QCA analysis could be performed in 63 (90%) cases. Seven cases had to be excluded due to inadequate view of the bifurcation, overlapping vessel segments, or presence of angiographic guidewires. LLL was 0.13±0.18 mm in the MV, 0.18±0.12 mm in the MB, and 0.08±0.06 mm in the SB (Table 5).
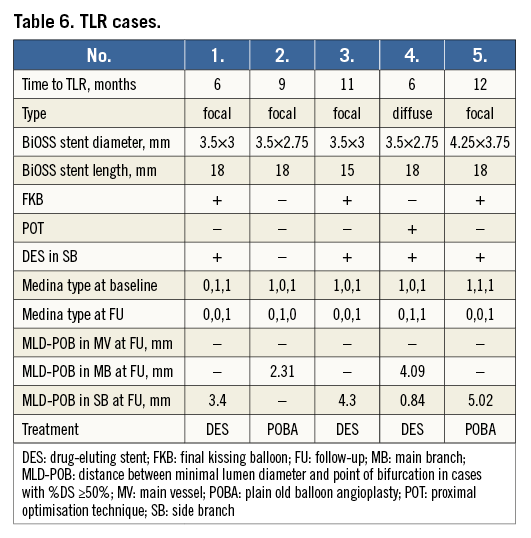
RESTENOSIS PATTERN
In one case the in-stent restenosis pattern was diffuse, while there was a focal restenosis in four of five (80%) cases. Restenosis was mainly located in the MB and in the SB. In four of five (80%) restenosis cases, a BiOSS LIM stent with a proximal diameter of 3.5 mm was implanted. Moreover, in four of five (80%) restenosis cases there was a second stent implanted in the SB, in which FKB was not performed except for one case. Moreover, POT was not performed in four (80%) restenosis cases (Table 6).
To verify whether the mid zone of the BiOSS LIM stent (at the position of the two joining struts) was a “weak” point prone to restenosis, we analysed the distance in each stent segment between the MLD and POB using QCA. The middle zone is located bilaterally 1.2 mm from the POB to the MV-MB. Figure 2 shows a scatter plot with the distance from the POB on the horizontal axis and the %DS at the MLD site in each segment on the vertical axis, indicating that the middle zone is not prone to restenosis (i.e., >50% restenosis).
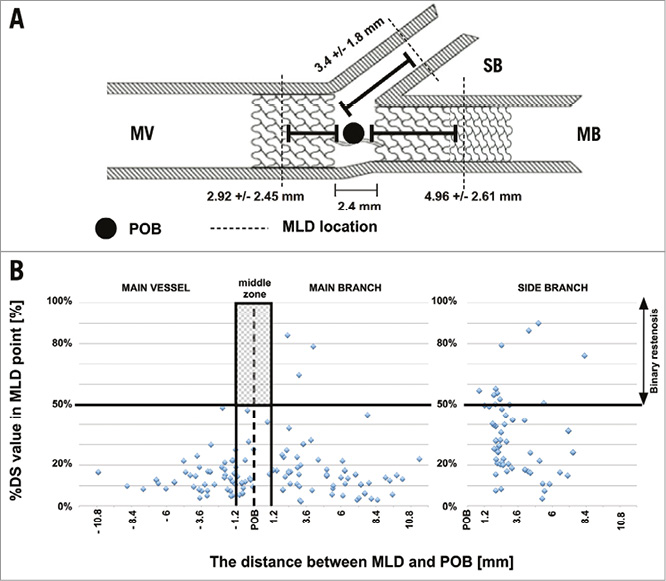
Figure 2. Restenosis pattern. A) Bifurcation scheme showing the mean distance between POB and MLD location at follow-up. B) Scatter plot showing no binary restenosis cases in the middle zone of the stent. MB: main branch; MLD: minimal lumen diameter; MV: main vessel; POB: point of bifurcation; SB: side branch
Discussion
The use of the BiOSS LIM stent for treatment of distal LM stenosis was associated with a high rate of device and angiographic success in a cohort with high-risk patients and lesions. At 12-month follow-up, the cumulative MACE rate was 9.5% without cardiac death or definite ST. The TLR rate was 6.8%, while the MI rate was 2.7%.
The vast majority of the included patients had concomitant comorbidities such as hypertension, diabetes, prior MI or prior CABG: these rates were at least comparable to studies with different DBS6,7 as well as to those in the paper by Bil et al analysing the performance of the BiOSS Expert stent in distal LM stenosis8.
It is worth mentioning that the implantation success rate of the BiOSS LIM stent in the current registry seems to be superior to other DBS in non-LM bifurcations7,12-17 as well as in LM bifurcations6. This suggests that the BiOSS stent is a user-friendly device. This might be explained by the lack of issues such as guidewire criss-crossing, improper device orientation or larger device profiles. Moreover, there was a relatively lower rate of SB stent implantation (14.9% of cases) than in other studies (30-50%)6,12,15. This might have been caused by the fact that this stent may provoke less carina displacement, because of the specific design of the BiOSS stent (with differences in proximal and distal diameters), adapting to the natural bifurcation geometry. However, the lower SB stenting rate might also be explained by the lower rate of side branch involvement, which was 56.8% on QCA in our study6,18-20. No apparent difficulties with rewiring of the SB were observed in our study, confirming the hypothesis that the BiOSS LIM stent is well-suited to the provisional T-stenting strategy20.
In the present paper, we found less periprocedural MI (1.4%) and increased levels of TnI (6.7%) compared to those we found in the BiOSS Expert LM registry (15.1% in-hospital increase of TnI, of which 9.4% met the criteria of periprocedural MI)8, which might be explained by the more aggressive protocol with higher rates of FKB used in the previous study.
The MACE rate in our current study was 9.5%, and the rate of TLR was 6.8%. In the first-in-man BiOSS LIM registry, these rates were 11.7% (MACE) and 8.3% (TLR)21; in the BiOSS Expert stent in LM study the rates were both 9.3%8. The somewhat higher rates of MACE and TLR in the FIM BiOSS LIM registry compared to the present paper might be explained by the difference in sirolimus concentration of the current BiOSS LIM stent. Initially, the concentration of sirolimus was 1 µg/mm2, while the concentration of the current version is 1.4 µg/mm2. This hypothesis is supported by the late lumen loss values obtained in the currently presented population. The LLL values we found in the current registry (MV: 0.13 mm, MB: 0.18 mm, SB: 0.08 mm) were smaller than those obtained in the BiOSS LIM registry in non-LM lesions (MV: 0.35 mm, MB: 0.34 mm, SB: 0.18 mm)21 as well as in the paclitaxel-eluting BiOSS Expert in LM study (MV: 0.20 mm, MB: 0.26 mm, SB 0.13 mm)8. These data might also suggest that the change of the drug (from paclitaxel to sirolimus) is associated with lower LLL and that the potential benefit of BiOSS over conventional DES might be greater in the LM (with more significant differences in diameters between the MV and the MB) than in non-LM bifurcation lesions22.
The TLR rate was comparable to other studies with values within the range of 6.6%-12%6,7,14-16. However, in other studies there were higher rates of predilatation and post-dilatation, as well as FKB7,12-16. Worth mentioning is the fact that the complexity of the coronary anatomy (SYNTAX score 22.4±4.4) was higher in our study compared to other trials6. Moreover, in the original paper of Serruys et al, the 12-month cumulative rate of MACE in a population of patients with low SYNTAX score (0-22) was 14.7% and with intermediate SYNTAX score (23-32) was 16.7%1. A recent study by Moynagh et al showed that the endpoint rate, defined as target vessel failure (cardiac death, target vessel MI, and clinically driven TLR), in LM with PES stenting was 16.3% and with EES stenting 7.6%23. Compared to these historical cohorts, the results of the BiOSS LIM we found seem to be favourable, although randomised data are needed to prove that.
Worth stressing is the fact that our LM study population was of intermediate to high complexity. To summarise the criteria for complex LM bifurcation lesions, we can use criteria proposed by Chen et al in the DEFINITION trial, which are as follows: Medina 1,1,1/0,1,1 with side branch diameter minimally 2.5 mm, side branch %DS ≥70% as well as SB lesion length ≥10 mm. Although the rate of true bifurcation was higher in the TRYTON LM registry (84% vs. 57%), in our study the SYNTAX score was higher (20 vs. 23 points)24.
Importantly, the pattern of restenosis did not reveal the middle zone (with only the two connection struts) to be the “weak point” of the BiOSS LIM stent. The MLD sites were located significantly further away from the POB at follow-up than pre-procedure (Figure 2). The restenosis cases were more frequently observed in stents with smaller nominal parameters (>50% restenosis in stents with proximal diameter ≤3.75 mm vs. >3.75 mm was 16.7% vs. 5.6%, respectively). Apart from the smaller nominal stent diameter, restenosis was associated with double stenting and the low rate of FKB and POT. Interestingly, the same pattern of restenosis was observed in the BiOSS Expert stent in LM registry8. These results were in agreement with the results of the NORDIC 3 study, in which FKB reduced angiographic side branch restenosis, especially in patients with true bifurcation lesions25 as well as with the results of the POLBOS I study, in which POT in the BiOSS group was associated with a lower rate of TLR26. The POT technique ensures better stent apposition in the MV, and improves the flow to the SB. This technique is recommended by the EBC27. Moreover, Kim et al as well as others have shown that the one-stent technique is better than the two-stent technique in distal LM treatment28,29.
QCA analyses were performed with dedicated bifurcation software. Dedicated bifurcation 2D QCA software is recommended since it was proven to be superior to straight vessel QCA applied in bifurcations30. Furthermore, a recent study has shown excellent inter-observer variability when using this software, even in distinct core labs31. Dedicated software enables integrated assessment of the reference function including the MV step-down, measurement of angulations between vessels and reporting in segmental models. Compared with the single-vessel analysis we performed in the BiOSS Expert stent in LM registry8, it enabled us to evaluate the MLD site relative to the POB. Furthermore, it allowed a more detailed analysis of bifurcation segments and angles.
Limitations
The present study has the limitations of a registry. These cases are selected and therefore may not reflect an “all-comer” LM bifurcation lesion population. There was no control group to compare the use of this dedicated bifurcation stent and stenting with other devices and techniques. Moreover, the number of patients enrolled in the registry was relatively low, and more studies, including randomised trials, are needed to draw more definitive conclusions. QCA was not possible in 100% of the cohort for the reasons explained above. The use of intravascular imaging (IVUS) techniques was not available in the majority of cases and not systematically analysed.
Conclusions
The use of the BiOSS LIM stent for treatment of distal LM stenosis is feasible and results in good acute angiographic results. The safety of the procedure and 12-month clinical outcome are acceptable in this high-risk PCI group. At 12-month follow-up, the cumulative MACE rate was 9.5% with no cardiac death or definite stent thrombosis. The TLR rate was 6.8% (n=5) and the MI rate was 2.7% (n=2). The 12-month QCA results of the MB were excellent and comparable with LLL values of best-in-class DES in non-bifurcation lesions.
| Impact on daily practice This report has shown that implantation of the dedicated bifurcation BiOSS LIM stent in distal left main stenosis in patients with a moderate SYNTAX score is safe and effective. Also, these results suggest that the sirolimus-eluting BiOSS LIM stent has better results than the paclitaxel-eluting BiOSS Expert stent. This stent might pose an interesting option in coronary bifurcation treatment, especially when there is a large difference in the diameter between the main vessel and the main branch. |
Guest Editor
This paper was guest edited by Jens Flensted Lassen, MD, PhD, The Heart Centre, Rigshospitalet, University Hospital of Copenhagen, Copenhagen, Denmark.
Conflict of interest statement
R.J. Gil was the medical consultant of the Balton Company. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

