
Abstract
Aims: Our aim is to investigate clinical outcomes following single versus dual stenting strategies for the treatment of true bifurcation distal left main coronary artery lesions.
Methods and results: The EBC MAIN study is a prospective, multinational, randomised clinical study of left main stem true bifurcation lesions (type 1,1,1 or 0,1,1: both left anterior descending and circumflex artery >2.75 mm diameter). The study hypothesis is that left main coronary bifurcation lesions are best treated with a planned single-stent strategy rather than a planned dual-stent strategy, with respect to death, target lesion revascularisation and myocardial infarction at one year. A total of 450 patients will be enrolled and treated either with a planned single or a planned dual zotarolimus-eluting stent strategy according to randomisation. The primary study endpoint is a composite of death, myocardial infarction and target lesion revascularisation at 12 months. Secondary endpoints are: death, myocardial infarctions, and target lesion revascularisation, each at 12 months; angina status, stent thrombosis, death, myocardial infarction, target lesion revascularisation at three- and five-year clinical follow-up.
Conclusions: EBC MAIN will be the first randomised clinical trial to compare single versus dual stenting strategies for the treatment of true bifurcation distal left main coronary artery lesions.
Abbreviations
Cx: circumflex artery
DES: drug-eluting stents
EBC: European Bifurcation Club
EBC MAIN: European Bifurcation Club Left Main Study
LAD: left anterior descending
LMS: left main stem
TLR: target lesion revascularisation
Introduction
With the advent of DES and the subsequent significant reduction in the occurrence of restenosis and need for TLR, left main stenting has become a viable elective alternative to coronary artery bypass surgery1-3. The majority of left main stenoses, however, occur at the bifurcation. Stenting results at the left main bifurcation are less favourable than those involving the ostium or shaft only4,5. Non-left main bifurcation lesions have been extensively studied. The consensus from these studies is that there is no systematic advantage to a more complex dual-stent implantation technique6-10. This question is now apposite with respect to the left main stem bifurcation.
Some non-randomised studies have suggested that a single-stent strategy may be superior to a dual-stent strategy for bifurcation lesions of the left main stem. However, these studies are naturally confounded by selection bias, and included many patients without “true” bifurcation lesions where there is >50% narrowing in both LAD and Cx coronary arteries in addition to the left main.
There are theoretical reasons why the left main coronary artery bifurcation may be more suited to a single- rather than a dual-stent technique, on account of the wider angle between the left main, LAD and Cx.
The EBC MAIN study hypothesis is that left main coronary bifurcation lesions (type 1,1,1 or 0,1,1: both branches >2.75 mm diameter) are best treated with a planned single-stent strategy rather than a planned dual-stent strategy, with respect to death, target lesion revascularisation and myocardial infarction at one year.
Study hypothesis
The hypothesis of this study is: “Left main coronary true bifurcation lesions (type 1,1,1 or 0,1,1: both LAD and Cx >2.75 mm diameter) are best treated with a planned single-stent strategy rather than a planned dual-stent strategy, with respect to death, target lesion revascularisation and myocardial infarction at one year”.
Study design
EBC MAIN (ClinicalTrials.gov Identifier: NCT02497014) is an international, multicentre, randomised comparison of two stent strategies for the treatment of left main coronary bifurcation disease. The trial is being conducted in accordance with the Clinical Investigational Plan, the Declaration of Helsinki and the applicable local legislation. The conduct of the trial will be approved by the local ethics committee at each site. The study sponsor is the EBC. The management of the study will be through the Cardiovascular European Research Center based in Paris, France, which will be responsible for the monitoring of electronic data capture, database and data management, as well as safety monitoring procedures. Additionally, there will be a core lab analysis of the left main angioplasty results. The study has been made possible by an unrestricted educational grant through Medtronic.
Study population
The trial will include patients with unprotected left main coronary bifurcation disease, requiring percutaneous coronary interventions. Patients with either stable or unstable angina (non-ST-elevation MI are included as well as ST-elevation MI >72 hours preceding) will be eligible. The left main disease should be of Medina type 1,1,1, or 0,1,1, and LAD and Cx coronary arteries should both be of diameter >2.75 mm, evaluated by visual estimation.
Patients with additional coronary disease may be considered for the study. The SYNTAX score for the lesions that are to be treated should be <32 and there should be no more than two additional coronary lesions requiring treatment, such that the main focus of the study will rest on the bifurcation left main stem disease. Lesions which are to be treated with PCI should be treated at the same time as, or preferably before, the index lesion. In the case of stable patients, this will allow randomisation to occur after treatment of non-index lesions. If other lesions are to be treated, study stents are to be used to reduce the risk of subsequent revascularisation unrelated to the index bifurcation disease.
Inclusion criteria
Patients must meet ALL of the inclusion criteria:
1. Bifurcation distal left main stem stenosis >50% and:
a. ischaemic symptoms, or
b. positive non-invasive imaging for ischaemia, or
c. positive fractional flow reserve, or
d. LMS IVUS minimal lumen area <6 mm²
2. Left main diameter ≤5.75 mm
3. True bifurcation lesion type 1,1,1 or 0,1,1
4. LAD and Cx diameter both >2.75 mm, evaluated by visual estimation
5. Unprotected left main
6. Patient ≥18 years old
Exclusion criteria
1. STEMI <72 hours preceding
2. Cardiogenic shock
3. Chronic total occlusion of either vessel
4. >2 other coronary lesions planned for treatment
5. SYNTAX score for planned lesions to be treated >32
6. LMS trifurcation if all vessels are ≥2.75 mm diameter
7. Either bifurcation vessel not suitable for stenting
8. Platelet count ≤50×109/mm3
9. Left ventricular ejection fraction ≤20%
10. Patient life expectancy less than 12 months
11. Participation in another investigational drug or device study
12. Patient unable to give informed consent
13. Women of child-bearing potential or lactating
Randomisation
Patients who fulfil inclusion and exclusion criteria and consent to the study will be randomised electronically via a 128-bit secure encrypted dedicated website according to a standard random number generation method (Figure 1). Patient randomisation will be stratified by participating centre.
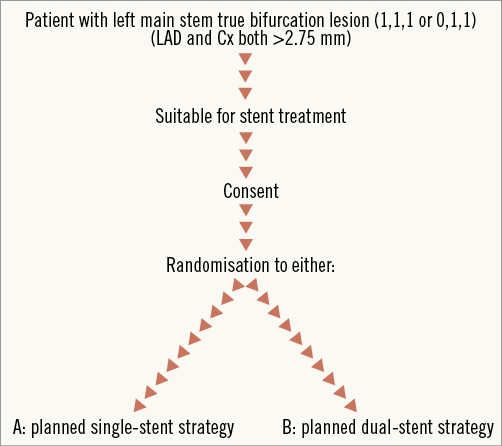
Figure 1. Randomisation scheme.
The revascularisation procedure
PCI may be undertaken via the access site of choice of the operator. Haemostatic technique and use of vascular closure devices will also be at the discretion of the operator. Operators in the trial should be undertaking a minimum of 150 coronary angioplasty cases annually. Planned single- and dual-stent techniques will be performed according to the recommendation of the EBC consensus11,12.
PLANNED SINGLE-STENT TECHNIQUE
Coronary guidewires should be passed to the LAD and Cx/intermediate arteries, respectively. One should be designated the main vessel and one should be designated the side vessel. Lesion preparation should be undertaken as required, but initial side vessel predilatation should not be undertaken unless considered essential by the operator. Stenting of the main vessel should be undertaken with a wire jailed in the side vessel to preserve side vessel flow and access. Stent diameter should be chosen according to the diameter of the main vessel immediately distal to the bifurcation (Figure 2).
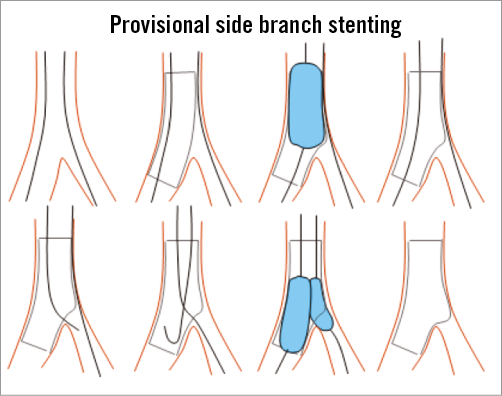
Figure 2. Provisional stenting scheme as recommended by the European Bifurcation Club.
Following stenting of the left main into the main vessel, the distal left main should be dilated with a short non-compliant balloon of appropriate size for the left main stem using the proximal optimisation technique11. Following this, the side vessel should be rewired, ideally through a distal stent strut, and a kissing balloon inflation should be undertaken when an angiographically significant (>75% DS or TIMI flow <3) ostial SB lesion remains after MV stenting according to the last EBC consensus12. Balloon sizes should be according to the diameter of the main and side vessel, respectively, with individual high-pressure inflation followed by a final lower-pressure kiss dilatation. Finally, the proximal stented portion in the left main coronary artery should be dilated to full expansion, if necessary using either low-pressure dilatation of the kissing balloon pair or a separate individual balloon. For all of these dilatations, it is preferable that non-compliant balloons are used in order to limit “overstretching” of the vessels.
Following kissing dilatation, the side vessel should not be treated further unless there is one of the following: TIMI flow <3 in the side vessel, severe ostial pinching of the side vessel (>90%), threatened side vessel vessel closure, side vessel vessel dissection >type A.
If one of these four situations applies, the operator may choose to implant a side vessel stent in a manner of their choosing (e.g., T, T and protrusion, culotte). Following implantation of a second stent, repeat kissing balloon inflation is mandatory, again ideally using non-compliant balloons as above, with individual high-pressure inflations followed by final kissing balloons at lower pressures.
Further treatment to proximal or distal aspects of the main vessel or side vessel can be continued at the discretion of the operator. At any stage, proximal or distal dissections may be treated as required with further stent implantations. At any stage, post-dilatations may be undertaken to optimise stent expansion.
PLANNED DUAL-STENT TECHNIQUE
Coronary guidewires should be passed to the LAD and Cx/intermediate arteries, respectively. One should be designated the main vessel and one should be designated the side vessel. Lesion preparation should be undertaken as considered necessary.
The planned dual-stent technique is at the discretion of the operator but should be either culotte (Figure 3A), DK mini-crush (Figure 3B), T or T and protrusion. Stent diameter should be chosen according to the diameter of the vessel immediately distal to the bifurcation. Wire jail, proximal optimisation technique, non-compliant balloons, high-pressure dilatations and final dilatation of the stented proximal left main should be used in accordance with the advice of the EBC.
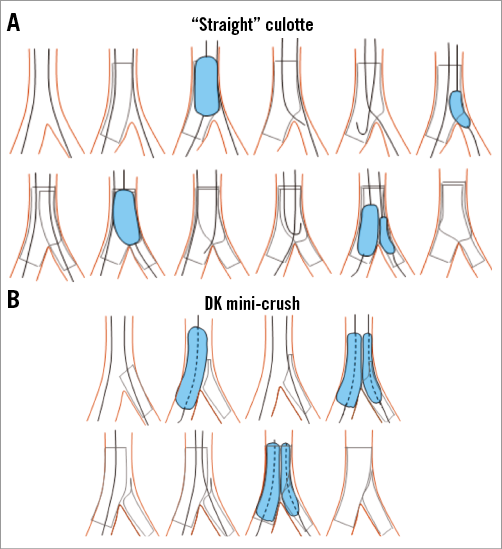
Figure 3. Dual-stenting techniques. Dual stenting using culotte (A) and DK mini-crush (B) techniques as recommended by the European Bifurcation Club.
Further treatment to proximal or distal aspects of the main vessel or side vessel can be continued at the discretion of the operator. At any stage, proximal or distal dissections may be treated as required with further stent implantations. At any stage, post-dilatations may be undertaken to optimise stent expansion.
IVUS-guided stenting, even if strongly recommended, if feasible, is left to the physician’s discretion.
STENT TYPE
The stent to be used is a zotarolimus-eluting stent (Resolute Onyx™ DES; Medtronic, Minneapolis, MN, USA). All sizes and lengths are allowed.
Follow-up
Patients will be followed up by telephone or in person at six months, 12 months, three and five years. At the six-month follow-up, patient symptoms and wellbeing will be established. The purpose of this midterm assessment is to ensure that patients are not suffering in silence during follow-up. Significant symptoms will trigger further investigation as required. At the one-, three- and five-year visits the following will be assessed: angina symptoms (CCS class), medications (angina index), endpoints.
Angina index is a scoring system where a point is scored for each of the following medications being taken by the patient: nitrate (sublingual or spray) used ≥1 during the week prior to question, nitrate (oral), blocker or ivabradine, calcium antagonist, nicorandil or other antianginal. The maximum angina index score on this system is therefore 5.
Primary and secondary study endpoints
The primary study endpoint will be a composite of death, myocardial infarction and TLR at 12 months. Secondary study endpoints will be death, MI, TLR each at 12 months, angina status, stent thrombosis, death, MI, TLR at three- and five-year clinical follow-up. Procedural endpoints will include procedural and technical success, procedural and in-hospital major adverse cardiac events and procedure duration, fluoroscopy and costs.
The third universal definition of myocardial infarction will be used to define MI in this study, except for the category of PCI-related MI (Type 4a) or CABG-related MI (Type 5)13. Under these circumstances, the expert consensus definition from the Society for Cardiovascular Angiography and Interventions (SCAI) will be used14.
The primary analysis will be on the intention-to-treat principle (i.e., once randomised to a treatment arm). The analysis will also be performed on a per-protocol basis (i.e., all randomised patients who are not protocol violators). The primary endpoint will be analysed at an intended mean or median of 12 months after randomisation, with a minimum patient inclusion period of six months. Depending on the duration of the recruitment phase, there will be some flexibility around the exact timing of database closure.
The secondary endpoints (stent thrombosis, angina status) will be analysed at 12 months on an intention-to-treat and per-protocol basis. The procedural endpoints and economic evaluation will be analysed after completion of the hospitalisation period of all patients in the intention-to-treat and per-protocol basis.
Calculation of sample size
Left main stem bifurcation data from published studies suggest that the primary endpoint will be reached in 14% (single) versus 25% (dual) of patients at one year15-17. Using these estimates, a two-sided significance (1-alpha) of 95% and 80% power, a sample size of 404 patients would be required. Allowing for a 10% loss-to-follow-up rate, a total of 450 patients should be recruited to the study.
Statistical analysis
Testing to see if treatment groups are comparable will be done by t-tests or chi-square tests, as appropriate. Kaplan-Meier survival curves will be plotted and the log-rank test for difference in event rates between the two treatment groups will be applied. The influence of explanatory variables on the endpoints will be modelled and tested using the Cox regression analysis method. Continuous variables will be analysed using analysis of variance techniques; however, whenever appropriate, non-parametric methods will be used. The statistical analysis method for the primary endpoint will be based on Kaplan-Meier methods, and the log-rank test will be used to test for treatment group differences.
Conclusions
The EBC MAIN study will be the first randomised clinical trial to compare single versus dual stenting strategies for the treatment of true bifurcation distal left main coronary artery lesions. The study started enrolment in the first quarter of 2016.
| Impact on daily practice No randomised clinical trial so far has investigated the optimal stenting strategy in bifurcation left main stenosis. Non-left main bifurcation lesions have, however, been extensively studied. The consensus from these studies is that in non-left main bifurcation lesions there is no systematic advantage to a more complex dual-stent implantation technique. This question is now apposite with respect to the left main stem bifurcation. The EBC MAIN study is the first prospective, multinational, randomised clinical study of left main stem true bifurcation lesions. The study hypothesis is that left main coronary bifurcation lesions are best treated with a planned single-stent strategy rather than a planned dual-stent strategy, with respect to death, target lesion revascularisation and myocardial infarction at one year (primary study endpoint). |
Appendix. List of centre principal investigators and participating centres
Hildick-Smith, David; Brighton and Sussex University Hospitals NHS Trust, Brighton, United Kingdom. Spence, Mark; Belfast City Hospital, Belfast, Northern Ireland, United Kingdom. Egred, Mohaned; Freeman Hospital Newcastle, Newcastle, United Kingdom. Banning, Adrian; John Radcliffe Hospital, Oxford, United Kingdom. Redwood, Simon; St Thomas’ Hospital,London, United Kingdom. Clesham, Gerald; Orwell Private Cardio-
thoracic Unit Essex, Essex, United Kingdom. Hovasse, Thomas; Institut Cardiovasculaire Paris Sud, Massy, France. Fajadet, Jean; Clinique Pasteur, Toulouse, France. Carrié, Didier; CHU Toulouse Rangueil, Toulouse, France. Brunel, Philippe; Clinique de Fontaine,Fontaine lès-Dijon, France. Finet, Gérard; HCL CHU Louis Pradel, Lyon, France. Koning, René; Clinique Saint-Hilaire, Rouen, France. Pan Alvarez Ossorio, Manuel; Hospital Universitario Reina Sofia, Córdoba, Spain. Masotti, Monica; Hospital Clinic de Barcelona, Barcelona, Spain. Serra, Antonio; Hospital de la Santa Creu i Sant Pau, Barcelona, Spain. Vaquerizo, Beatriz; Hospital del Mar, Barcelona, Spain. Lassen, Jens Flensted; Rigshospitalet, University of Copenhagen, Copenhagen, Denmark. Christiansen, Evald; Aarhus University Hospital, Aarhus, Denmark. Stankovic, Goran; Clinical Center of Serbia, Division of Cardiology, Diagnostic and Catheterisation Laboratory, Belgrade, Serbia. Chieffo, Alaide and Colombo, Antonio; San Raffaele Hospital, Milan, Italy. Tamburino, Corrado; Ferrarotto Hospital, University of Catania, Catania, Italy. Burzotta, Francesco; Universita’ Cattolica del Sacro Cuore, Rome, Italy. Erglis, Andrejs; Pauls Stradins Clinical University Hospital, Riga, Latvia. Naber, Christoph; Elisabeth-Krankenhaus Essen, Essen, Germany. Ferenc, Miroslaw; Universitaets-Herzzentrum Freiburg-Bad Krozingen, Bad Krozingen, Germany.
Funding
The study has been made possible by an unrestricted educational grant through Medtronic.
Conflict of interest statement
The authors have no conflicts of interest to declare.

