
Abstract
Aims: We aimed to investigate the association between the use and findings of IVUS with clinical outcomes in the PCI arm of a randomised trial of LMS PCI.
Methods and results: The NOBLE trial randomised patients with LMS disease to treatment by PCI or CABG. Of 603 patients treated by PCI, 435 (72%) underwent post-PCI IVUS assessment, 224 of which were analysed in a core laboratory. At five years, the composite of MACCE was 18.9% if post-PCI IVUS was performed versus 25.0% if it was not performed (p=0.45, after adjustment). Overall repeat revascularisation was not reduced (10.6% vs 16.5%, p=0.11); however, LMS TLR was (5.1% vs 11.6%, p=0.01) if IVUS was used. For comparison of stent expansion, LMS MSA was split into tertiles. We found no significant difference in MACCE, death, myocardial infarction or stent thrombosis between tertiles. There was a significant difference between the lower and upper tertiles for repeat revascularisation (17.6% vs 5.2%, p=0.02) and LMS TLR (12.2% vs 0%, p=0.002).
Conclusions: Post-PCI IVUS assessment and adequate stent expansion are not associated with reduced MACCE; however, there is an association with reduced LMS TLR. The use of intracoronary imaging to prevent stent underexpansion in LMS PCI is likely to improve outcomes.
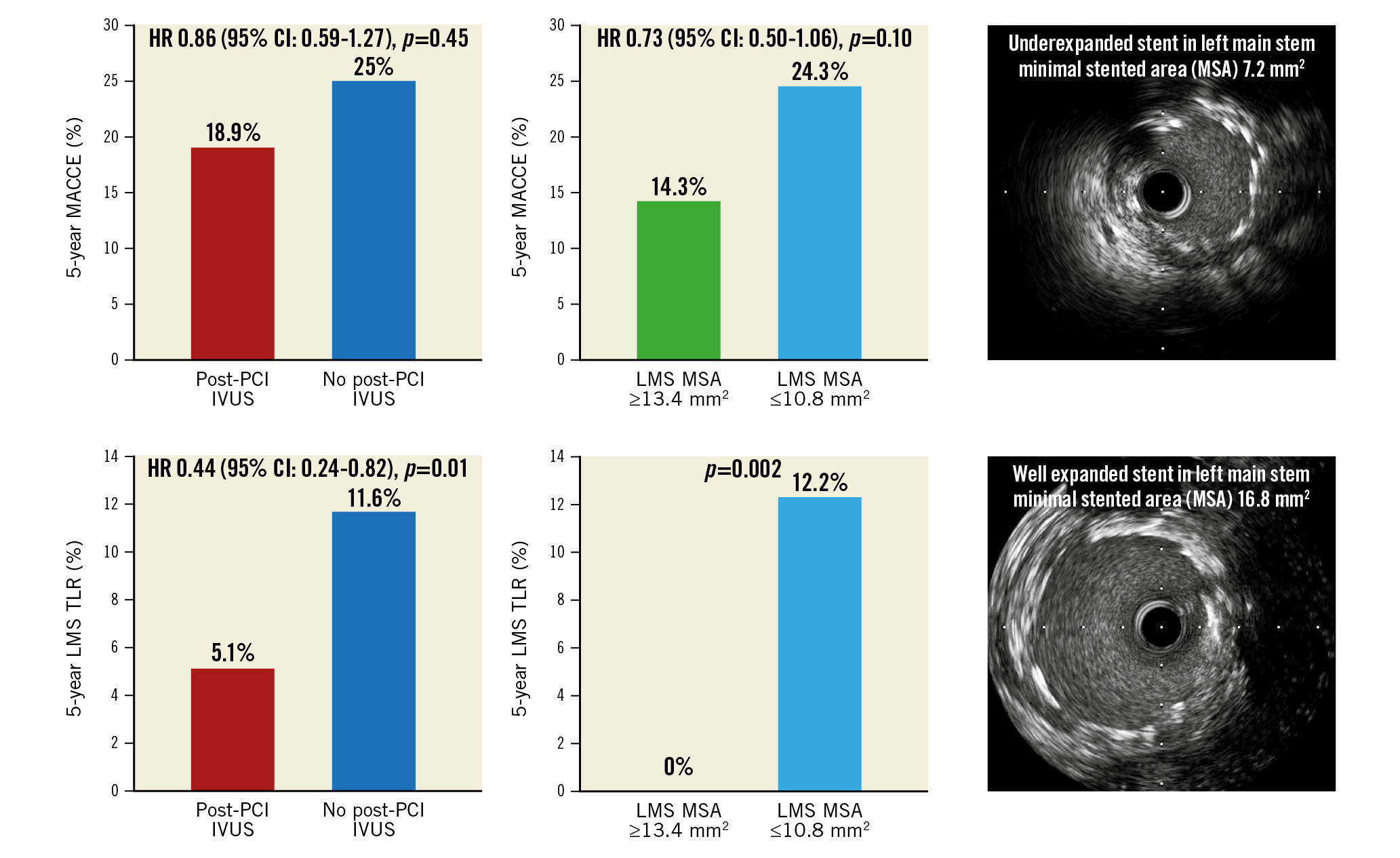
Visual summary. Rates of MACCE (top) and left main stem target revascularisation (bottom) for patients with and without post-PCI IVUS assessment (left) and lower and upper LMS minimal stented area tertiles (right).
Introduction
Percutaneous coronary intervention (PCI) of unprotected left main stem (LMS) coronary disease is now an acceptable treatment with comparable medium-term survival to coronary artery bypass graft surgery (CABG)1,2. It is also frequently undertaken in patients in whom the risks of CABG are prohibitively high3. However, composites of major cardiovascular events, in particular repeat revascularisation (the majority of which is target lesion revascularisation [TLR]1,2), are inferior in those treated by PCI relative to CABG1,2. Intravascular ultrasound (IVUS) measurements of stent expansion are predictive of restenosis and TLR4,5, a finding which appears to remain applicable in patients who have undergone LMS stenting6. The use of IVUS in LMS PCI is recommended; its use in this setting has been associated with improved survival at long-term follow-up7,8.
The Nordic-Baltic-British left main revascularisation study (NOBLE) is a prospective, randomised, open-label, non-inferiority trial. It enrolled 1,201 patients with unprotected LMS disease and randomised them in a 1:1 fashion to PCI or CABG1. Rates of major adverse cardiac or cerebrovascular events (MACCE) were higher in the PCI arm than in the CABG arm1. IVUS was not used to guide all PCI procedures in NOBLE. This is a substudy of NOBLE with the aim of investigating (1) whether the use of IVUS to guide optimisation of the post-stenting result was associated with superior clinical outcomes compared with angiographic guidance alone, and (2) the relationship between post-PCI IVUS findings, in particular stent expansion, and clinical outcomes.
Methods
STUDY DESIGN
The NOBLE study is a trial comparing PCI and CABG in the treatment of unprotected LMS coronary disease1. The study protocol has been described in detail previously1. Briefly, patients were eligible for study enrolment if they presented with stable angina, or an acute coronary syndrome, and were found to have a stenosis of ≥50% diameter or fractional flow reserve ≤0.80 in the LMS, with no more than three additional non-complex lesions (lesions not involving chronic total occlusions, bifurcations requiring a two-stent technique or excessive calcification or tortuosity). Participants were excluded if they had suffered an ST-elevation infarction within 24 hours, were considered too high risk for CABG or PCI or expected to survive for <1 year. All enrolled patients provided written informed consent. The study complied with Good Clinical Practice and the Declaration of Helsinki. It was approved by The Central Denmark Region Committees on Health Research Ethics, and by national or local ethics committees for the individual sites (as appropriate), and by the Danish Data Protection Agency. The trial was registered with ISRCTN87206264 and ClinicalTrials.gov identifier: NCT01496651.
PROCEDURES
Patients were treated with the intention of achieving complete revascularisation of all vessels with significant stenoses. Ostial and mid-shaft lesions were treated with a single-stent strategy. Distal bifurcation lesions could be treated with two-stent techniques at the discretion of the operator, with a preference for the culotte technique. Final kissing balloon dilation was mandatory when a two-stent technique was used. IVUS was strongly recommended pre and post stent deployment but was not mandatory. Suggested criteria for stent expansion were not included in the trial protocol. Use of drug-eluting stents was mandatory. After treatment of 73 patients with PCI, the Biolimus-eluting stent (BioMatrix Flex™; Biosensors, Morges, Switzerland) became the recommended study stent.
Patients treated with PCI were eligible to be included in the IVUS core lab analysis if they had post-PCI IVUS assessment of the LMS. Site investigators would send anonymised IVUS images to the core lab (Royal Victoria Hospital, Belfast, UK). Once analysed, IVUS data were linked to adjudicated study follow-up data.
OUTCOMES
The primary endpoint was a composite of MACCE (death from any cause, non-procedural myocardial infarction [MI])9, repeat revascularisation, or stroke). Other clinical endpoints were the individual components of the primary MACCE endpoint and definite stent thrombosis. Procedural MIs were documented (post hoc). Repeat revascularisations were categorised as TLR, LMS TLR, or de novo lesion revascularisation.
INTRAVASCULAR ULTRASOUND
Immediate post-stenting IVUS imaging was performed, using motorised transducer pullback (0.5 mm/s) and Atlantis™ SR Pro 40 MHz rotating 3.2 Fr catheters (Boston Scientific, Marlborough, MA, USA) or a synthetic-aperture array, 20 MHz, 3.2 Fr catheter (Eagle Eye®, In-Vision Gold [Philips Volcano, San Diego, CA, USA]). Off-line IVUS analysis was performed using DICOM analysis software for computerised planimetry (Rubo Medical Imaging, Aerdenhout, the Netherlands).
Minimal lumen area or MSA was measured in the LMS and, when available, in the bifurcation core segment6, the ostial LAD (defined as ≤5 mm from the carina), and the ostial LCx (defined as ≤5 mm from the carina). An MSA <8 mm2 in the LMS, <7 mm2 in the bifurcation core segment, <6 mm2 in the ostial LAD and <5 mm2 in the ostial LCx has previously been associated with adverse events6. Those in whom IVUS had demonstrated a smaller MSA at any of these sites were identified and outcomes compared with those in whom all MSA measurements exceeded these values. At the site of the MSA, the cross-sectional areas of external elastic membrane (EEM) and residual plaque were measured by 2D planimetry. Residual plaque burden was calculated as plaque/EEM x100 expressed as a percentage (Figure 1). At each measured segment, calcification was noted to be present or absent in binary form; the extent of calcification at the MSA was measured in degrees, the maximum being 360˚. This was recorded as a summed total if not a continuous arc. Malapposition was defined as a separation of at least one metallic strut from the vessel wall in the absence of a side branch. We did not routinely record whether IVUS assessment resulted in further optimisation of the stenting result. All analysed IVUS images were of the final stenting result.
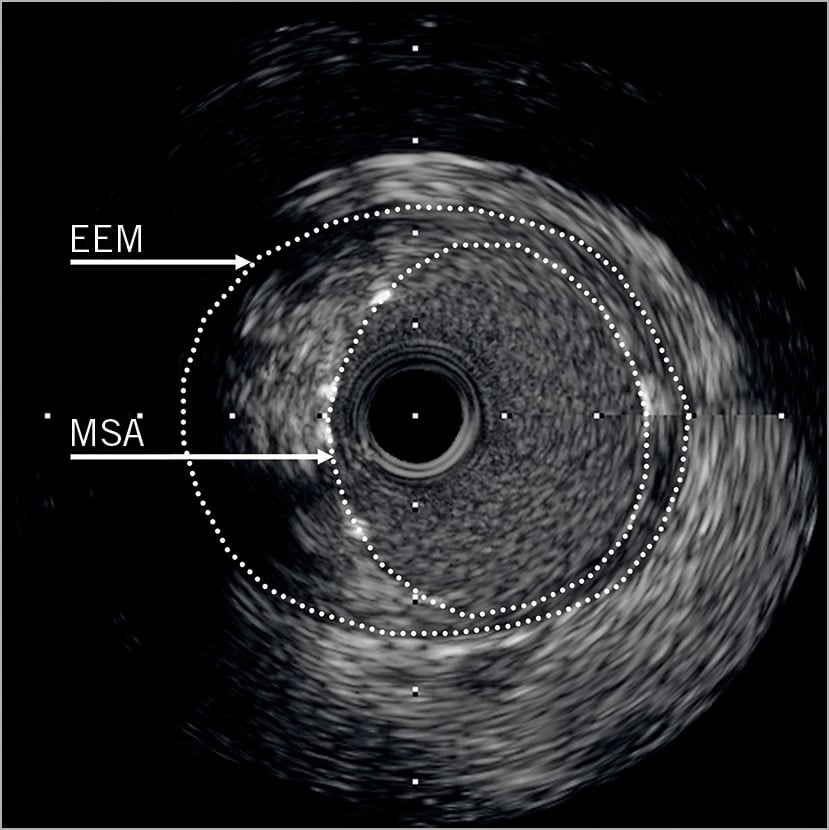
Figure 1. IVUS image taken post LMS PCI. Both MSA and EEM areas are highlighted. The residual plaque burden is the area outside the MSA, but within the EEM, expressed as a proportion of the total EEM area.
STATISTICAL ANALYSIS
Continuous variables are reported as mean (SD) and were compared by t-test if they followed a Gaussian distribution. Continuous variables not following a Gaussian distribution are reported as their median value (IQR) and compared using the Mann-Whitney test. Continuous variables were compared amongst three or more groups using a one-way ANOVA test or Kruskal-Wallis test as appropriate. Binary variables are reported as counts and percentages, and baseline and in-hospital differences between the two groups were assessed with the χ2 or Fisher’s exact test if a cell value was <5. Follow-up began at randomisation. In the analysis of individual endpoints, follow-up continued until the date of a clinical endpoint event, death, or five years after randomisation, whichever occurred first. All patients were followed for at least one year. Cumulative rates of MACCE were stratified into three tertile groups based on LMS MSA and presented using Kaplan-Meier curves. MACCE and its components are reported using five-year Kaplan-Meier estimates and hazard ratio by unadjusted Cox regression analysis with 95% confidence intervals. In addition, adjusted hazard ratios are stated, calculated using a multivariate Cox proportional hazards model. Only the significant variables were retained in the final model. A p-value of <0.05 was considered significant. All analyses were carried out using Stata 12 (StataCorp, College Station, TX, USA).
Results
In the NOBLE trial, 603 patients received PCI, including 13 allocated to CABG, and were included in the present analysis. Of those, 474 underwent IVUS guidance in some form (pre-PCI in 270 [45%] and post-PCI in 435 [72%]). Characteristics of those who did and did not undergo post-PCI IVUS assessment are presented in Supplementary Table 1. Of the participants who underwent post-PCI IVUS, 224 (37% of the total PCI population and 52% of those in whom post-PCI IVUS was undertaken) had images submitted to the IVUS core lab for analysis. Demographics and clinical characteristics for those included in the core lab analysis and those who underwent post-PCI IVUS but were not analysed in the core lab are listed in Supplementary Table 2.
THE USE OF IVUS TO GUIDE LMS PCI
Table 1 and Figure 2 summarise clinical event rates for those patients in the NOBLE trial who underwent LMS PCI and had post-PCI IVUS assessment, and for those who did not. There was a numerical reduction in MACCE, non-procedural MI and repeat revascularisation in the IVUS group; however, after adjustment, none reached conventional statistical significance. There was, however, significantly reduced repeat revascularisation of the LMS (LMS TLR) in favour of post-PCI IVUS. There was no difference in survival between groups.
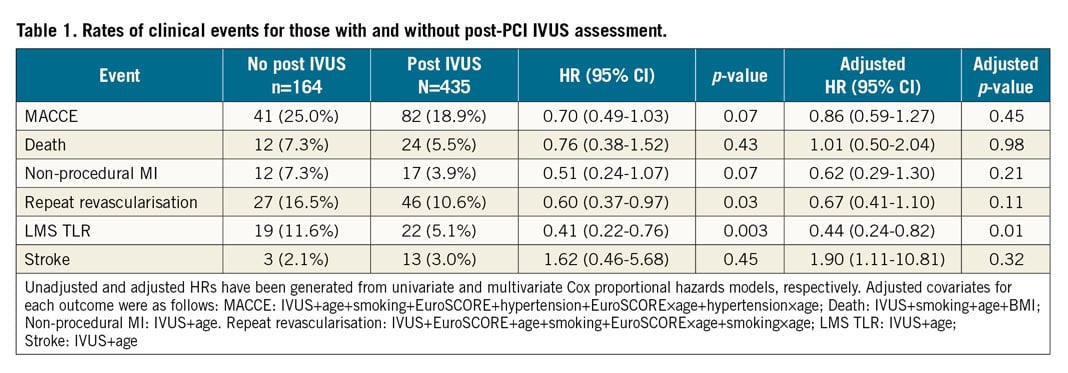
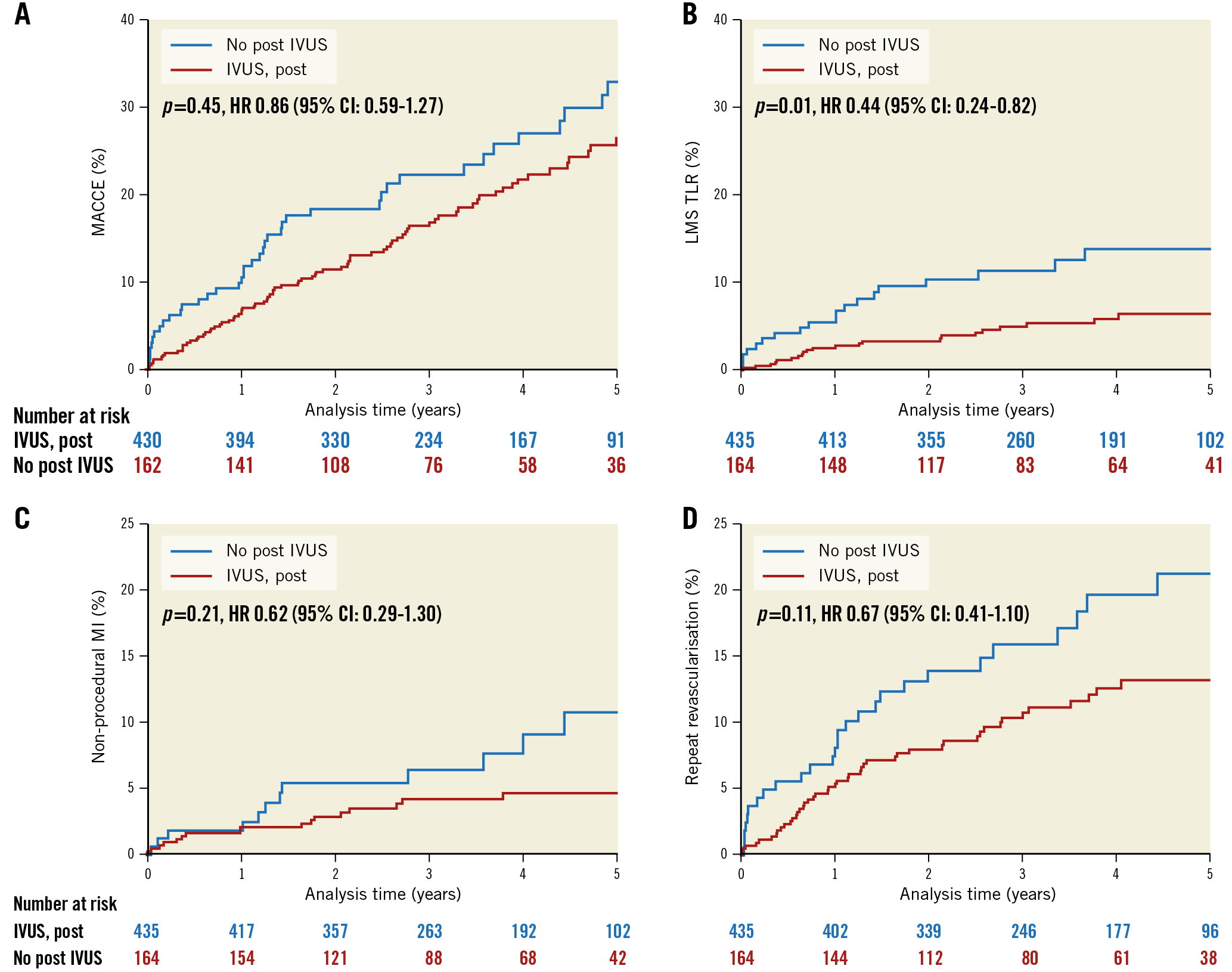
Figure 2. Kaplan-Meier curves showing five-year MACCE (A), LMS TLR (B), non-procedural MI (C) and repeat revascularisation (D), comparing those who underwent post-PCI IVUS assessment with those who did not.
Of the 435 patients who underwent post-PCI IVUS assessment, 243 had IVUS assessment both pre and post stenting. There were an additional 29 patients who only had IVUS assessment prior to stenting. There was no difference in MACCE or LMS TLR between those with only post-stenting IVUS assessment and those with both pre and post (Supplementary Figure 1, Supplementary Figure 2).
POST-PCI IVUS FINDINGS AND CLINICAL OUTCOMES
MALAPPOSITION
Of the 224 patients with post-PCI IVUS images analysed in the core lab, we identified malapposition in 12 (5.4%). We demonstrated no difference in MACCE at five years between those with and without malapposition: 33% (4) versus 21% (44), hazard ratio 1.47 (0.53-4.10), p=0.47. Individual components of the composite endpoint are detailed in Supplementary Table 3. None of the differences reached statistical significance.
PREVIOUSLY IDENTIFIED PREDICTORS OF ADVERSE EVENTS
Mean MSA measurements in the LMS, bifurcation core segment, ostial LAD and ostial LCx are detailed in Supplementary Table 4A, including number of failures to achieve an MSA in each segment in excess of those previously associated with adverse events6 (<8 mm2 in the left main stem, <7 mm2 in the bifurcation core segment, <6 mm2 in the ostial LAD and <5 mm2 in the LCx). Of the 224 patients analysed in the IVUS core lab, 19 (8.5%) failed to achieve an MSA above these values in ≥1 vessel segment. The rates of adverse events for those with at least one “failure to achieve MSA” compared with those without a failure are compared in Supplementary Table 4B. Numerically, event rates were higher in those in whom there was stent underexpansion in ≥1 vessel segment. However, there was no statistically significant difference in MACCE, death, non-procedural MI or repeat revascularisation between groups, although the numbers of events and failures to achieve MSA were both small.
IVUS AND CLINICAL PREDICTORS OF EVENTS
Of the 224 patients with post-PCI IVUS images analysed in the core lab, univariate predictors of MACCE were age in years, logistic EuroSCORE and hypertension. When incorporated into a multivariable proportional hazards model, only age (HR 1.03, 95% CI: 1.00-1.06, p=0.048) and hypertension (HR 2.23, 95% CI: 1.07-4.65, p=0.03) remained significant predictors (Supplementary Table 5).
Univariate predictors of LMS TLR included LMS MSA and LMS maximal residual plaque burden. Residual plaque burden was removed from the model due to collinearity with LMS MSA, leaving LMS MSA as the only significant multivariable predictor of LMS TLR (HR 0.75, 95% CI: 0.61-0.92, p=0.006) (Supplementary Table 6).
STENT EXPANSION IN THE LMS
The mean MSA in the LMS measured 12.5±3.0 mm2 with a mean maximal stented area of 15.6±3.8 mm2. The mean maximal stented LMS luminal diameter was 4.8±0.6 mm; 151 of 224 patients (67.4%) included in the substudy had a maximal stented LMS diameter of ≥4.5 mm. LMS stent brand and platform/diameter were known in 199/224 patients. Of those patients in whom the maximal LMS stented diameter was ≥4.5 mm (n=133) and stent brand and platform/diameter were known, the largest stent platform with the greatest overexpansion capacity (3.5/4.0 for BioMatrix Flex, 4.0 for PROMUS Element™ [Boston Scientific], >4.0 for TAXUS™ Liberte™ [Boston Scientific], 3.0/3.5/4.0 for Resolute Integrity® [Medtronic, Minneapolis, MN, USA], 3.5/4.0 for CYPHER® [Cordis, Cardinal Health, Milpitas, CA, USA]10) was used in 119/133 (89.5%). In the 14 cases where this was not the case, there was no malapposition found by post-procedural IVUS imaging and no association with MACCE.
To investigate further the relationship between stent expansion and events at follow-up, patients were split into tertiles according to the post-PCI LMS MSA. Details of the three tertile groups are shown in Supplementary Table 7. With respect to the IVUS characteristics, EEM area increased with each tertile and the residual plaque burden expressed as a percentage (EEM-MSA)/EEM) was higher in the lower tertiles. The frequency and extent of visible calcification on IVUS at the MSA site was higher in the lower tertiles.
Outcomes at 30 days are presented in Supplementary Table 8 and five-year follow-up with a median follow-up of four years is detailed in Table 2 and Figure 3. At 30 days, event rates were very low and there was no difference in outcomes between tertiles. At a median follow-up of four years, there was no significant difference in MACCE, death, MI or stent thrombosis between tertiles. However, there was a trend towards a difference in MACCE between the low and upper tertiles. There was a significant difference between the low and upper tertiles for repeat revascularisation, and specifically LMS TLR.
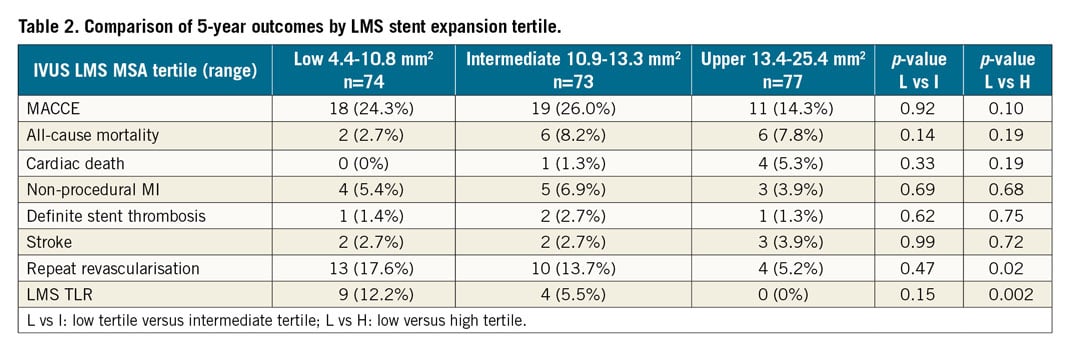
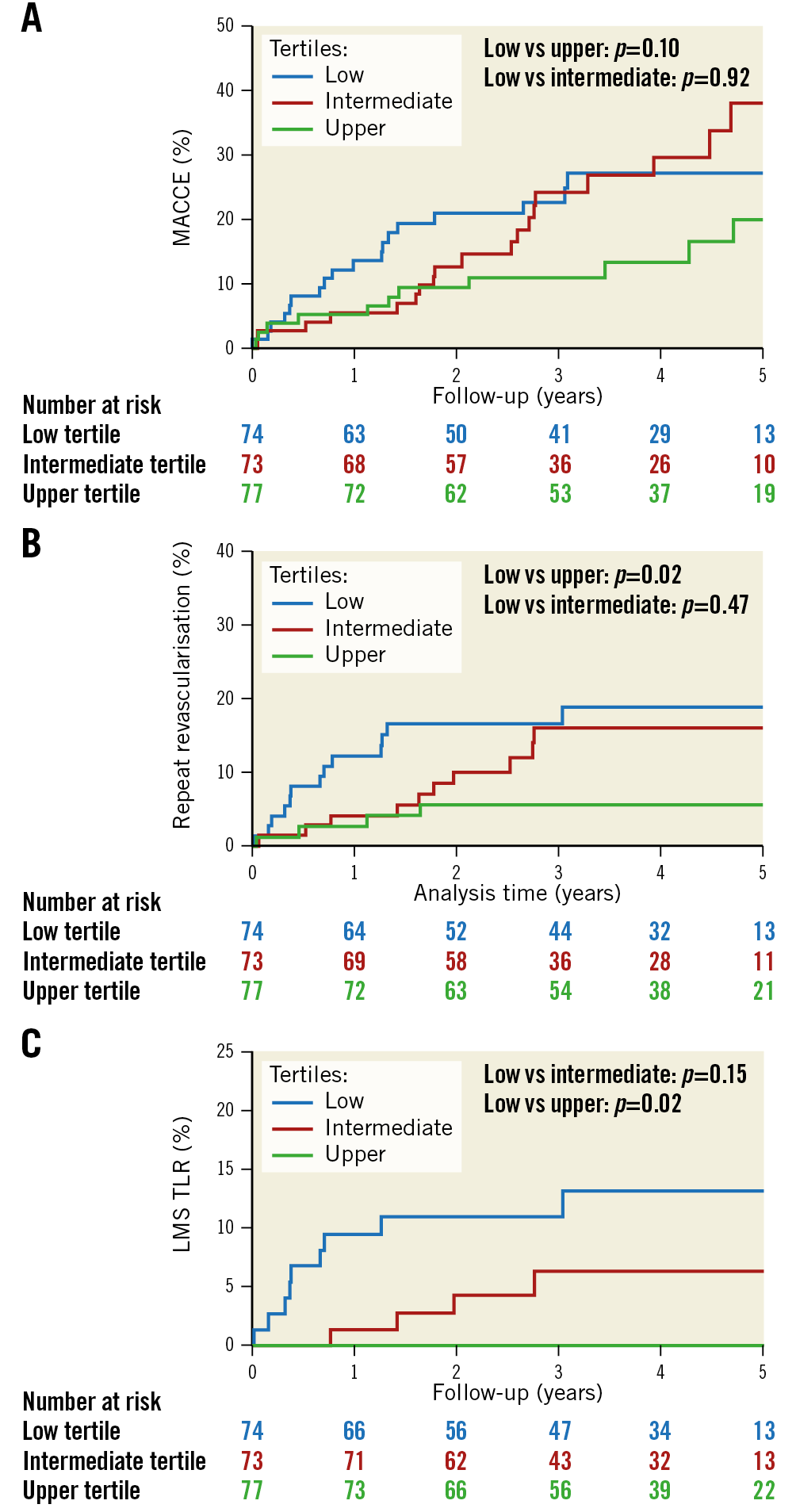
Figure 3. Kaplan-Meier curves showing five-year MACCE (A), repeat revascularisation (B) and LMS TLR (C) for low, intermediate and upper LMS MSA tertiles.
Discussion
The main findings of this NOBLE substudy were that 1) a final IVUS assessment was associated with a reduction in LMS TLR, 2) an association between better stent expansion and superior clinical outcomes was demonstrated, in particular for TLR at the site of IVUS assessment (the LMS), 3) inferior stent expansion in the LMS was associated with a greater extent of calcification in the LMS, and 4) in what is a sample of convenience of those in the NOBLE trial who underwent IVUS-guided PCI, overall stent expansion was good relative to other publications in the field6,11(Maehara A. IVUS-guided Left Main and Non-left Main Stenting in the EXCEL Trial: Lessons From the EXCEL IVUS Core Laboratory; presented at the 2016 Transcatheter Cardiovascular Therapeutics conference, Washington, DC, USA).
POST-PCI IVUS ASSESSMENT AND IMPROVED OUTCOMES
We have demonstrated an association between post-PCI IVUS examination and reduced LMS TLR. The data are not randomised, and we did not record whether a post-PCI IVUS resulted in further optimisation. Nevertheless, the finding of increased LMS TLR if we do not assess for optimal stent expansion and the same increased TLR if we do assess it, but accept a smaller MSA, provides a powerful message regarding the importance of optimising our PCI result in the LMS with intracoronary imaging. The findings are consistent with what we know about predictors of restenosis whether the PCI involves the LMS6 or not4,12. They also raise the question of whether we might have achieved better clinical outcomes in the PCI arm of the NOBLE trial if all operators had followed the recommendation of pre- and post-PCI IVUS assessment.
THE ASSOCIATION BETWEEN STENT EXPANSION AND CLINICAL OUTCOMES
Stent underexpansion has been shown to be associated with increased rates of restenosis and stent thrombosis13. We demonstrated an association between LMS MSA and repeat revascularisation, most notably repeat revascularisation at the site of underexpansion (LMS TLR). A lower MSA may reflect an anatomically smaller LMS rather than underexpansion, and indeed the lower LMS MSA tertiles do have correspondingly lower LMS EEM areas. However, the residual plaque burden expressed as a percentage (EEM-MSA)/EEM is higher in the lower tertiles, suggesting that these are not just smaller LMS, but that the stented segment was less well expanded. We did not identify a difference in overall MACCE or harder endpoints such as MI or stent thrombosis between tertiles. A similar analysis reported from the EXCEL trial (Maehara A. IVUS-guided Left Main and Non-left Main Stenting in the EXCEL Trial: Lessons From the EXCEL IVUS Core Laboratory; presented at the 2016 Transcatheter Cardiovascular Therapeutics conference, Washington, DC, USA) did show an excess in MACCE, and the individual endpoints of MI, stent thrombosis and all-cause death in the lowest LMS MSA tertile, but no difference in TLR.
We also demonstrated a greater frequency and extent of calcification at the site of the LMS MSA in the lower MSA tertiles (Supplementary Table 7). This is a possible explanation for poorer stent expansion. Calcification in itself has been associated with restenosis and stent thrombosis13, as well as stent underexpansion14. Once a calcified stenosis is stented, it can be more challenging to address the underexpansion than it would have been to modify the calcific segment prior to stenting15; post-PCI IVUS assessment might highlight the problem too late. A lower threshold for calcification modification techniques such as rotablation or intracoronary lithotripsy16 may be important in achieving adequate stent expansion and reducing LMS stent failure. Alternatively, if good stent expansion cannot be achieved, favouring CABG in patients with a heavily calcified LMS might be appropriate.
NOBLE IVUS RESULTS IN COMPARISON WITH OTHER STUDIES
In any trial involving PCI, it is always possible to question the quality of the PCI procedure. A simple metric, given the established association between adequate stent expansion and clinical outcomes13, is a comparison of LMS MSA. Mean LMS MSA in this substudy was 12.5 mm2, greater than the IVUS substudy taken from the EXCEL trial11, which measured <10 mm2. Similarly, compared with the study from which binary measurements of underexpansion in the LMS, bifurcation core segment, ostial LAD and ostial LCx associated with adverse events were derived6, underexpansion in this substudy was much less frequent at 8.5%, compared with 33.8%6. The most vulnerable anatomical site for underexpansion is the ostium of the LCx; the frequency of IVUS assessment in the LCx in this substudy was low. Nevertheless, the measurements of stent expansion presented here compare favourably with similar studies6,11. This could be explained by the patient population in NOBLE being from the United Kingdom or Scandinavia1 and therefore larger than in Korea6, or even the population included in the EXCEL trial2. However, the EEM measurements at the site of the LMS MSA presented here are very similar to those presented in the IVUS substudy of the EXCEL trial (Maehara A. IVUS-guided Left Main and Non-left Main Stenting in the EXCEL Trial: Lessons From the EXCEL IVUS Core Laboratory; presented at the 2016 Transcatheter Cardiovascular Therapeutics conference, Washington, DC, USA).
It is interesting that we have demonstrated an association between stent underexpansion and TLR, but not the harder endpoints of MACCE, death, MI and stent thrombosis, as have been described in the IVUS substudy of the EXCEL trial (Maehara A. IVUS-guided Left Main and Non-left Main Stenting in the EXCEL Trial: Lessons From the EXCEL IVUS Core Laboratory; presented at the 2016 Transcatheter Cardiovascular Therapeutics conference, Washington, DC, USA). It is difficult to explain this discrepancy, although our results are in keeping with the results of other studies6. A possible explanation might be that the XIENCE stent platform used in EXCEL when ≤3.0 mm has an upper overexpansion limit (ca. 4.4 mm diameter) which is lower than most other stent platforms including those used in NOBLE10. This might have resulted in a greater risk of malapposition and underexpansion in EXCEL cases if 3.0 mm XIENCE stents were brought back into the LMS.
Limitations
This study is limited by its small size and non-randomised nature. Interpretation of the association between stent malapposition and adverse events is limited by both the low rate of malapposition and the low event rate. In addition, not all segments were imaged in all patients (particularly the ostium of the circumflex artery), so the prevalence of stent underexpansion may have been higher than reported. Whilst our findings of an association between LMS stent expansion, post-PCI IVUS assessment and repeat revascularisation are interesting and consistent with other similar studies14 (Maehara A. IVUS-guided Left Main and Non-left Main Stenting in the EXCEL Trial: Lessons From the EXCEL IVUS Core Laboratory; presented at the 2016 Transcatheter Cardiovascular Therapeutics conference, Washington, DC, USA), they can only be considered hypothesis-generating. An assessment of the frequency with which post-PCI IVUS assessment resulted in further optimisation would have been valuable, but these data were not collected. Finally, this study does not provide a threshold LMS MSA above which we should strive to prevent TLR; the number of events is too small.
Conclusions
Undertaking post-LMS PCI IVUS assessment is not associated with reduced MACCE; however, it is associated with reduced TLR. If undertaken, LMS stent underexpansion is associated with both LMS calcification and increased TLR. Use of IVUS in addressing and preventing stent underexpansion in LMS PCI is likely to result in improved outcomes. Stent expansion in the NOBLE trial compares favourably with other studies of LMS PCI.
|
Impact on daily practice This substudy supports the suggestion that post-procedural intracoronary imaging and optimal stent expansion improve long-term durability of LMS PCI. In order to attain optimal stent expansion and reduce TLR, patient selection, adequate lesion preparation and stent optimisation guided by intracoronary imaging are all likely to be important. |
Funding
Biosensors provided an institutional research grant for the trial.
Conflict of interest statement
A. Erglis reports grants from Abbott Vascular, Boston Scientific, and HeartFlow, Inc., and personal fees from Biosensors, outside the submitted work. E. Christiansen reports grants from Biosensors, outside the submitted work. C. Hanratty reports personal fees from Boston Scientific, outside the submitted work. A. Ladwiniec reports personal fees from Boston Scientific, outside the submitted work. M. Spence reports grants and personal fees from Medtronic, personal fees from Edwards Lifesciences, and grants and personal fees from Boston Scientific, outside the submitted work. S. Walsh reports personal fees from Boston Scientific, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

