
Abstract
Interventional cardiology and coronary stent insertion have an increasing role in the optimal management of left main coronary artery (LMCA) stenosis. Assessing the extent of obstructive disease of the LMCA by angiography alone can be challenging. However, in contrast to the two-dimensional, shadow graphic nature of coronary angiography, intravascular ultrasound (IVUS) is an accurate tomographic technique for assessing both the coronary lumen and the vessel wall characteristics. Consequently, it is a particularly useful technique in imaging the LMCA before, during and after intervention. The European Bifurcation Club (EBC) recommends the use of IVUS during most LMCA interventions. The purpose of this consensus document is to review the available IVUS data on LMCA disease evaluation and treatment. It is a practical guide to show “how and when” to use the imaging modality. It is hoped that a standardisation of the practical approach to imaging may allow consolidation of learning and, ultimately, improve patient outcomes.
Abbreviations
AUC: area under the curve
CI: confidence interval
DS: diameter stenosis
FFR: fractional flow reserve
ISR: in-stent restenosis
IVUS: intravascular ultrasound
LAD: left anterior descending
LCx: left circumflex
LMCA: left main coronary artery
MACE: major adverse cardiac events
MLA: minimum lumen area
MLD: minimum lumen diameter
MI: myocardial infarction
MSA: minimal stent area
OR: odds ratio
RR: relative risk
SB: side branch
ST: stent thrombosis
TLR: target lesion revascularisation
Introduction
Atherosclerotic obstruction of the left main coronary artery (LMCA) is worthy of particular consideration compared to stenoses elsewhere in the coronary tree as it usually provides blood supply to >75% of the left ventricle; untreated patients with obstructive LMCA disease have a particularly poor prognosis. Recent trial data have highlighted the potential role of coronary stenting in the LMCA setting, particularly in patients with less complex LMCA disease and patients unsuitable for surgery1. LMCA disease is difficult to assess angiographically because of the possible lack of a proximal reference; atherosclerosis within the LMCA is usually diffuse, with frequent involvement of the bifurcation. Calcific disease is also common in the LMCA making lesions more difficult to dilate; consequently, optimal stent expansion is more challenging. Notably, acute complications occurring during LMCA intervention may have a rapid progression towards haemodynamic instability.
Even when using modern equipment, LMCA assessment by angiography can be challenging2,3. For example, in a series of 38 patients with an angiographically normal or nearly normal LMCA, 10 patients had intravascular ultrasound (IVUS) evidence of diffuse LMCA disease4.
In contrast to the two-dimensional, shadow graphic nature of coronary angiography, IVUS is an accurate, tomographic technique for assessing both the coronary lumen and wall characteristics; it has a higher tissue penetration compared to optical coherence tomography (OCT) which uses infrared light. Consequently, IVUS is a particularly useful technique in imaging the LMCA.
The European Bifurcation Club (EBC) recommends the use of IVUS during most LMCA interventions. The purpose of this consensus document is to review the available IVUS data on LMCA disease evaluation and treatment.
THE NORMAL LMCA, PATHOGENESIS, AND PLAQUE DISTRIBUTION
The sizing of the normal LMCA and its bifurcation into the LAD and LCx is predictable using fractal geometry and Murray’s Law5. IVUS dimensions for a normal (or minimally diseased) LMCA and the subtended LAD and LCx are remarkably consistent4,6,7 and have been confirmed in cross-sectional data from the PROSPECT study (Supplementary Table 1).
PROSPECT8 also suggests that, when atheroma develops, the LMCA undergoes positive remodelling in response to plaque deposition to preserve lumen dimensions, a process originally described by Glagov et al9. Conversely, an ostial stenosis may be a consequence of negative remodelling (sometimes without significant plaque) and can occur at all three segments – ostial LMCA, ostial LAD, and ostial LCx10-12. Shorter anatomic LMCAs tend to develop ostial narrowings while longer anatomic LMCAs tend to develop distal bifurcation stenoses10.
Careful IVUS imaging from both the LAD and the LCx back to the LMCA have demonstrated that bifurcation disease is rarely focal: in 140 patients and irrespective of angiographic Medina classification13, the carina and both sides of the flow divider were almost always disease-free. Continuous plaque from the LMCA into the proximal LAD was seen in 90% and from the LMCA into the LCx in 66%, with disease from the LMCA into both the LAD and LCx in 62% (Figure 1, Figure 2)14. Importantly, plaque localised to either the LAD or LCx ostium and not involving the distal LMCA was seen in only 9% of LAD and 17% of LCx arteries. In practice, a stent in the proximal LAD can move as much as 5.5 mm between systole and diastole15. This reality, together with recognition of typical atheroma distribution, explains why attempting to place a stent accurately at the true LAD ostium is usually unsuccessful and results in angiographic and clinical results which are commonly suboptimal.
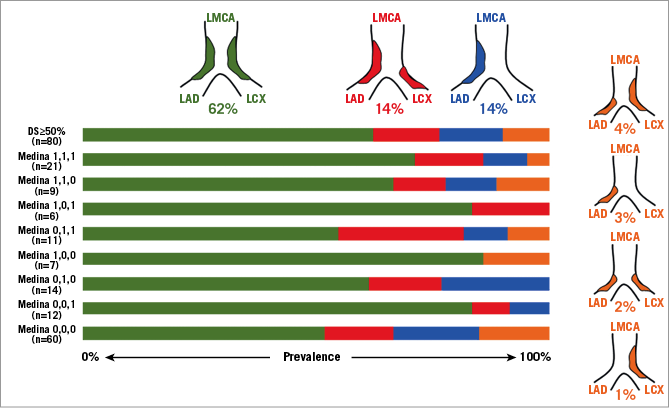
Figure 1. Irrespective of the Medina classification13, plaque in the distal LMCA is continuous into the ostium of the LAD and LCX in 62%. The other patterns are less common comprising only 10% of distal LMCA lesions. (Adapted with permission from Oviedo et al14).
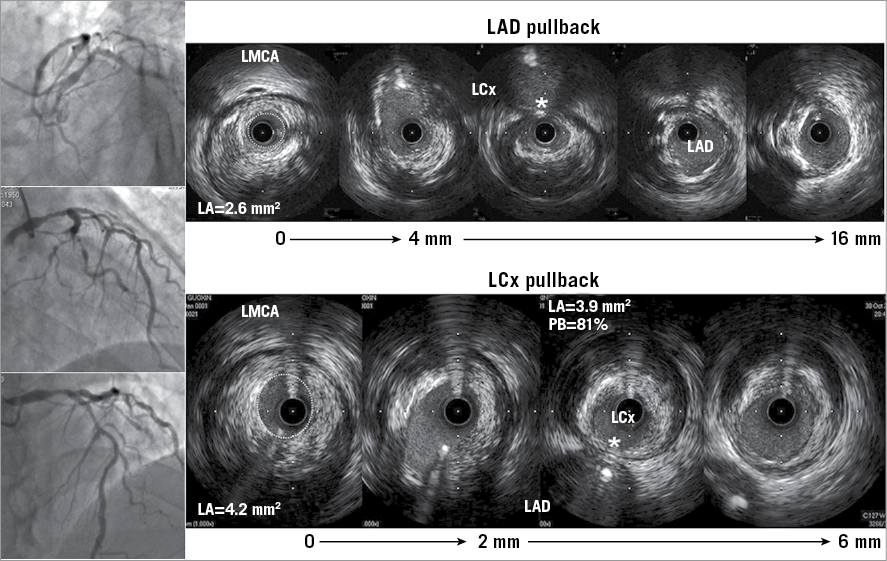
Figure 2. In this patient with a distal LMCA stenosis, the MLA in the LMCA measured during the LAD pullback was round and smaller (dotted line, 2.6 mm2) than the oval-shaped MLA when imaged during the LCx pullback (dotted line, 4.2 mm2) because of the sharp turn from the LCx into the LMCA creating an oblique IVUS image, an oval-shaped lumen, and a larger and oval-shaped artery compared to the LAD pullback. Despite the apparently normal-looking LCx on the angiogram (as well as in the LMCA to LAD pullback), the plaque burden at the ostium of the LCx measured 81%. In addition, and irrespective of the angiographic appearance, plaque at the ostium of the LAD and LCx is opposite the flow divider, and the carina (shown as a white asterisk in both pullbacks) is disease-free.
Examples of complete IVUS assessment are shown in Figure 2 and Figure 3.
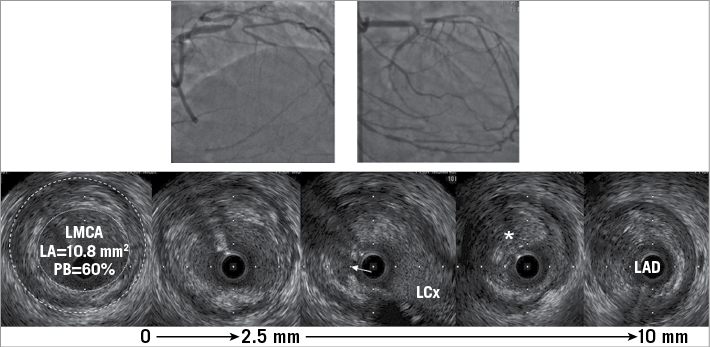
Figure 3. A patient who presented with a chronic total occlusion (CTO) of the LAD. IVUS imaging indicated that the cause was plaque rupture at the polygon of confluence (white arrow) with chronic thrombus at the LAD ostium (asterisk) and plaque in the distal LMCA (lumen: dotted white line and external elastic membrane dashed white line) where the plaque burden measured 60%.
Summary statements/recommendations
–Sizing of the normal LMCA is predictable using fractal geometry.
–LMCA bifurcation disease is rarely focal and almost always extends from the LMCA to the LAD (with variable involvement of the LCx), but the carina and both sides of the flow divider are commonly disease-free.
IVUS ASSESSMENT OF LMCA STENOSIS: INDICATION FOR TREATMENT
There have been six published IVUS studies assessing LMCA severity, including three with clinical follow-up7,16,17. These are reviewed in Supplementary Appendix 1 and Figure 4.
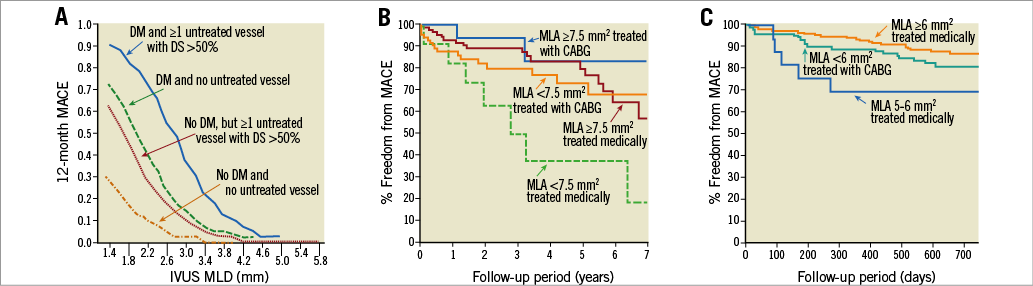
Figure 4. Three studies have related IVUS minimum lumen area (MLA) in the left main coronary artery to long-term clinical events. Abizaid et al (A, with permission16); Fassa et al (B) (adapted with permission7); and C) an update of the LITRO Registry17 in a presentation by de la Torre Hernandez at TCT2017.
IVUS ASSESSMENT OF THE LMCA: PRACTICAL GUIDE
Three considerations are important from a technical standpoint when evaluating LMCA lesion severity. First, when studying an ostial LMCA stenosis, it is important to disengage the guiding catheter to avoid confusing the guiding catheter with an ostial stenosis, and it is important to maintain a coaxial relationship between the IVUS catheter and the LMCA ostium. Secondly, it is not reliable to use IVUS imaging of either the LCx or the LAD to infer the status of the other vessel tangentially during pullback, as either ostial lumen dimension or plaque burden assessment can be misleading18. Third, a discrepancy between the minimum lumen area (MLA) in the LMCA when imaging from the LAD or LCx can be generated by the oblique plane of the IVUS beam when the transducer turns from the daughter vessel with the greatest angle into the LMCA. This oblique transducer position can create an artificially large MLA, but not one that is artificially small; therefore, the smallest MLA is the most accurate. An example is shown in Figure 2.
As part of procedure planning, IVUS can be used before stent implantation to assess the following:
1)Risk of side branch (SB) compromise. Lesions proximal or distal to the SB or ostial SB stenoses affect the risk of SB compromise after main vessel stenting. Patients who have a “vulnerable” carina – the eyebrow sign19) or significant calcium20 identified by IVUS longitudinal reconstruction – are at particular risk of adverse carina shift towards the LCx.
2)Stent length. When using automated pullback, proposed stent length can be measured to limit residual stenosis in adjoining segments (i.e., geographic miss).
3)Stent diameters. Segmental stent diameters can be based on proximal and distal reference size measurements.
4)Reference size and length measurements can be used to plan the size and length of the “proximal optimisation technique (POT) balloon” to ensure that it fits within the stent from carina to the proximal stent edge.
Summary statements/recommendations
– The EBC recommends IVUS guidance for patients undergoing LMCA intervention.
– Given the unique prognostic implications of LMCA disease, the EBC recommends using a threshold MLA cut-off of 6 mm2 to indicate an LMCA that should be treated with revascularisation in a European population.
– Disengage the guiding catheter prior to image acquisition and ideally image from both the LAD and LCx to the LMCA with at least one pullback to the aorta.
– The vessel with the angiographically least apparent disease should be imaged back to the LMCA as a minimum guide for bifurcation strategy.
EVIDENCE THAT USING IVUS GUIDANCE DURING LMCA INTERVENTION IMPROVES OUTCOMES
The EBC recommends IVUS in all elective LMCA cases especially when clinical practice is evolving and especially when procedural complications occur or there is uncertainty.
Single-centre data suggest that higher-volume operators get better outcomes when stenting patients with LMCA disease with less benefit from IVUS guidance, replicating some historical randomised trial data that did not clearly support IVUS imaging in every case21. However, Ye et al performed a meta-analysis of 10 studies indicating that IVUS guidance of LMCA stenting reduced the risk of all-cause mortality by 40% and cardiac death by 53% compared with conventional angiography-guided procedures22. In addition, IVUS guidance was associated with lower risks of TLR and definite or probable stent thrombosis but not MI or TVR. Among these included studies, one was a small randomised trial23; the others were either single-centre or multicentre registries24-28. Five were conference abstracts and only one study was later published as a manuscript28. This analysis was performed by the same group that assessed the impact of operator volume21.
These data were supported by a complex lesion meta-analysis performed by Fan et al29. In a propensity score-matched substudy including three of the four LMCA articles that were also included by Ye24-26 plus one additional study30, they also showed a significantly lower incidence of all-cause mortality, MI, ST, and MACE when LMCA intervention was informed by IVUS.
Most studies included in both meta-analyses had a significant number of distal LMCA lesions, but only the propensity score-matched IVUS-TRONCO-ICP looked at this subgroup specifically25. In the subgroup of distal lesions irrespective of treatment, IVUS guidance reduced the composite of death+MI+TLR from 19% to 11% (p=0.03), a difference that was magnified in distal LMCA lesions treated with two stents. Most recently, Andell et al (using registry data) have reported a reduced incidence of a combined primary endpoint of mortality, ST, and restenosis over a period of five years when LMCA intervention was IVUS-guided27.
SPECIFIC PROCEDURAL OPTIMISATION: ONE VERSUS TWO STENTS
A “provisional” approach is recommended by the EBC for bifurcation treatment, including treatment of the LMCA. A trial comparing this approach with a systematic two-stent strategy has been initiated by the EBC and is currently recruiting. The provisional approach when applied to the LMCA will involve wiring both the LAD and the LCx. Stenting towards the LAD will be the usual strategy with a stent sized according to the LAD diameter. However, occasionally when the LAD appears to be spared from disease on IVUS and the LCx is large and/or dominant, stenting to the LCx may be the initial strategy. The type of stent selected should allow post-dilation to a diameter suitable for the LMCA – commonly >5 mm. POT should precede rewiring of the SB; rewiring through a distal strut is optimal.
After stent implantation, IVUS can be used to rule out residual edge stenosis or edge dissection (geographic miss), evaluate stent expansion and apposition, rule out accidental abluminal rewiring, assess complications, and in some cases verify guidewire position after SB recrossing. When a second stent must be implanted, kissing balloon inflation with two balloons is mandatory. A final POT is recommended. IVUS can then be used to evaluate the SB ostium, assess stent expansion (particularly important at the LCx ostium) and apposition, and rule out longitudinal compression.
Kang et al attempted to inform on the likelihood of needing a second stent after initial stenting to the LAD across the LCx. They reported 23 LMCA bifurcation lesions with a preprocedural angiographic DS <50% at the LCx ostium evaluated using pre- and post-stenting IVUS in both the LAD and the LCx31. The MLA within the LCx ostium significantly decreased from 5.4 mm2 to 4.0 mm2 post stenting (p<0.001). This was associated with a significant decrease in vessel area and increase in vessel eccentricity, but no increase in plaque mass (although there was an increase in plaque burden related to the decrease in vessel area), indicating that the main mechanism of lumen area loss at the LCx ostium was carina shift during crossover stenting. Importantly, in 43 patients with a pre-PCI ostial LCx angiographic DS <50%, a post-stent crossover FFR <0.80 in the LCx was predicted by a preprocedural ostial LCx MLA <3.7 mm2 or a preprocedural plaque burden of >56%32. Sato et al reported on patients who underwent single stent crossover and IVUS pullback of only the LAD to LMCA20. Post-stenting narrowing at the LCx ostium (defined as a >50% angiographic DS) occurred in 27 patients (35%) who more frequently had IVUS-identified calcified plaque at the culprit with a greater arc of calcium. On multivariable analysis, a calcium arc >60° was an independent predictor of LCx ostium narrowing. In two-stent procedures, whether or not pre-planned, IVUS evaluation after each rewiring may be useful.
An example of how to use pre-intervention IVUS for treatment planning is shown in Figure 5.
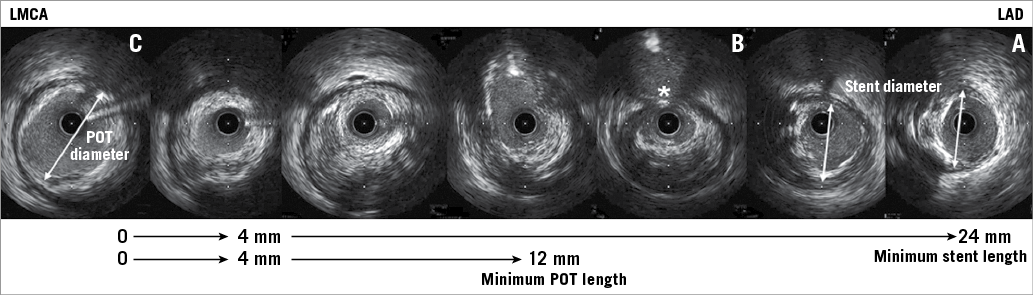
Figure 5. IVUS imaging to select the stent and POT balloon size and length using the case shown in Figure 2. A) The distal landing zone in the LAD. B) The carina (white asterisk). C) The proximal landing zone in the LMCA. Stent size should be 3.5 mm in diameter (based on the LAD mid wall measurements, double-headed arrow in panel A as well as the nearby cross-section) and at least 24 mm in length (to cover LMCA disease [C]). The POT balloon should be 5 mm in diameter (based on the LMCA measurements, double-headed arrow in panel C) and at least 12 mm in length.
Summary statements/ recommendations
– A “provisional” approach is the default treatment strategy for the LMCA.
– Sizing of the stent should be based on the diameter of the branch vessel. The selected stent type should allow POT as the final dilation of all LMCA procedures (POT or re-POT).
– IVUS can be used before stenting to inform the operator about the likelihood of needing a two-stent technique.
– POT and kissing balloon post-dilation are mandatory in two-stent bifurcation procedures.
SPECIFIC PROCEDURAL OPTIMISATION: RISK OF RESTENOSIS/THROMBOSIS
These data are discussed in Supplementary Appendix 2 and summarised above and in Figure 6.
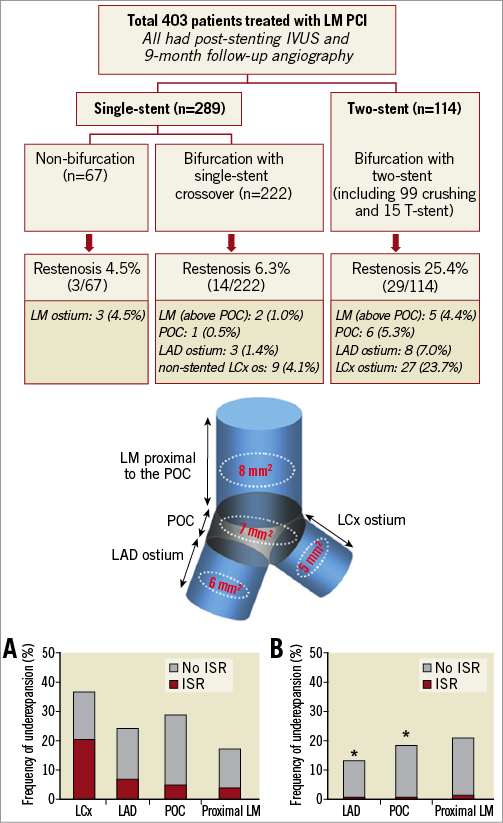
Figure 6. A comprehensive analysis of LMCA lesions performed by Kang et al. A) Two stents; B) one stent. They related the frequency of angiographic restenosis to underexpansion at one of four sites (cartoon): LMCA, polygon of confluence (POC), ostial LAD, and ostial LCX (adapted with permission from Kang et al33).
Conclusions
The EBC believes that IVUS guidance is useful at each step of an LMCA interventional procedure: (1) to decide whether or not revascularisation is necessary, (2) to decide whether a one-stent crossover technique (the default strategy) is sufficient or whether a two-stent technique may be more appropriate, (3) to size the stent (diameter and length) and select the optimum landing zones, and (4) to optimise the final result (expansion, apposition, and geographic miss). While randomised trials are limited, data suggest that IVUS guidance is superior to angiographic guidance in terms of death, MI, TLR, ISR, and ST.
| Impact on daily practice Treatment of the left main with stents is an increasing part of our interventional practice. Use of IVUS within the left main has a strong evidence base but there is limited practical guidance about how to use it. This practical guideline based on principles from the European Bifurcation Club provides important clinical information for interventional cardiologists and provides momentum towards changes in clinical practice that might improve outcomes. |
Conflict of interest statement
G. Mintz has received honoraria from Boston Scientific, Volcano, and Infraredx. The CRF receives grant/fellowship support from Boston Scientific and Volcano. A. Banning has received honoraria from Boston Scientific, Volcano and partial funding from NIHR Oxford BRC. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. IVUS assessment of LMCA stenosis: indication for treatment.
Supplementary Appendix 2. Specific procedural optimisation: risk of restenosis/thrombosis.
Supplementary Table 1. Measurements of a normal or minimally diseased LMCA and the corresponding proximal LAD and LCx.
To read the full content of this article, please download the PDF.

