Introduction
The following article, in the EuroIntervention Tools & Techniques series, is a contemporary overview of the established and emerging diagnostic tools for left main stem (LMS) intervention for the clinician. The paper highlights key salient messages in the management of LMS disease, ranging from risk stratification, in conjunction with the SYNTAX trial pioneered “Heart Team” approach, to principles of intravascular ultrasound (IVUS), optical coherence tomography (OCT) and physiological assessments of lesions – either invasively (pressure wire assessment) or non-invasively (Heartflow™ technology [HeartFlow Inc., Redwood City, CA, USA] applied to computed tomography angiography) – and how this influences distal LMS bifurcation management and even the interpretation of the SYNTAX score. Correlation of intravascular imaging findings with IVUS grey scale, IVUS virtual histology (IVUS-VH) and computed tomography angiogram findings are also discussed. Furthermore, new and emerging intravascular imaging techniques of vulnerable plaque and issues pertaining to “plaque-sealing” are discussed in the context of the LMS. An abridged version is detailed below – the full unabridged version is available online at www.pcronline.com/eurointervention.
Risk stratification
The SYNTAX trial pioneered Heart Team approach in the management of LMS disease has recently been incorporated as a class I recommendation in the latest myocardial revascularisation guidelines.1 Risk stratification is a key component within this process.
The important findings from the clinician’s perspective for the LMS population within the SYNTAX trial include:
1. The LMS population of the SYNTAX trial involved a population of patients with isolated LMS disease, or associated with one, two or three vessel disease (3VD).2,3
2. A low-intermediate SYNTAX score (i.e., SYNTAX score <33) in the LMS population was associated with comparable surgical and percutaneous clinical outcomes (Death & MACCE) at three years;2,4 and is currently being investigated in the on-going EXCEL randomised trial.
3. A high SYNTAX score (i.e., SYNTAX score ≥33) (Figure 1) and a high EuroSCORE are both able to individually identify a high risk population within the LMS PCI population at three years.5,6

Figure 1. Outcomes by tertiles of risk of the SYNTAX Score alone within the randomised LMS PCI population. Kaplan Meier curves for cumulative rates of Death (left) and MACCE (major adverse cardiovascuar and cerebrovascular events) (right) at 3-year follow-up stratified to tertiles of the SYNTAX score (event rate ±1.5 SE). A high SYNTAX score was able to identify a high-risk population within the LMS PCI population for Death and MACCE at three years.33
4. The combination of clinical variables with the SYNTAX score, such as with the EuroSCORE, potentially improves the predictive ability of the SYNTAX score.7,9
5. The outcomes of the LMS PCI population appear to predominantly affected by the increasing prevalence of 3VD, and its association with clinical co-morbidities and anatomical complexities, such as the presence of multiple bifurcations/total occlusions/distal LMS bifurcation disease.8,9
Functional SYNTAX Score
The Fractional Flow Reserve (FFR) versus Angiography for Guiding PCI in Patients with Multivessel Evaluation study (FAME) determined the potential prognostic impact of PCI guided by FFR measurements to determine the functional significance of an individual coronary lesion before intervention.10,11 The newly dubbed “functional SYNTAX score” essentially incorporates FFR measurements in the SYNTAX score calculation, and was recently shown to potentially improve the risk stratification of patients, by re-classifying higher-risk groups into lower-risk categories without any adverse sequelae, in terms of MACE and death or MI at one year.12 It should however be emphasised that no patients with LMS were involved in this study and prospective validation of the functional SYNTAX score in LMS (and multivessel disease) are required.
Non-invasive calculation of the functional SYNTAX score have recently been proposed (Figure 2) and allows for the simultaneous assessment of anatomy and measurement of the haemodynamic significance of lesions, to permit the non-invasive computation of FFR, utilising computational fluid dynamic techniques applied to the coronary CT angiography. Preliminary validation data has proven promising,13 larger studies are currently underway.
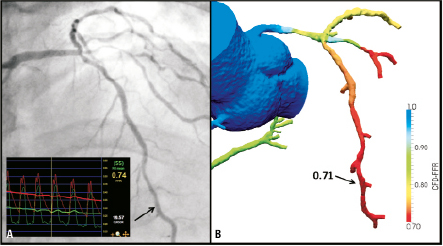
Figure 2. The principle of non-invasive FFR measurement utilising CT coronary angiography and HeartFlow™ technology. 2D coronary angiography (left image, A) and CT coronary angiography (B) demonstrated moderate distal LMS/ostial LAD disease. Subsequent FFR measurements were both suggestive of haemodynamically significant disease on invasive (inset left image, A) and non-invasive (right image, B) FFR measurements; a good correlation between both FFR measurements were also evident (FFR 0.74, 0.71 respectively). Courtesy of Dr. Bon Kwon Koo, Seoul National University, Republic of Korea and Dr. John H. Stevens, HeartFlow Inc., Redwood City, CA, USA
IVUS and FFR guided LMS intervention: anatomical vs. functional approach
A “simpler is better” approach,14 utilising the provisional T-stenting technique to LMS PCI, with IVUS/FFR guidance, is discussed below; further details, and the use of other intravascular techniques, including MSCT, as well as OCT are discussed in detail in the online version.
Ultrasound guidance LMS intervention
The potential advantages of IVUS guided LMS interventions are their characterisation of plaque morphology and plaque distribution which can guide us in lesion preparation and the choice of stent technique, assist the stenting itself (using the media to media distance); and finally provide an assessment of stent apposition as well as expansion, side branch (SB) compromise, and the presence of coronary dissections. As an approximate rule of thumb, an IVUS-derived minimal luminal area (MLA) of <6.0 mm2 post stent deployment has been associated with optimal stent expansion and apposition, as previously determined by its strong correlation with the haemodynamic significance assessed by FFR;15 this in turn has been associated with a reduced incidence of revascularisation, stent thrombosis and possibly survival in non-randomised registry data.16-18 Randomised controlled trials are, however, still lacking.
FFR guidance LMS intervention
As FFR is a physiological parameter, reflecting both the severity of epicardial stenosis and the amount of myocardium supplied, the same cut-off point can be applied for both the main branch (MB) and SB of a coronary bifurcation.19 Importantly, at up to five years follow-up, comparable mortality rates were reported in patients with LMS disease with FFR ≥0.80 treated medically (with other lesions treated with PCI if needed), and other patients with LMS stenosis and FFR <0.80 treated with CABG.20 The use of FFR in assessing the significance of LMS lesions should be performed with certain principles in mind as discussed in Figure 3.
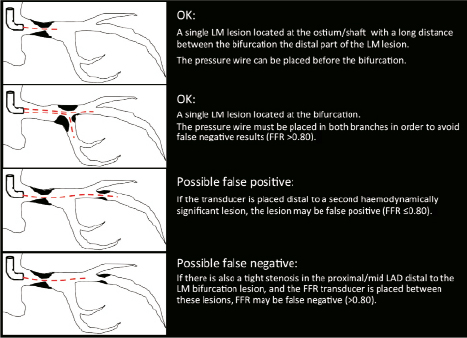
Figure 3. The principles of performing pressure wire assessment of LMS disease, involving positioning of the pressure wire transducer. Further intravascular imaging may be required in the lower two scenarios, such as with IVUS as previously described.
Angiographic appearances of the SB ostium after MB stenting have been demonstrated to be unreliable, with only a quarter (27%) of cases with a residual angiographic narrowing of ≥75% in the SB being found to have a functionally significant narrowing in pressure wire studies;21 furthermore discrepancies between angiographic percentage diameter stenosis and FFR in jailed LCx lesions after LMS-to-LAD cross-over stenting have recently been reported.22 Two cases demonstrating these issues are illustrated (Figures 4 and 5), with the mechanism to explain this concept illustrated with 3-dimensional frequency domain OCT imaging (Figure 6).23
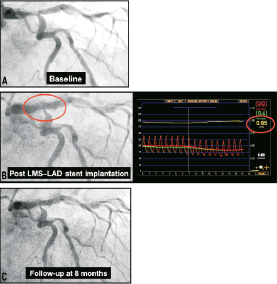
Figure 4. Scenario 1: Provisional T-stenting approach of cross-over LMS-LAD stent implantation. Baseline image (A), followed by cross-over LMS-LAD stenting, this lead to angiographic pinching of the LCx ostium, which looked angiographically significant (left image, B); functional assessment with FFR however suggested no haemodynamic compromise (FFR: 0.95) (right image, B) – no further intervention was carried out. At 8-month follow-up, the patient remained symptom free; coronary angiogram demonstrated no interval change in the angiographic appearances of the LCx ostium (C). Courtesy of Dr. Chang Wook Nam, Kyeimyung University Hospital, South Korea.
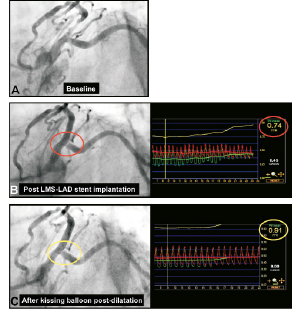
Figure 5. Scenario 2: Provisional T-stenting approach of cross-over LMS-LAD stent implantation. Baseline images (A), followed by cross-over LMS-LAD stent implantation, lead to angiographic pinching of the LCx ostium which looked angiographically significant (left image, B); in this case functional assessment with FFR suggested haemodynamic compromise (FFR: 0.74) (right image, B). Subsequent KBPD, lead to an improved angiographic appearances of the LCx ostium (left image, C) and the near normalisation of the FFR in the LCx (0.91) (right image, C). Courtesy of Dr. Chang Wook Nam, Kyeimyung University Hospital, South Korea
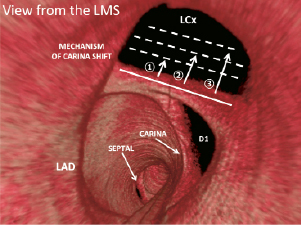
Figure 6. Three-dimensional frequency-domain OCT of the distal left main stem illustrating the mechanism of carina shift if LMS-LAD cross-over stenting was to be undertaken. Varying degrees of carina shift (broken lines, numbered from 1 to 3) would lead to pinching of the LCX ostium, or even closure if carina shift was complete. Kissing balloon post-dilatation would potentially reverse the carina shift, and if performed with a large enough angioplasty balloon, would potentially reverse the pinching and restore any haemodynamic compromise, as illustrated in Figure 5. It may be hypothesised that a “functional” restoration of flow to the LCx ostium, may be at the expense of a reduced MLA and risk of restenosis due to the the “bigger is better” paradigm.34 Potential compromise of the first diagonal (D1) would also be a risk with cross-over LMS to LAD stenting in this case example (not illustrated). LMS: left main stem; LAD: left anterior descending artery; LCx: left circumflex; D1: first diagonal. Reproduced and adapted from Farooq et al23.
OCT and IVUS-virtual histology for the assessment of vulnerable plaques
The use of intravascular imaging modalities to identify vulnerable plaque and lipid rich regions are innovative areas of on-going research, in potentially guiding stent placement and “plaque sealing” or the “shielding” of vulnerable plaque. As of present, despite case reports, and pilot trials suggesting a possible benefit,24-26 the use of intravascular imaging modalities to adopt such an approach remains hypothetical and probably should be avoided.
Within the LMS, Valgimigli et al have shown that plaque composition – in particular volume of necrotic core, as assessed by IVUS-VH – favouring propensity to vulnerability, are more commonly located in the distal LMS (especially if a long LMS is evident as illustrated in Figure 7) and proximal coronary vasculature; this may explain the greater likelihood for plaque erosion or rupture to occur in these territories, as previously reported in post mortem studies.27-30 The most plausible theory to explain the apparent paradox between the distal LMS and proximal coronary vasculature are the well-established roles shear stress modulation has at the coronary bifurcations, – distally in the LMS and more proximally in the coronary vasculature due to these locations being the site of the major coronary bifurcations – leading to pockets of low oscillatory shear stress, with the subsequent risk of unfavourable plaque progression, plaque vulnerability and rupture. Importantly, the severity of the coronary stenosis has not emerged as a criterion for plaque vulnerability, thereby diminishing the value of both angiography and FFR measurements in the detection of vulnerable lesions that may be responsible for future cardiac events.31
How should the presence of vulnerable plaques –as assessed by intracoronary imaging (Figure 7)– impact on our treatment strategy? PROSPECT32 is to date the only large scale clinical trial that investigated the prognostic value of IVUS-VH derived plaque parameters, and demonstrated the presence of IVUS-VH derived TCFAs to be one of the predictors (HR 3.31, 95% CI 1.77-6.36, p <0.001) for the occurrence of major cardiovascular events at three-year follow-up. Expressed in terms of absolute numbers: of 51 non-culprit lesion related recurrent events occurring in the imaged segments, 26 (51%) occurred at sites with TCFAs. Yet, considering that a total of 595 TCFAs were identified in the total PROSPECT study population, the lack of specificity precludes us to derive any clinical recommendations on how to proceed with vulnerable plaques. It should also be emphasised that the results of PROSPECT are less applicable to the LMS as the LMS was not investigated. Furthermore, the relatively low event rates in the PROSPECT study, should remind us of the paramount importance of secondary prevention, namely the induction and surveillance of state-of-the-art cardiovascular medication and secondary prevention measures.

Figure 7. Plaque rupture in the distal LMS seen on 2D and 3D OCT reconstructions (A). Matched OCT (left, B) and IVUS-VH (middle, B) and fusion imaging (right, B) cross-sections, depicting a vulnerable plaque (thin cap fibroatheroma - TCFA) in a non-culprit vessel of a patient presenting for primary PCI. Detailed description of the images are available on the online version. Fusion images courtesy of Dr Jung Ho Heo, ThoraxCenter, Erasmus MC, Rotterdam, The Netherlands, and Dr. Lorenz Räber & Prof. Stephan Windecker, Bern University Hospital, Bern, Switzerland, NCT00962416.
Conflict of interest statement
The authors have declared no conflict of interest.
Acknowldegements
V. Farrooq thanks the Dickinson Trust Travelling Scholarship, Manchester Royal Infirmary, Manchester, England, UK. L. Raber is the recipient of a research fellowship (SPUM) funded by the Swiss National Science Foundation (Grant 33CM30- 124112).

