Abstract
Background: Intravascular ultrasound (IVUS)-guided PCI improves the prognosis of left main stem (LMS) PCI and is currently recommended by international guidelines. Although OCT resolution is greater than that of IVUS, this tool is not yet recommended in LMS angioplasty due to the absence of data.
Aims: This pilot study aimed to analyse the feasibility, safety and impact of OCT-guided LMS PCI.
Methods: This prospective, multicentre trial investigated whether patients might benefit from OCT-guided PCI for mid/distal LMS according to a pre-specified protocol. The primary endpoint was procedural success defined as follows: residual angiographic stenosis <50% + TIMI 3 flow in all branches + adequate OCT stent expansion (LEMON criteria).
Results: Seventy patients were included in the final analysis (median age: 72 [64-81] years, 73% male). The OCT pre-specified protocol was applied in all patients. The primary endpoint was achieved in 86% of subjects. Adequate stent expansion was observed in 86%, significant edge dissection in 30% and residual significant strut malapposition in 24% of the cases. OCT guidance modified the operators’ strategy in 26% of the patients. The rate of one-year survival free from major adverse clinical events was 98.6% (97.2-100).
Conclusions: This pilot study is the first to report the feasibility and performance of OCT-guided LMS PCI according to a pre-specified protocol.
Introduction
Percutaneous coronary intervention (PCI) represents a valid alternative for the treatment of left main stem (LMS) lesions in selected patients1 and is currently considered by the European guidelines in patients with low or intermediate SYNTAX score2. These interventions require accurate analysis of lesion anatomy, adequate stenting strategy and optimal assessment of the results3.
The guidance and optimisation of LMS PCI by intravascular ultrasound (IVUS) can improve the overall procedure quality by allowing more precise device selection and identification of early stenting pitfalls that favour adverse clinical events4,5,6,7. Optical coherence tomography (OCT) is considered as non-applicable for coronary artery ostia and might be limited in case of a large vessel; however, the technique outmatches IVUS in identification of thrombus, coronary dissection and incomplete stent apposition due to its better spatial resolution5. Hence, mid and distal LMS can be adequately studied by this imaging modality with comparable, if not superior, results to IVUS8,9. Moreover, online three-dimensional (3D) reconstruction of OCT images clarifies the device configuration within coronary bifurcations and precisely identifies the guidewire recrossing point into the stent-jailed side branch (SB)10. However, there is a lack of available data and consensus regarding the use of OCT guidance for LMS PCI9,11.
In the current study, we aimed to assess the feasibility, performance and safety of a standardised OCT-guided protocol for completion and optimisation of LMS PCI.
Material and methods
STUDY DESIGN AND INCLUSION/EXCLUSION CRITERIA
The LEMON study (LEft Main Oct-guided iNterventions) was a prospective, multicentre, open-label, interventional, non-randomised trial that investigated OCT guidance for LMS PCI in 10 French interventional cardiology centres. The aim of the study was to assess the applicability of a predefined standardised protocol. Inclusion criteria were: 1) age >18 years and informed consent; 2) stable or non-stable mid/distal LMS lesion (Medina classification: 1,0,0, 1,1,0 or 1,0,1 ) requiring PCI with a one- or two-stent strategy or stable or non-stable ostial left anterior descending artery (LAD) and/or circumflex artery (Cx) lesion (Medina classification 0,1,0 or 0,0,1) requiring PCI with involvement of the distal LMS; and 3) a SYNTAX angiographic score <23.
The exclusion criteria included any of the following: ostial LMS lesion; acute ST-elevation myocardial infarction (STEMI); cardiogenic shock; severe chronic renal failure (Cr Cl <30 ml/min/m²); anticipated technical contraindication to OCT (highly calcified lesions, severe proximal tortuosity); contraindication to drug-eluting stent implantation.
The research protocol was approved by the CHU Kremlin-Bicêtre ethics committee and the participants gave written informed consent. The study is registered at ClinicalTrials.gov (identifier: NCT04248777).
THE LEMON STANDARDISED PCI PROTOCOL
The LMS PCI strategy was guided by three pre-specified OCT runs (Figure 1, Figure 2A).
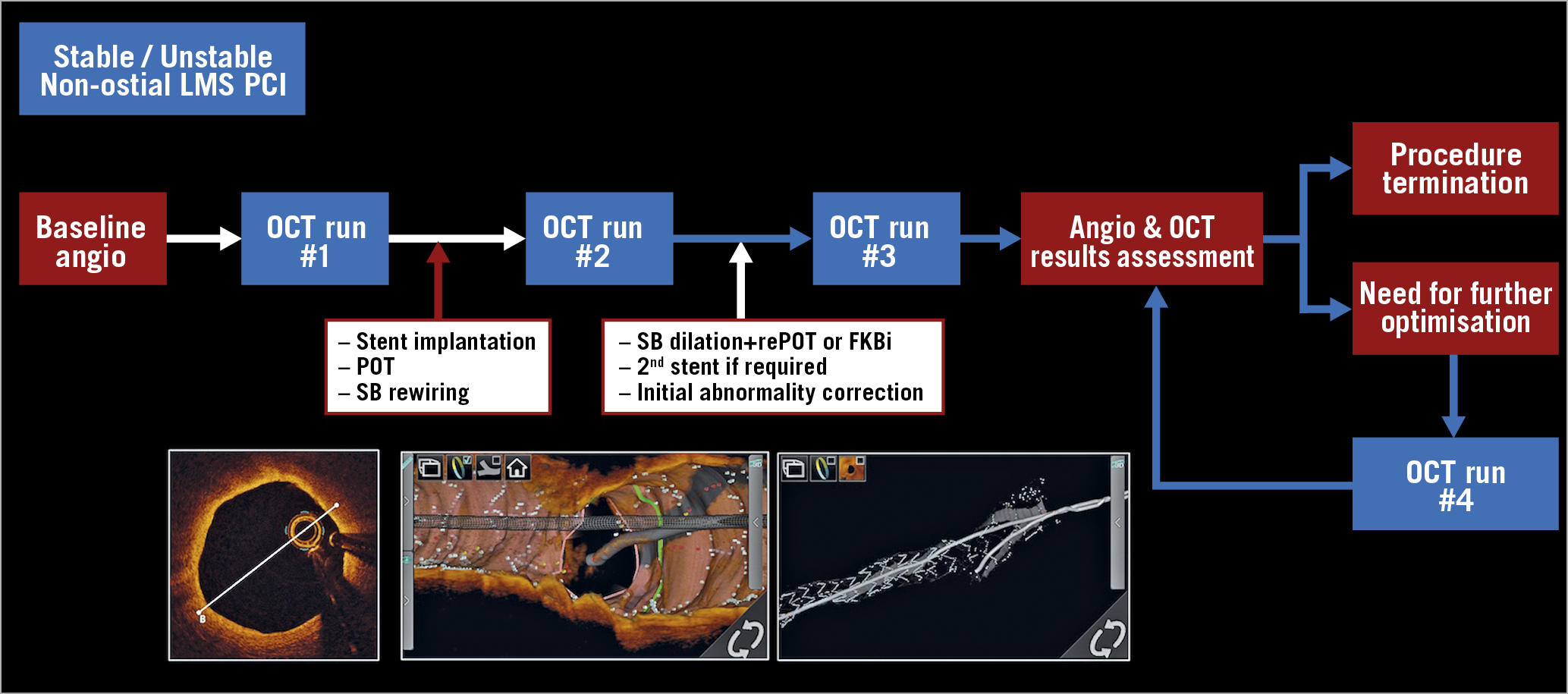
Figure 1. Global overview of the LEMON protocol.
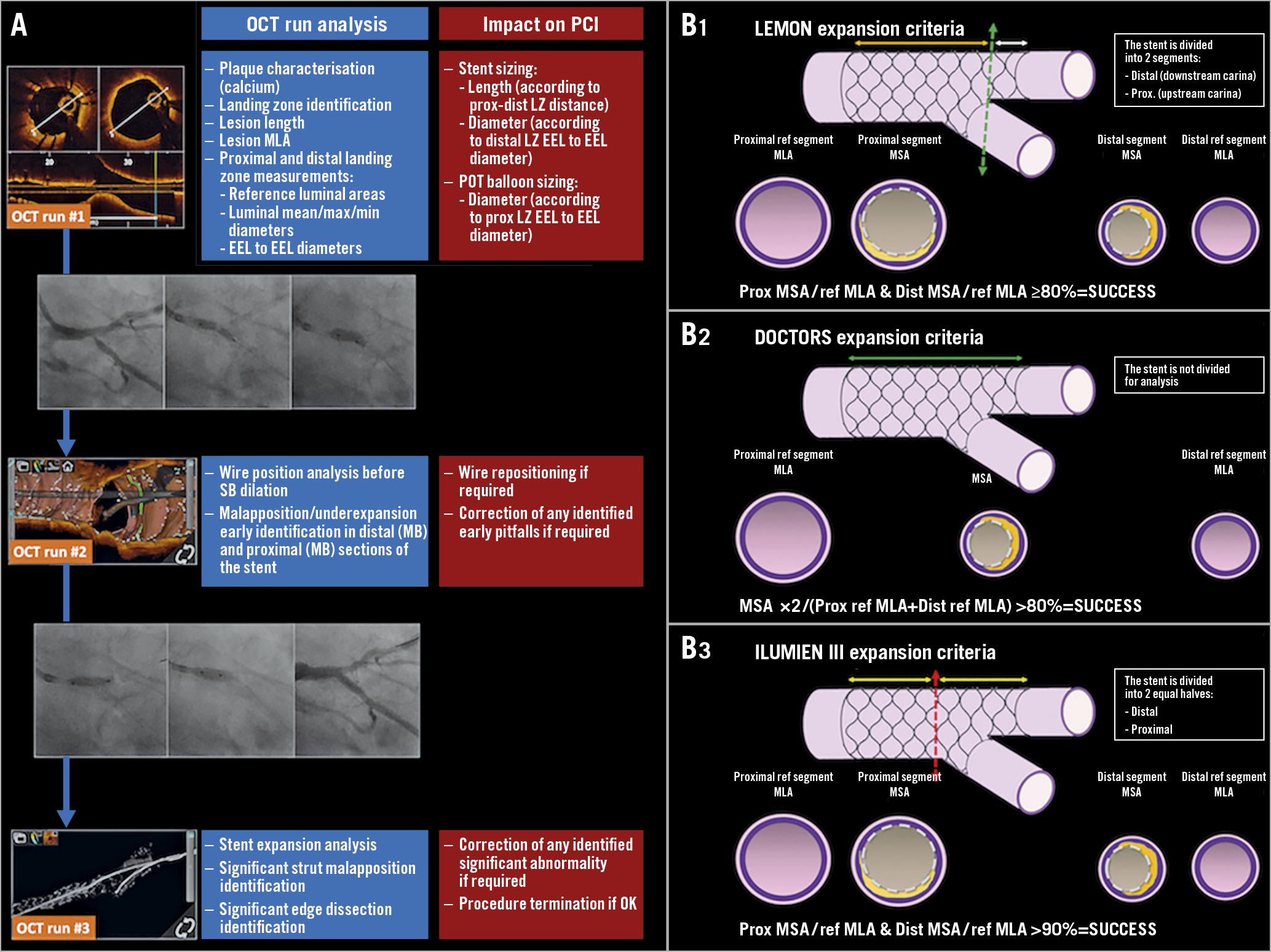
Figure 2. OCT guidance in LMS PCI in the LEMON trial. A) Roles of the pre-specified OCT runs on the decision-making process during LMS PCI. B1) - B3) Comparison of the calculation of the LEMON, DOCTORS and ILUMIEN III criteria for stent expansion evaluation in bifurcated lesions.
– Run #1 was performed from the main vessel towards the LMS before any stent implantation to analyse plaque characteristics, identify proximal and distal landing zones (LZ), measure lesion length, reference segment dimensions and determine stent dimensions and the proximal optimisation technique (POT) balloon diameter12. SB OCT analysis was left to the discretion of the operator.
– Run #2 was performed after the stent was implanted, POT was applied and the SB was rewired. It aimed to assess an adequate guidewire recrossing point into the stent-jailed SB.
– Run #3 was performed after PCI optimisation to analyse stent expansion and identify significant strut malapposition and edge dissection. In case of additional stent optimisation, a supplementary OCT run was acquired to assess the final result. The detailed protocol is provided in Supplementary Appendix 1.
ANGIOGRAPHIC ANALYSIS
The LMS bifurcation was analysed according to the latest European Bifurcation Club (EBC) consensus document, including grading using the Medina classification and segmentation in the proximal main branch (PMB/LMS), SB and distal main branch (DMB)3. The PCI strategy (one or two stents) was left to the operators’ discretion and involved implantation of XIENCE Sierra™ everolimus-eluting stents (Abbott Vascular, Santa Clara, CA, USA).
The angiography data were centrally reviewed by two independent operators. The stenosis degree was calculated by a dedicated quantitative coronary angiography (QCA) software (Centricity CA1000; GE Healthcare, Buc, France).
OCT ACQUISITION AND ANALYSIS
The OCT analysis methodology is provided in Supplementary Appendix 2.
STENT EXPANSION
Minimal stent area (MSA) was assessed in the different stent sections (MB and DMB) and stent expansion was analysed by three different methods: LEMON, DOCTORS and ILUMIEN III12,13. The LEMON criteria have been proposed to overcome the inherent difficulties in stent expansion assessment within bifurcated lesions (where proximal and distal reference segments display discrepant areas)14. The stent was split into two sections, using the carina as the cut-off point. The MSA was then measured in the proximal (upstream carina) and distal (downstream carina) sections. The ratio between MSA and reference minimum luminal area (RefMLA) was calculated for both sections. The expansion was considered successful if the MSA/RefMLA was ≥80% in both proximal and distal stent sections (Figure 2B).
ENDPOINTS
The clinical and procedural characteristics were entered into a predefined standardised case report form. Clinical follow-up was obtained by clinic visits and/or by telephone contact.
The primary endpoint was procedural success, defined as follows: Thrombolysis In Myocardial Infarction (TIMI) 3 flow in all vessels + residual stenosis <50% by QCA + adequate stent expansion according to LEMON criteria.
Secondary endpoints included: 30-day and one-year incidence of major adverse cardiovascular events (MACE: a composite of cardiovascular death/stent thrombosis/target vessel revascularisation), percentage of appropriate wire position on run #2, stent expansion according to DOCTORS and ILUMIEN III criteria, contrast agent volume and radiation dose (safety endpoints).
STATISTICAL ANALYSIS
Statistical analysis was performed with SPSS, Version 21.0 software (IBM Corp., Armonk, NY, USA). Continuous numerical data are expressed as median±interquartile range and qualitative data as percent. Normal distribution of continuous variables was tested by the Kolmogorov-Smirnov test. The differences between the variables were compared by the chi-square or Fisher’s test for qualitative variables and by the Mann-Whitney U, Kruskal-Wallis, Student’s t-test or Student’s paired t-test for quantitative variables. The agreement between local operator and core lab for the wire recrossing point into the jailed SB was evaluated by the kappa coefficient. Binary logistic regression analysis was used to test the relationships between inadequate stent deployment incidence and clinically relevant variables. Univariable regression analyses were performed first to test the relationship between outcome and selected parameters, then all covariates with a p-value of <0.10 were included in the multivariable backward stepwise elimination regression model. A value of p<0.05 indicated a statistically significant difference.
Results
BASELINE CHARACTERISTICS
Between May 2018 and January 2019, a total of 117 patients were screened for inclusion; the final analysis included 70 patients. The flow chart of the study is given in Supplementary Figure 1. The distal LMS was stenosed in 93% of patients; the remaining 7% of patients presented a Medina 0,1,1 lesion. The population baseline characteristics are given in Table 1.
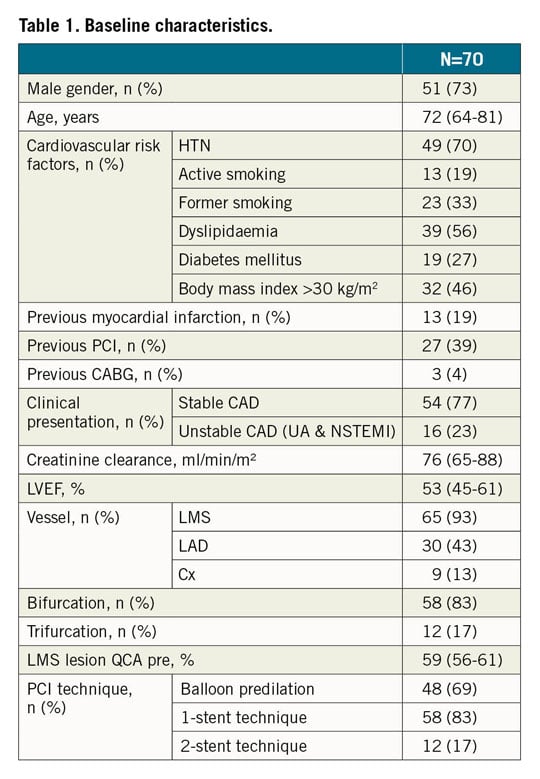
There was no predilation before OCT run #1. OCT analyses revealed that the culprit lesion was most frequently a mixed plaque. The stenosis length was 19 mm (14-27 mm) and the maximal LMS luminal and EEL/EEL diameters were 4.5 mm (4.1-4.9 mm) and 4.9 mm (4.5-5.5 mm), respectively. The MB and SB were both imaged before PCI in two patients.
The provisional one-stent strategy was the preferred operator strategy. When a two-stent strategy was decided on, the T-stenting and TAP techniques were applied in all patients (Supplementary Table 1). POT/Side/rePOT sequence was performed in 37 patients and POT/final kissing balloon inflation (FKBi) in 33 patients (12 patients with a two-stent technique and 21 patients with a one-stent technique). The median main stent diameter and length were 3.5 mm (3.0-3.5 mm) and 23 mm (18-28 mm), respectively (Supplementary Table 1).
PRIMARY ENDPOINT AND IMPACT OF OCT ON OPERATOR STRATEGY
The pre-specified protocol was respected in all the patients. Further PCI optimisation was provided in 26% of the cases following OCT run #2 or run #3, suggesting that OCT guidance modified the operators’ strategy in more than one patient out of four.
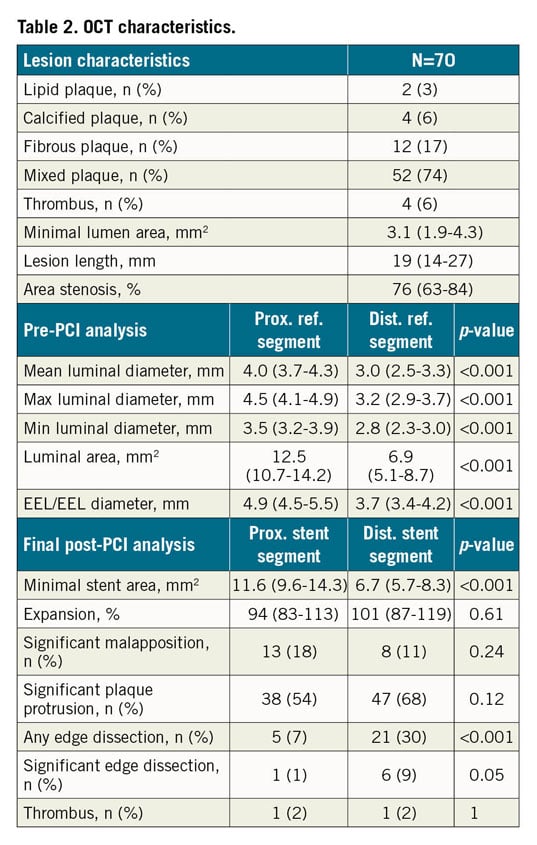
The primary endpoint was achieved in 86% of the cases. This percentage was driven by the achievement of optimal stent expansion: the expansion was considered appropriate according to the LEMON criteria in 86% of the cases, but this percentage decreased to 40% and 37% when analysed with the DOCTORS and ILUMIEN III criteria, respectively. Angiographic residual LM stenosis <50% and final TIMI 3 flow were achieved in all patients. The stenosis percentage decreased from 61% (53-76%) to 16% (0-21%) by QCA and from 76% (63-84%) to 6% (0-17%) by OCT (p<0.0001). Multivariable analysis revealed that larger proximal reference EEL/EEL diameter was a predictor of inadequate stent expansion (Supplementary Table 2). We also observed that proximal reference segment MLA was larger and LM stent MSA smaller in patients with suboptimal LM stent expansion compared to others (Supplementary Table 3). However, a large MSA was observed in the vast majority of patients (Supplementary Table 4).
MALAPPOSITION AND EDGE DISSECTION
The stent proximal edge was visualised in all cases. The final OCT analysis findings (after corrective actions) are presented in Table 2. Edge dissection was present in 30% of the cases, mostly on the distal part of the stent. However, the incidence of significant dissection was lower - 1% on the proximal edge and 9% on the distal edge. Residual significant strut malapposition was observed in 17 patients (24%), 9 (13%) in the proximal part, 4 (6%) in the distal part and 4 (6%) in both sections of the stent. However, when comparing post-POT OCT analysis (OCT run #2) with the final OCT run, all malapposition parameters were significantly reduced (Supplementary Table 5, Supplementary Figure 2). Finally, there was no influence of FKBi or POT/Side/rePOT strategies on post-PCI OCT parameters (Supplementary Table 6).
SECONDARY ENDPOINTS
The wire position could be analysed in 68 patients (97% of the cohort) on run #2. The core lab analysis showed that the wire was in an appropriate position towards the SB in 81% of the cases and inappropriate in 19%. The local operators reported wire position analysis in 60 patients (Supplementary Table 7). In this subset, there was an agreement on wire position in 44 patients and the k coefficient was 0.3. The operators proceeded to a wire repositioning in 15% of the cases on the basis of the OCT analysis.
The median contrast agent volume, procedure duration and radiation dose were 220 ml (180-260 ml), 65 mins (54-82 mins) and 4,374 cGy/cm2 (2,200-7,868 cGy/cm2), respectively.
Follow-up was achieved in all patients. No MACE were recorded at 30 days following PCI. One patient died from a non-cardiovascular cause (fatal traumatic subdural haematoma at day #98). One patient suffered from a target vessel revascularisation (related to LMS intra-stent restenosis). Thus, the one-year MACE rate was 1.4% and the 12-month actuarial survival free from MACE was 98.6% (97.2-100%) (Figure 3).
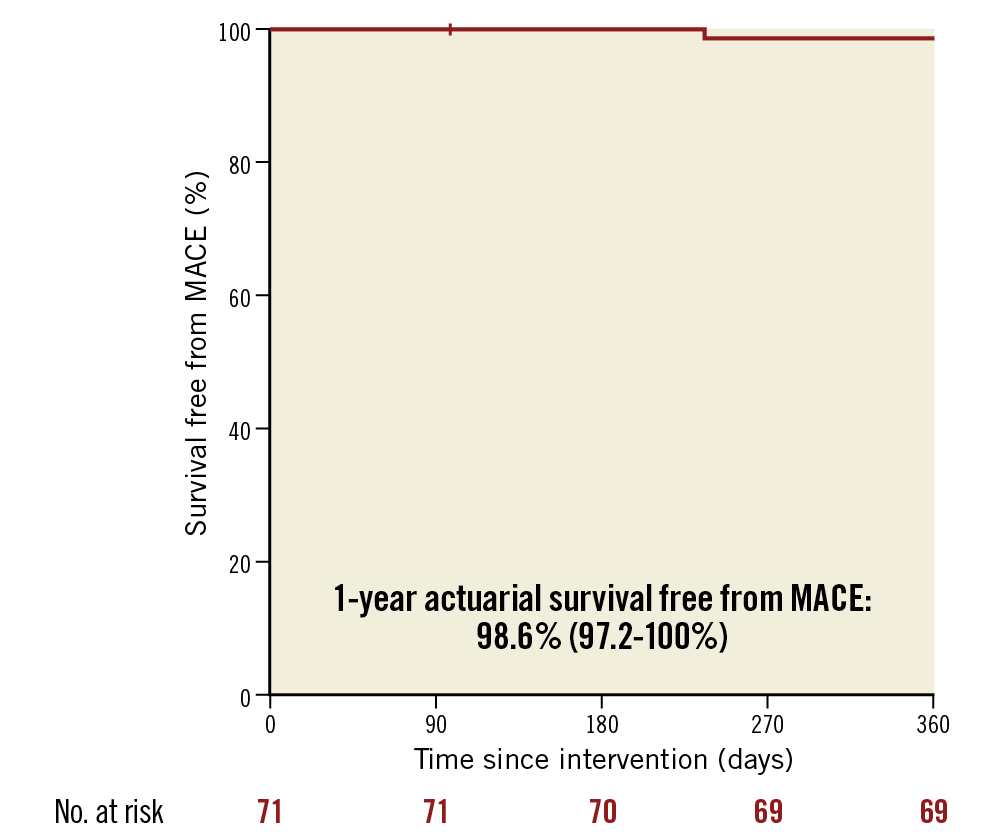
Figure 3. One-year incidence of MACE (cardiovascular death/target vessel revascularisation/stent thrombosis) in the LEMON cohort.
Discussion
The pilot LEMON study was conducted to assess the possible applicability of a pre-specified standardised OCT protocol for LMS PCI in a multicentre cohort. The main results of the study can be summarised as follows: 1) OCT guidance for LMS PCI was feasible and safe, and was successful in 86% of patients; 2) an optimal guidewire recrossing point into the stent-jailed side branch was observed in 81% of the cases at the first attempt following POT, but modest agreement was observed between the core lab and local operators; 3) OCT guidance impacted on operator strategy in one patient out of four despite adequate/acceptable angiographic results and the operators being experienced.
Intracoronary imaging (ICI) guidance (either by OCT or by IVUS) improves the quality of PCI7. The potential benefits of ICI-guided PCI are more pronounced in case of complex/high-risk lesions, which includes bifurcated and LMS lesions. LMS lesions display specific features (diameter discrepancies, tapered anatomy, plaque eccentricity and a higher probability of calcifications) that are difficult to analyse correctly by angiography alone15. Hence, the use of IVUS to guide LMS stent implantation provides a significant clinical advantage according to the multiple studies conducted so far6,7,16 and is currently recommended by international guidelines2. The higher resolution of OCT compared to IVUS confers greater sensitivity for detection of thrombus, stent underexpansion, strut malapposition and edge dissection, suggesting that it might be a valuable option for LMS PCI guidance. LMS OCT analysis is feasible in its mid/distal, but not ostial portions8,17,18 and is more accurate than IVUS for post-stenting assessment9. Recently, Cortese et al observed in the ROCK-1 retrospective series of non-standardised OCT-guided distal LMS PCI11 that the procedure detected a substantial number of cases of acute strut malappositon and device underexpansion and led to lower lumen late loss at follow-up11. To the best of our knowledge, the LEMON trial is the first study to have prospectively evaluated a specific OCT protocol with pre-specified optimisation criteria. It thus reinforces the interest of standardised protocols for ICI guidance in LMS PCI15.
Our current results confirm, on a large scale, the feasibility, safety and efficacy of the procedure. The success rate of the procedure was very encouraging, as the primary endpoint was achieved in 86% of the cases. Interestingly, the failures were related to non-achievement of the pre-specified device expansion criteria. Hence, optimal stent expansion was observed in 86% of the cases, according to the LEMON criteria, but in only 40% and 37% with the DOCTORS and ILUMIEN III criteria, respectively. These results are explained by the differences in the calculation of these indices: the LEMON criteria were conceived for bifurcated lesions, whereas the DOCTORS and ILUMIEN III criteria were not. Hence, these discrepancies highlight the difficulty in assessing optimal expansion by ICI within the LMS. Although the LEMON criteria were conceived to integrate some of the geometrical features of bifurcated lesions, they might not be completely adapted to the LMS as they do not take into account the anatomy and shape of the vessel and do not include specific analysis of the polygon of confluence (POC). Hence, tapered or funnel shapes display luminal area variations along the LMS that might influence the calculation of the degree of expansion14. However, the percentage of optimal expansion achieved in LEMON remains higher than in other “simpler” lesion series such as ILUMIEN III or DOCTORS12,19, and is in line with the most recently observed results in LMS ICI-guided PCI7. Altogether, these data advocate for the need of further improvement in our understanding of LMS anatomy and design of more adapted stent expansion criteria. Hence, ongoing trials will integrate a dedicated bifurcation expansion algorithm that will specifically analyse (ILUMIEN IV) or not (OCTOBER) the POC20.
The operators reported that post-PCI OCT analysis influenced their strategy and led to further optimisation despite satisfactory angiographic results in 26% of the cases. These results are in line with the ILUMIEN I trial data, in which post-PCI OCT analysis prompted PCI optimisation in 27% of the patients21. However, in LEMON, we cannot assess the influence of pre-PCI OCT analysis on the stenting strategy (especially in terms of device sizing) as this was not a pre-specified endpoint. Nevertheless, our study confirms the impact of periprocedural OCT on physician decision making, which is more pronounced for complex lesions21. OCT guidance is currently proposed by the EBC to support bifurcation lesion PCI and this might be expanded to distal LMS lesions14. In addition to its higher resolution, OCT analysis can also provide 3D online reconstructions to assess the distal recrossing point into a stent-jailed side branch. In this series, the core lab analysis identified an appropriate wire position after POT in 81% of the cases, hence validating the AptiVue™ E5 software. Once again, these results are in line with previous reports10 and might be related to the stent design as well as the systematic use of POT before SB rewiring3. However, we also recognise the modest agreement between core lab and local operators, suggesting that continuous physician education, better experience in handling these new tools and potential upgrades in online software are required to improve the results.
Limitations
Several limitations of this study deserve consideration. First, this pilot study was not randomised and did not include a control angiography guidance group, as our main task was to evaluate the feasibility of standardised OCT-guided LMS PCI. Future studies in the field will integrate control groups and longer follow-up. Moreover, the strict inclusion criteria might have induced a degree of patient selection. However, although we did not include STEMI, extremely tight and unstable lesions requiring predilation, high SYNTAX score, severe renal failure patients, extremely complex lesions or other bifurcation PCI techniques (such as DK crush or culotte) in LEMON, we believe that the spectrum of patients we analysed covers a large proportion of the individuals treated in our cath labs. Finally, our study only included mid and distal LMS lesions. Although this situation might evolve in the future, ostial LMS lesion PCI should only be performed under IVUS guidance.
Conclusions
The prospective multicentre LEMON study is the first to report the feasibility and performance of OCT-guided LMS PCI according to a predefined standardised protocol. The impact of this strategy on clinical outcome compared to conventional angiography-guided or IVUS-guided LMS PCI has to be evaluated in future, larger randomised trials.
|
Impact on daily practice Although OCT is accurate to guide PCI, there is a lack of available data regarding its use in the LMS. The LEMON study demonstrates for the first time the possible use of OCT for guidance of LMS PCI according to a pre-specified standardised protocol. This procedure was feasible and safe but its impact on clinical outcome has to be investigated in future, larger randomised trials. |
Funding
The LEMON study was funded by a research grant from Abbott Medical.
Conflict of interest statement
N. Amabile reports consulting/lecturing fees (modest) from Abbott Vascular and Boston Scientific, and a research grant from Abbott Vascular. G. Souteyrand reports consulting/lecturing fees (modest) from Terumo, Abbott Vascular and Boston Scientific. N. Meneveau reports consulting fees (modest) from Abbott Vascular. P. Motreff reports consulting fees (modest) from Abbott Vascular and Terumo. G. Cayla reports lectures fees (modest) from Abbott. T. Lefèvre reports honoraria/consultation, personal fees (modest) from Terumo and Boston Scientific. C. Caussin reports consulting/lecturing fees (modest) from Abbott and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

