Abstract
Background: Data on left main (LM) percutaneous coronary interventions (PCI) have mostly been obtained in studies using drug-eluting stent (DES) platforms without dedicated large-vessel devices and with limited expansion capability.
Aims: Our study aimed to investigate the safety and efficacy of LM PCI with the latest-generation Resolute Onyx DES.
Methods: ROLEX (Revascularization Of LEft main with resolute onyX) is a prospective, multicentre study (ClinicalTrials.gov: NCT03316833) enrolling patients with unprotected LM coronary artery disease and a SYNTAX score <33 undergoing PCI with the Resolute Onyx zotarolimus-eluting coronary stent, that includes dedicated extra-large vessel platforms. The primary endpoint (EP) was target lesion failure (TLF): a composite of cardiac death, target vessel myocardial infarction (TVMI) and ischaemia-driven target lesion revascularisation (ID-TLR), at 1 year. All events were adjudicated by an independent clinical event committee. An independent core lab analysed all procedural angiograms.
Results: A total of 450 patients (mean age 71.8 years, SYNTAX score 24.5±7.2, acute coronary syndrome in 53%) were enrolled in 26 centres. Of these, 77% of subjects underwent PCI with a single-stent and 23% with a 2-stent technique (8% double kissing [DK] crush, 6% culotte, 9% T/T and small protrusion [TAP] stenting). Intravascular imaging guidance was used in 45% (42% intravascular ultrasound [IVUS], 3% optical coherence tomography [OCT]). At 1 year, the primary EP incidence was 5.1% (cardiac death 2.7%, TVMI 2.7%, ID-TLR 2.0%). The definite/probable stent thrombosis rate was 1.1%. In a prespecified adjusted subanalysis, the primary EP incidence was significantly lower in patients undergoing IVUS/OCT-guided versus angio-guided PCI (2.0 vs 7.6%; hazard ratio [HR] 0.28, 95% confidence interval [CI]: 0.13-0.58; p<0.001).
Conclusions: In this large, multicentre, prospective registry, LM PCI with the Resolute Onyx DES showed good safety and efficacy at 1 year, particularly when guided by intracoronary imaging.
Introduction
Percutaneous coronary intervention (PCI) with drug-eluting stent (DES) implantation is a guideline-recommended option to treat patients with unprotected left main (LM) coronary artery disease (CAD) and a low-intermediate SYNTAX score1. DES implantation in the LM is known to pose specific challenges due to the large target vessel size, the common involvement of bifurcation with large and important branches, and the potential major clinical impact of complications2. To date, available data on LM PCI derive from previous studies on patients mostly receiving DES without dedicated large-vessel platforms and with limited expansion capability345. In fact, only a few DES manufacturers provide large-diameter stents whose nominal range falls within the typical LM size (4.5-5.0 mm). The new-generation, Resolute Onyx (Medtronic) zotarolimus-eluting stent includes large (3.5-4.0 mm – expansion limit 5.0 mm) and extra-large vessel (4.5-5.0 mm – expansion limit 6.0 mm) stent platforms and is designed to optimise the treatment of larger vessels, such as the LM.
On such a basis, we designed a prospective, international, multicentre study aimed at assessing the results of LM PCI obtained with the Resolute Onyx DES.
Methods
Study population
The ROLEX (Revascularization Of LEft main with resolute onyX) registry is a prospective, international, multicentre study enrolling patients with LM de novo CAD undergoing PCI with the Resolute Onyx zotarolimus-eluting coronary stent at 26 centres. According to the study protocol, we included patients aged >18 years with unprotected LM CAD up to intermediate anatomical complexity (defined by a SYNTAX score <33) considered amenable for PCI with the Resolute Onyx DES. Significant LM CAD was defined as angiographic diameter stenosis >50%. If LM diameter stenosis was between 50% and 70%, evidence of ischaemia by fractional flow reserve (FFR) with intracoronary adenosine administration <0.80 or intravascular ultrasound (IVUS) minimal lumen area <6.0 mm2 was recommended. The Heart Team was involved in every case and the decision to perform PCI was shared with the patient after informing them about all the therapeutic options (i.e., patients did not have to be refused for surgery to be enrolled in the study). The study inclusion and exclusion criteria are reported in Table 1. Patients with an indication for PCI of other non-LM lesions could be enrolled, as long as the SYNTAX score was <33 and all lesions were treated with the Resolute Onyx DES. The study received institutional review board/ethics committee approval at each participating site.
Table 1. Detailed study inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Unprotected de novo LM CAD with angiographic diameter stenosis >50% (if stenosis 50-70%, evidence of FFR <0.80 or IVUS minimal lumen area <6.0 mm2 is recommended) | – Prior LM PCI or CABG– Left main diameter stenosis <50%– SYNTAX score ≥33 |
| Silent ischaemia, stable angina, unstable angina or non-ST elevation myocardial infarction | – Concomitant indication to cardiac surgery (severe heart valve disease, etc.) |
| Ability to provide written informed consent and comply with follow-up for at least 2 years | – Cardiogenic shock (Killip >2) |
| Age >18 years | – Severe CKD (GFR <30 ml/min)– Left ventricular ejection fraction <30%– Inability to tolerate or comply with dual antiplatelet therapy for at least 1 year– Pregnancy or intention to become pregnant– Known intolerance to aspirin, heparin, zotarolimus, or contrast material– Life expectancy less than 1 year– Subject participating in other investigational drug or device studies that have not reached their primary endpoint |
| CABG: coronary artery bypass grafting; CAD: coronary artery disease; CKD: chronic kidney disease; FFR: fractional flow reserve; GFR: glomerular filtration rate; IVUS: intravascular ultrasound; LM: left main; PCI: percutaneous coronary intervention | |
Study device and procedure
The Resolute Onyx is the latest-generation zotarolimus-eluting stent built on the Resolute Integrity (a premounted composite of cobalt alloy and platinum-iridium alloy) platform. Resolute Onyx was the first drug-eluting stent available in 4.5 mm and 5.0 mm diameter sizes among major manufacturers, with a maximal expansion capability up to 6.0 mm. The 3.5-4.0 mm platform also has excellent expansion capability (up to 5.0 mm).
In case of LM bifurcation lesions, the technique selection was left to the operator’s judgment. However, the following technical considerations were strongly suggested based on emergent best practices2: 1) a provisional technique was recommended whenever possible, followed by the proximal optimisation technique (POT), using a post-dilating balloon with a diameter selected 1:1 to the LM reference diameter; 2) if deemed necessary by the operator to appropriately treat complex LM bifurcation anatomies, an intentional double-stenting technique (T stenting, T and small protrusion [TAP], double kissing [DK] mini crush, or culotte) could be adopted. Final kissing balloon inflation (preferably with non-compliant balloons) was strongly advised, as was final POT. Imaging-guided PCI (by either IVUS or optical coherence tomography [OCT]) to optimise stent sizing, expansion and apposition in the LM segment and for all complex non-LM lesions was not mandatory per protocol, yet strongly advised. Remaining non-LM lesions amenable for PCI could be treated at the time of the index intervention or staged. Antiplatelet therapy after PCI had to follow the latest guidelines1. An independent core laboratory analysed all procedural angiograms, both of the index and subsequent diagnostic or interventional coronary procedures during follow-up. Quantitative coronary angiography analyses were performed using the CAAS Workstation 8.4 (Pie Medical Imaging).
Study endpoints
The primary endpoint (EP) was target lesion failure (TLF): a composite of cardiac death, target vessel myocardial infarction (TVMI) and ischaemia-driven target lesion revascularisation (ID-TLR) at 1 year. Secondary EP were all-cause mortality, TVMI, ID-TLR, periprocedural MI, stroke rate and (definite or probable) stent thrombosis (ST). Follow-up was prospectively performed at 30 days (±7 days) and 1 year (±30 days) with outpatient visits or telephone interviews. Definitions of individual endpoints can be found in the study protocol (Supplementary Appendix 1). Routine follow-up angiography was not recommended. All events were adjudicated by an independent clinical events committee, after review of original source documentation.
Statistical analysis
For the ROLEX study, the sample size calculation was based on the primary EP at 1 year. An upper limit for primary EP incidence rates was set at 7%, consistent with reported event rates in the literature678. An overall sample size of 404 patients, increased to 450 to account for a 10% dropout rate, was expected to allow an estimation of the primary EP incidence rate of 7% with the precision of 2.5% and a confidence level (1-alpha) set at alpha=0.05.
According to the study protocol, several subanalyses were prespecified. In particular, subanalyses of patients with isolated LM CAD (with or without involvement of the proximal bifurcation main branch) receiving a 4.5/5.0 mm diameter stent and undergoing either IVUS or OCT imaging-guided LM PCI were planned. According to the study plan, a minimum of 150 patients with isolated LM CAD (with or without involvement of the proximal bifurcation main branch) was set in order to ensure that the EP incidence rate would be estimated in such subgroups with a precision of 3.5%, according to previous literature findings678.
Descriptive statistics were reported as mean ± standard deviation (SD) or median and 1st and 3rd interquartile ranges (IQR) for continuous variables and percentages for discrete variables. The Wilcoxon test was performed to compare the distribution of continuous variables. The chi-squared or Fisher’s exact tests were performed to compare the distribution of categorical variables. P-values underwent the Benjamini-Hochberg procedure to control for false discovery rates. The incidence of the primary endpoint was evaluated using cumulative incidence functions (CIF) to account for competing risks. To adjust the results for possible confounders, a covariate balance propensity score (CBPS) was estimated, accounting for age, gender, diabetes, 3-vessel CAD (vs 1- or 2-vessel CAD), acute coronary syndrome (vs chronic coronary syndrome) and the use of intravascular imaging. The balancing performance for the CBPS was assessed via common support propensity score visualisation and reporting in a plot of the mean difference of the covariate before and after the adjustment. The covariates having a balanced mean difference within 0.1 in absolute variables were defined as well balanced after the propensity estimation procedure. Inverse probability of treatment weighted (IPTW) Cox regression models were estimated. A shared frailty random effect term was calculated to account for the cluster effect within the same centre. Results were reported as hazard ratios (HR), 95% confidence intervals (CI) and p-values. Computations were performed with the R 3.4.2 system and the CBPS and WeightIt packages (Microsoft).
Results
Baseline clinical characteristics
Between November 2017 and December 2020, a total of 450 patients with LM CAD were enrolled in the ROLEX study at 26 centres. Detailed demographics and clinical characteristics are described in Table 2. In summary, patients were aged 71.8±10.7 years, 83% were male, the mean European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 2.7±3.3. Diabetes mellitus was present in one-third of the study population (9% insulin-dependent). Thirty-seven percent of subjects had a history of previous percutaneous coronary revascularisation, and baseline left ventricular ejection fraction (LVEF) was >50% in over two-thirds of patients. Clinical indications for LM PCI were acute coronary syndrome (ACS) in 53%, and 15% of patients had an NYHA Functional Class III-IV on admission.
Table 2. Baseline demographics and clinical characteristics.
| Characteristic | N=450 | |
|---|---|---|
| Age | 71.8±10.7 | |
| Female gender | 75 (17%) | |
| BMI (kg/m2) | 28.0±17.3 | |
| EuroSCORE II | 2.7±3.3 | |
| Current smoker | 95 (21%) | |
| Diabetes mellitus | 134 (30%) | |
| Insulin-dependent | 41 (9%) | |
| Non-insulin-dependent | 93 (21%) | |
| Hypertension | 355 (79%) | |
| Hypercholesterolaemia | 313 (70%) | |
| Glomerular filtration rate (ml/min) | 75 [57,90] | |
| Previous stroke | 27 (6%) | |
| Previous MI | 111 (25%) | |
| Previous PCI | 165 (37%) | |
| Peripheral vascular disease | 77 (17%) | |
| COPD | 30 (7%) | |
| LVEF | Good (>50%) | 292 (65%) |
| Fair (30-50%) | 158 (35%) | |
| Chronic coronary syndrome | 212 (47%) | |
| CCS 0 | 238 (53%) | |
| CCS 1 | 44 (10%) | |
| CCS 2 | 66 (14%) | |
| CCS 3 | 53 (12%) | |
| CCS 4 | 49 (11%) | |
| Acute coronary syndrome | 238 (53%) | |
| Unstable angina | 63 (14%) | |
| NSTEMI | 175 (39%) | |
| NYHA Class at admission | I | 243 (54%) |
| II | 140 (31%) | |
| III | 58 (13%) | |
| IV | 9 (2%) | |
| Dual antiplatelet therapy at the time of PCI | 252 (56%) | |
| Clopidogrel | 150 (33%) | |
| Ticagrelor | 93 (21%) | |
| Prasugrel | 8 (2%) | |
| Ticlopidine | 1 (0.2%) | |
| Oral anticoagulation | 31 (7%) | |
| Values are expressed as mean±SD, median [IQR] or n (%). BMI: body mass index; CCS: Canadian Cardiovascular Society Angina Score; COPD: chronic obstructive pulmonary disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; IQR: interquartile range; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SD: standard deviation | ||
Angiographic characteristics
Details on baseline angiographic characteristics are reported in Table 3. The mean SYNTAX score of our study population was 24.5±7.2, with isolated LM CAD in 10% of patients. LM disease involved the ostium in 20% and the bifurcation in 78% of subjects. In 187 patients, the lesion involved either only the LM or the LM and the proximal part of its bifurcation main branch. Among the adverse lesion features, severe calcifications were present in 12%, LM thrombus was observed in 6% and severe tortuosity was found in 1% of patients. At quantitative coronary angiography analysis, baseline LM diameter stenosis was 61.9±16.9%, LM reference vessel diameter was 4.1±0.7 mm, and the angle of the LM bifurcation was 79.9±26.6 degrees.
Table 3. Angiographic characteristics.
| Characteristic | N=450 | |
|---|---|---|
| CAD distribution | Isolated LM disease | 45 (10%) |
| LM+single-vessel CAD | 142 (31%) | |
| LM+dual-vessel CAD | 152 (34%) | |
| LM+triple-vessel CAD | 111 (25%) | |
| SYNTAX score | 24.5±7.2 | |
| <23 | 162 (36%) | |
| 23-32 | 288 (64%) | |
| LM CAD distribution | Ostial LM disease | 92 (20%) |
| LM shaft disease | 117 (26%) | |
| Distal LM disease | 350 (78%) | |
| Medina 1,0,0 | 76 (17%) | |
| Medina 1,1,0 | 153 (34%) | |
| Medina 1,0,1 | 36 (8%) | |
| Medina 0,1,1 | 21 (5%) | |
| Medina 1,1,1 | 64 (14%) | |
| LM calcifications | None | 126 (28%) |
| Mild/moderate | 270 (60%) | |
| Severe | 54 (12%) | |
| LM thrombotic lesion | 27 (6%) | |
| Tortuosity | None | 343 (76%) |
| Mild/moderate | 103 (23%) | |
| Severe | 4 (1%) | |
| Baseline QCA | LM diameter stenosis (%) | 62±17 |
| LAD diameter stenosis (%) | 56±30 | |
| LCx diameter stenosis (%) | 35±31 | |
| LM RVD (mm) | 3.7±0.7 | |
| LAD RVD (mm) | 3.2±0.8 | |
| LCx RVD (mm) | 2.9±0.6 | |
| LM minimal lumen diameter (mm) | 2.0±0.8 | |
| LAD minimal lumen diameter (mm) | 1.7±0.9 | |
| LCx minimal lumen diameter (mm) | 2.0±0.8 | |
| LM lesion length (mm) | 9.0±5.7 | |
| LAD lesion length (mm) | 11.1±9.7 | |
| LCx lesion length (mm) | 5.5±6.1 | |
| Bifurcation angle (°) | 79.9±26.6 | |
| Values are expressed as mean±SD or n (%). CAD: coronary artery disease; LAD: left anterior descending artery; LCx: left circumflex artery; LM: left main; QCA: quantitative coronary angiography; RVD: reference vessel diameter | ||
Procedural data
LM PCI was performed in 77% of cases through the radial approach, mostly (82%) with a 6 Fr guiding catheter (Table 4). By intention, the stepwise provisional approach was applied in 79% of patients. Seventy-seven percent of LM PCI were completed with a single-stent strategy (without involving the LM bifurcation in 11% of cases), while 23% of cases required a 2-stent strategy. Double kissing (DK)-crush was the most commonly used 2-stent technique (8%), followed by culotte and TAP (both 6%) and, finally, T stenting (3%). The majority of LM PCI were performed with a 3.5 or 4.0 mm stent, and the extra-large DES platform (4.5-5.0 mm) was implanted in 17% of subjects. Final kissing balloon inflation was adopted in 64% of cases (93% in the cases of a 2-stent strategy), and final proximal optimisation technique (POT) was used in 87% of procedures. Over half of the patients underwent additional PCI not involving the LM, and the mean number of implanted stents was 2.2±1.2. Intraprocedural death occurred in 3 (0.7%) patients.
At the time of PCI, 7% of patients were taking an oral anticoagulant (OAC), while 56% were already on dual antiplatelet therapy (DAPT). The prescription time for DAPT at discharge was 1-3 months for 20 patients (all with an indication for OAC), 6 months for 45 subjects, and ≥12 months for the rest of the study population. At 1-year follow-up, 77% of patients were taking 2 antiplatelet drugs.
Table 4. Procedural characteristics.
| Characteristic | N=450 | |
|---|---|---|
| Access | Femoral | 104 (23%) |
| Radial | 346 (77%) | |
| Guiding catheter | 6 Fr | 369 (82%) |
| 7 Fr | 81 (18%) | |
| Balloon predilation | 333 (74%) | |
| Intravascular imaging | IVUS | 188 (42%) |
| OCT | 12 (3%) | |
| FFR/iFR | 28 (6%) | |
| Rotational atherectomy | 19 (4%) | |
| Initial treatment strategy | Provisional | 356 (79%) |
| Two-stent strategy | 94 (21%) | |
| Final treatment strategy | One-stent | 347 (77%) |
| LM only | 49 (11%) | |
| LM-LAD | 278 (62%) | |
| LM-LCx | 20 (4%) | |
| Two-stent | 103 (23%) | |
| T stenting | 12 (3%) | |
| TAP stenting | 27 (6%) | |
| DK crush | 36 (8%) | |
| Culotte | 27 (6%) | |
| Kissing stenting | 1 (0.2%) | |
| Nominal LM stent diameter | ≤3.0 mm | 53 (12%) |
| 3.5-4.0 mm | 319 (71%) | |
| 4.5-5.0 mm | 78 (17%) | |
| LM stent length (mm) | 22 [18,26] | |
| Side branch stent length (mm) | 18 [15,22] | |
| POT | 391 (87%) | |
| POT balloon diameter (mm) | 4.5 [4.0,5.0] | |
| Final kissing balloon | 288 (64%) | |
| After two-stent strategy | 95 (93%) | |
| Additional PCI (not involving LM) | 249 (55%) | |
| LAD | 152 (34%) | |
| LCx | 72 (16%) | |
| RCA | 25 (5%) | |
| Number of implanted stents | 2.2±1.2 | |
| Total stent length (mm) | 46.1±25.5 | |
| Fluoroscopy time (min) | 22 [16,30] | |
| Contrast volume (cc) | 220 [170,282] | |
| Number of guidewires used | 2 [2,3] | |
| Final TIMI flow | 0-1 | 2 (0.4%) |
| 2 | 4 (0.9%) | |
| 3 | 444 (99%) | |
| Any remaining coronary dissection | 10 (2%) | |
| Acute side branch occlusion | 4 (0.8%) | |
| Post-PCI QCA | LM diameter stenosis (%) | 3±7 |
| LAD diameter stenosis (%) | 5±11 | |
| LCx diameter stenosis (%) | 11±19 | |
| LM minimal lumen diameter (mm) | 4.0±0.6 | |
| LAD minimal lumen diameter (mm) | 3.3±0.7 | |
| LCx minimal lumen diameter (mm) | 2.8±2.2 | |
| Residual SYNTAX score | 3.4±5.6 | |
| Gp IIb/IIIA inhibitors | 9 (2%) | |
| Mechanical circulatory support | 58 (13%) | |
| Planned | 49 (11%) | |
| Unplanned | 9 (2%) | |
| Clinical device success | 444 (99%) | |
| Procedural success | 446 (99%) | |
| Intraprocedural death | 3 (0.7%) | |
| Values are expressed as mean±SD, median [IQR] or n (%). DK: double kissing; FFR: fractional flow reserve; iFR: instantaneous wave-free ratio; Gp: glycoprotein; IVUS: intravascular imaging; LAD: left anterior descending artery; LCx: left circumflex; LM: left main; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; POT: proximal optimisaton technique; QCA: quantitative coronary analysis. RCA: right coronary artery; TAP: T and small protrusion | ||
Outcomes
The rates of primary and secondary EP are reported in Table 5 and Supplementary Table 1. At 1 year, the incidence of the primary EP was 5.1% (Figure 1). Cardiac death occurred in 2.7% of patients (Supplementary Table 2), 2.7% experienced a TVMI, while 2.0% underwent ID-TLR (PCI: 1.3% and coronary bypass grafting [CABG]: 0.7%). The rate of definite/probable ST was 1.1%. In particular, 1 acute, 2 subacute and 2 late ST were reported during follow-up. Significant bleeding was observed at 1 year in 4.2% of the study population. Follow-up was completed in all patients at 1 year.
Table 5. 1-year outcomes.
| Outcome | N=450 | |
|---|---|---|
| Primary endpoint | ||
| Target lesion failure | 23 (5.1%) | |
| Cardiac death | 12 (2.7%) | |
| TVMI | 12 (2.7%) | |
| ID-TLR | 9 (2.0%) | |
| Secondary endpoints | ||
| All-cause death | 28 (6.2%) | |
| Periprocedural MI | 17 (3.8%) | |
| Stroke | 5 (1.1%) | |
| Stent thrombosis (definite/probable) | 5 (1.1%) | |
| Definite | 3 (0.7%) | |
| Probable | 2 (0.4%) | |
| Acute | 1 (0.2%) | |
| Subacute | 2 (0.4%) | |
| Late | 2 (0.4%) | |
| Bleeding | 19 (4.2%) | |
| BARC 2 | 3 (0.6%) | |
| BARC 3A | 11 (2.4%) | |
| BARC 3B | 4 (0.8%) | |
| BARC 3C | 1 (0.2%) | |
| CCS | 0 | 347 (84%) |
| 1 | 44 (11%) | |
| 2 | 14 (3.4%) | |
| 3 | 4 (1.0%) | |
| 4 | 2 (0.5%) | |
| Values are expressed as n (%). BARC: Bleeding Academic Research Consortium; CCS: Canadian Cardiovascular Society Angina Score; ID-TLR: ischaemia-driven target lesion revascularisation; MI: myocardial infarction; TVMI: target vessel myocardial infarction | ||
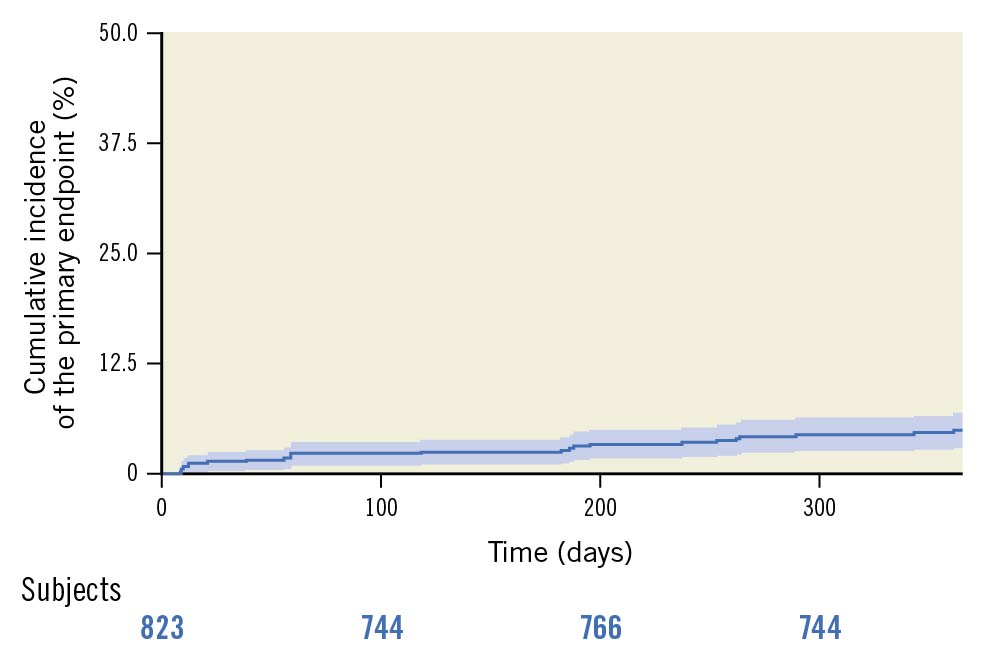
Figure 1. Primary endpoint incidence estimated using the cumulative incidence function accounting for death as a competing risk.
Angio-guided versus intracoronary imaging-guided LM PCI
Intravascular imaging guidance was used in 200 patients (45% of the entire study population; IVUS: 42%, OCT: 3%). The baseline and procedural characteristics of patients undergoing angiography-guided versus intracoronary imaging-guided LM PCI are reported in Supplementary Table 3-Supplementary Table 5. As depicted in Figure 2, after CBPS weighting (Supplementary Figure 1) and adjustment for the cluster effect within the same centre, the rate of the primary EP was significantly lower in patients undergoing intracoronary imaging-guided versus angio-guided PCI (2.0 vs 7.6%; HR 0.28, 95% CI: 0.13-0.58; p<0.001). Also, the 1-year incidence of cardiac death (1.0 vs 4.0%; HR 0.28, 95% CI: 0.10-0.78; p=0.015), TVMI (1.0 vs 4.0%; HR 0.25, 95% CI: 0.09-0.70, p=0.009), ID-TLR (1.0 vs 2.8%; HR 0.35, 95% CI: 0.11-1.14; p=0.081) and stent thrombosis (0.5 vs 1.6%, p=0.4) were lower in the study subgroup undergoing intracoronary imaging-guided PCI.
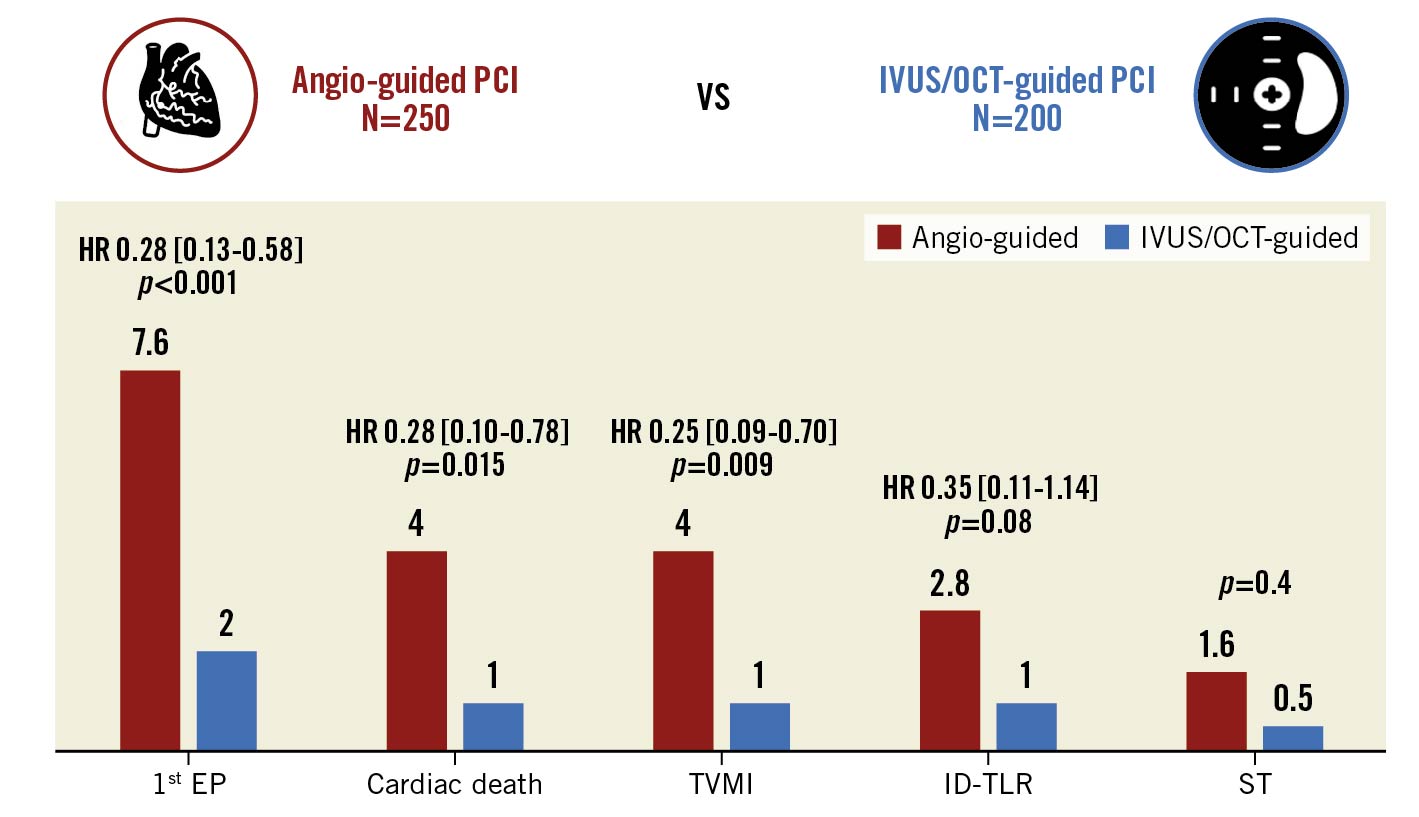
Figure 2. One-year outcomes of patients undergoing angiography-guided vs intravascular imaging-guided left main percutaneous coronary interventions. Inverse probability treatment weighted Cox regression models. Propensity score has been estimated by considering as confounding factors age, gender, diabetes, 3-vessel CAD and ACS. ACS: acute coronary syndrome; CAD: coronary artery disease; EP: endpoint; HR: hazard ratio; ID-TLR: ischaemia-driven target lesion revascularisation; IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; ST: stent thrombosis; TVMI: target vessel myocardial infarction
Other prespecified subanalyses
A total of 187 patients (41% of the study population) had isolated LM CAD (with or without involvement of the proximal main branch). As shown in Figure 3, these subjects had similar outcomes at follow-up, compared to patients with more diffused disease (HR 1.03, 95% CI: 0.58-1.86; p=0.91 for the primary EP). Baseline characteristics are reported in Supplementary Table 6-Supplementary Table 8 (propensity score diagnostic plots in Supplementary Figure 2).
Only 78 patients received a 4.5 or 5.0 mm DES. There was no significant difference in the rates of the primary EP between patients receiving a 4.5 or 5.0 mm versus a ≤4.0 mm DES (HR 1.6, 95% CI: 0.88-2.90; p=0.12 for the primary EP) (Supplementary Figure 3, Supplementary Figure 4, Supplementary Table 9-Supplementary Table 11).
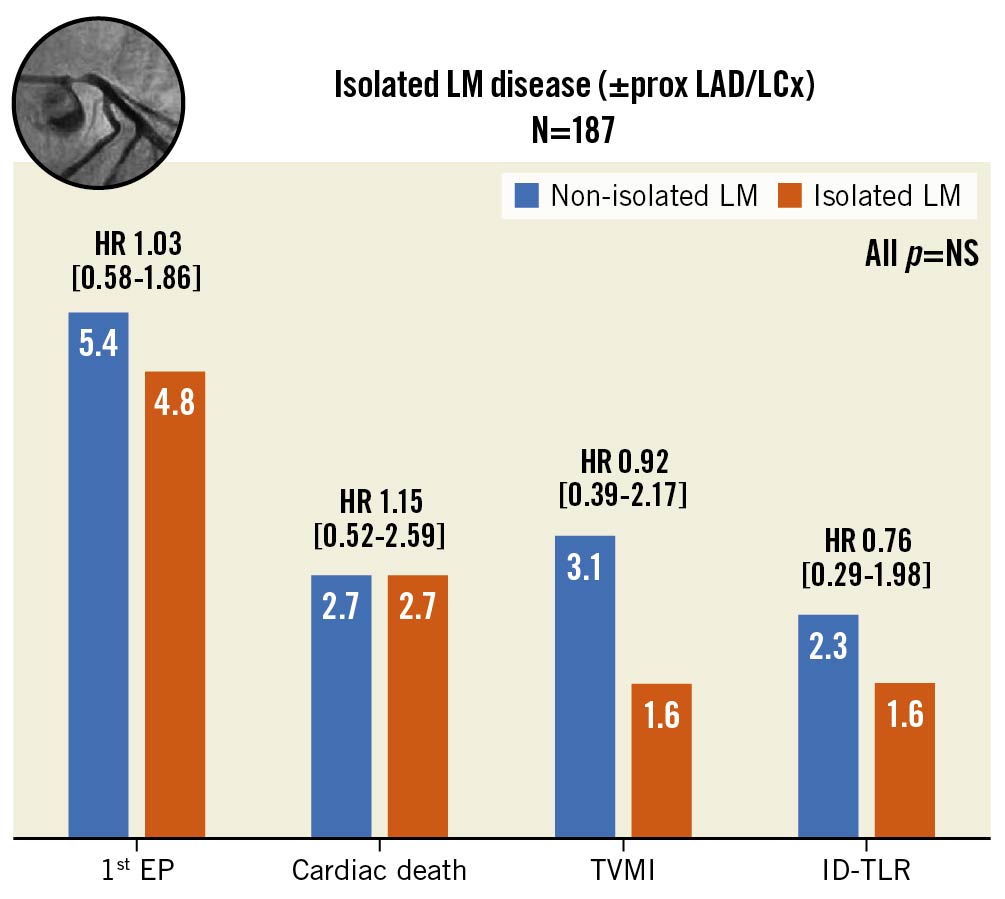
Figure 3. Incidence of the composite primary endpoint and its single components in patients treated for isolated left coronary artery disease (with or without involvement of the proximal segment of the bifurcation main branch). Inverse probability treatment weighted Cox regression models. Propensity score has been estimated by considering as confounding factors age, gender, diabetes, ACS and use of intravascular imaging. ACS: acute coronary syndrome; DES: drug-eluting stent; EP: endpoint; HR: hazard ratio; ID-TLR: ischaemia driven target lesion revascularisation; LAD: left anterior descending; LCx: left circumflex; LM: left main; NS: nonsignificant; PCI: percutaneous coronary intervention; prox: proximal; TVMI: target vessel myocardial infarction
Discussion
The improvement of LM PCI represents a hot topic in interventional cardiology and large, prospective, multicentre studies may integrate the randomised trial findings and provide important insights. In the present manuscript, we reported the final results of the largest prospective study on LM PCI with the latest-generation Resolute Onyx DES (covering all LM sizes). The main findings of the ROLEX study can be summarised as follows: 1) adverse events noticed up to 1 year were low (5.1% combined primary composite EP of cardiac death and/or TVMI and/or ID-TLR); 2) after adjustment for possible confounders, the use of intracoronary-imaging was associated with a lower incidence of adverse events at 1 year (Central illustration).
Left main CAD represents a high-risk subset with significant morbidity and mortality if not treated in a timely manner, as it supplies over 80% of the left ventricular myocardium9. PCI is an accepted treatment option in selected patients with unprotected LM CAD based on available clinical evidence from prospective registries and randomised trials5678. Nevertheless, most LM PCI observational registries included subjects treated with previous-generation DES without a specific design for the treatment of larger vessels345.
In the ROLEX study, we observed low rates of adverse events at mid-term follow-up with LM PCI performed with a latest-generation zotarolimus-eluting stent. At 1 year, the primary study EP, TLF, occurred in 5.1% of the patients, so the study hypothesis was met. As expected, outcomes compared favourably with those reported in the LM subgroup of the SYNTAX trial (15.8% incidence of major cardiovascular events at 1 year)10, in which first-generation DES were implanted. Observed TLF rates were also in line with those described in more recent LM PCI trials, as well as in an individual patient data meta-analysis of LM randomised controlled studies11 whose study population had similar baseline and procedural features compared to the ROLEX registry. In particular, 1-year TLF rates of 6-7% were reported in the PCI arm of the Evaluation of XIENCE Everolimus Eluting Stent Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL)6 and the Nordic-Baltic-British Left Main Revascularization Study (NOBLE)7 trials, where other new-generation DES lacking dedicated large vessel platforms were implanted. Notably, patients in the ROLEX registry reported significant symptomatic relief at 1-year follow-up, with a Canadian Cardiovascular Society (CCS) Angina Score of 0-1 in 95% of cases, as well as a higher rate of complete revascularisation (mean residual SYNTAX score 3.4), as compared to previous trials12.
Since stent thrombosis is a dreadful complication especially when the LM represents the target lesion, it is important to note that the ST rate at 1 year was as low as 1.1%, similar to that of the European Bifurcation Club (EBC) main study (1.5%), which tested a provisional versus 2-stent strategy in LM bifurcation lesions with the same DES platform13. Notably, our incidence of definite/probable ST is just slightly higher than that reported in the EXCEL trial (0.8% at 1 year) with a thin-strut everolimus-eluting stent, and lower than that observed in the NOBLE trial, where a thicker-strut stainless steel biolimus-eluting stent was implanted in about 90% of patients67. Importantly, in these 2 randomised trials, the use of intravascular imaging was as high as 77%.
Improved LM PCI outcomes observed in our study and other recent studies can be explained by the advancements in stent technology, technical refinements and adjunctive drug therapy141516. Latest-generation DES adopt thinner-strut platforms, improved delivery systems, and more biocompatible polymers than their predecessors. Stent design may impact expansion capability, an important attribute in the setting of LM PCI, where proximal post-dilation of the stent is normally necessary to match the proximal reference diameter and optimise stent apposition, as large diameter mismatches increase the risk of over-stretching or underexpansion with malapposition. In fact, according to a large observational IVUS study, the mean LM diameter is 5 mm and ranges between 3.5 and 6.5 mm17. Bench tests have confirmed the ability of different DES platforms with a nominal size of 3-4 mm to maintain structural integrity when expanded to higher diameters181920, but major concerns exist regarding specific expansion limits, suboptimal plaque coverage (secondary to reduced metal-artery ratio) and the preservation of drug-elution kinetics. Only a few manufacturers provide large-diameter stents whose nominal range falls within the typical LM size (4.5-5.0 mm). The Resolute Onyx DES was among the first with an extra-large vessel platform (4.5-5.0 mm nominal diameter with a maximal expansion capability of 6.0 mm with minimum foreshortening), thus presenting an advantage in the treatment of the LM.
Beyond DES technical advances, PCI result optimisation and improved bifurcation stenting techniques might concur to explain the low rate of events observed in the ROLEX study. Despite the involvement of many centres and operators, slightly less than half of the enrolled patients received intravascular imaging-guidance (42% IVUS, 3% OCT). Such rates of intravascular imaging use look quite high (considering the observational nature of our study) and are superior to that reported in the recent EBC13 and DKCRUSH-V21 randomised controlled trials (38% and 41%, respectively), where mainly high-volume centres were enrolled. Intravascular imaging provides unique insights into the extent and distribution of coronary atherosclerosis, lesion morphology, stent sizing and technique, and mechanisms and complications of stent implantation at the LM. In keeping with this, in the ROLEX registry the use of intravascular imaging increased the use of larger stent platforms and prompted further interventions to optimise the final result in one-third of cases. In our prespecified subanalysis, angiography-guided LM PCI was associated with significantly higher rates of the composite endpoint (7.6 vs 2.0%), as compared to IVUS/OCT-guided intervention, and a similar trend was noticed for all secondary endpoints (Figure 2). While the non-randomised design of the ROLEX study suggests caution in the interpretation of these results, the lower rates of adverse events in the intravascular imaging-guided LM PCI group were confirmed after propensity score analysis adjustment for potential confounders.
Importantly, the rate of definite/probable ST numerically increased 3-fold when intravascular imaging was not performed. These findings add to the previous body of evidence suggesting that IVUS-guided LM stenting is associated with a clinically detectable benefit, with reduction of long-term mortality and improvement in clinical outcomes as compared with angiographic guidance alone2223 and extend them also to the latest-generation dedicated large-vessel stent platforms, reinforcing current guideline recommendations1. As a final remark, the more frequent use of IVUS as compared to OCT in our study is likely due to the challenges of OCT in the setting of LM, where, due to the need to create a blood-free space by dye, artefacts in the proximal LM segment are known to be common24.
The other relevant technical issue which is potentially able to affect clinical outcomes in LM interventions is bifurcation stenting techniques2. In the ROLEX study, LM bifurcation involvement was frequent and similar to other recent large randomised trials725. As expected in a study where operators were free to select the stenting strategy according to the individual yield, the ROLEX investigators often adopted the stepwise provisional strategy and reserved an upfront 2-stent strategy mainly to treat the subgroup of patients with more extensive, true LM bifurcation lesions. Of note, the techniques were selected according to the operator’s preference and were in agreement (as underlined by the 87% of POT and 93% of kissing with non-compliant balloons in 2-stent implantation) with best practices2, which were indeed recommended according to the study protocol. Such a “tailored” approach was associated with a low procedural complication rate and resulted in excellent angiographic results as described by quantitative coronary angiography analysis. The latter showed single-digit percent diameter stenosis values in the LM-LAD axis and as low as 11±19% in the left circumflex artery (LCx).
Moving from procedure to the broad issue of LM patient management, the high prevalence (77%) of patients on dual antiplatelet therapy at 1 year in our study population should be underlined, since it also likely contributed to the low rate of thrombotic events (in particular, ST) during follow-up. Of note, in the ROLEX registry there were 2 definite ST following premature DAPT discontinuation (1 because of major bleeding and 1 for urgent non-cardiac surgery). In fact, a recent analysis including over 5,000 patients undergoing bifurcation PCI showed that DAPT discontinuation before 6 months in stable CAD patients and before 12 months in ACS patients was associated with a higher risk of adverse events26. Moreover, two-thirds of patients on oral anticoagulation had a DAPT time prescription of 1-3 months. Yet, in our study, significant bleeding beyond 30 days from the index procedure was observed in 1.3% of patients. Accordingly, DAPT duration should be decided based on clinical presentation, baseline bleeding risk, stenting strategy and use of intracoronary imaging27.
In another prespecified subanalysis, we failed to find a significant difference in the primary EP incidence between patients with isolated LM CAD (with or without involvement of the proximal bifurcation main branch) and those with 2- or 3-vessel involvement. This hypothesis was based on the observation of a meta-analysis of individual patient-level data of LM CAD patients included in the PRECOMBAT-2 (Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease)15 and SYNTAX (Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery)8 trials, showing that in this subgroups of patients, PCI was associated with a 60% reduction in all-cause mortality and a 67% reduction in cardiac mortality when compared to CABG28. While this result might be explained by the low event rate in our study, it is noteworthy that in the 45 patients with isolated ostial or mid-shaft LM disease, no cardiac death and just 1 cardiovascular event (an ID-TLR at 8 months after the index procedure) was observed.
Finally, the results of the remaining subanalysis on patients receiving an extra-large vessel stent platform cannot be considered informative, given the low number of patients treated with a 4.5/5.0 mm DES. The higher number of patients (51% observed difference) receiving an extra-large vessel platform in the intravascular imaging-guided versus the angiography-guided LM PCI group, despite comparable reference vessel diameters at quantitative coronary angiography, suggests that the 4.5/5.0 mm DES might have been underutilised in our study (probably a result of the operators' tendency to routinely use 3.5-4.0 mm platforms as the other DES types lack larger sizes).
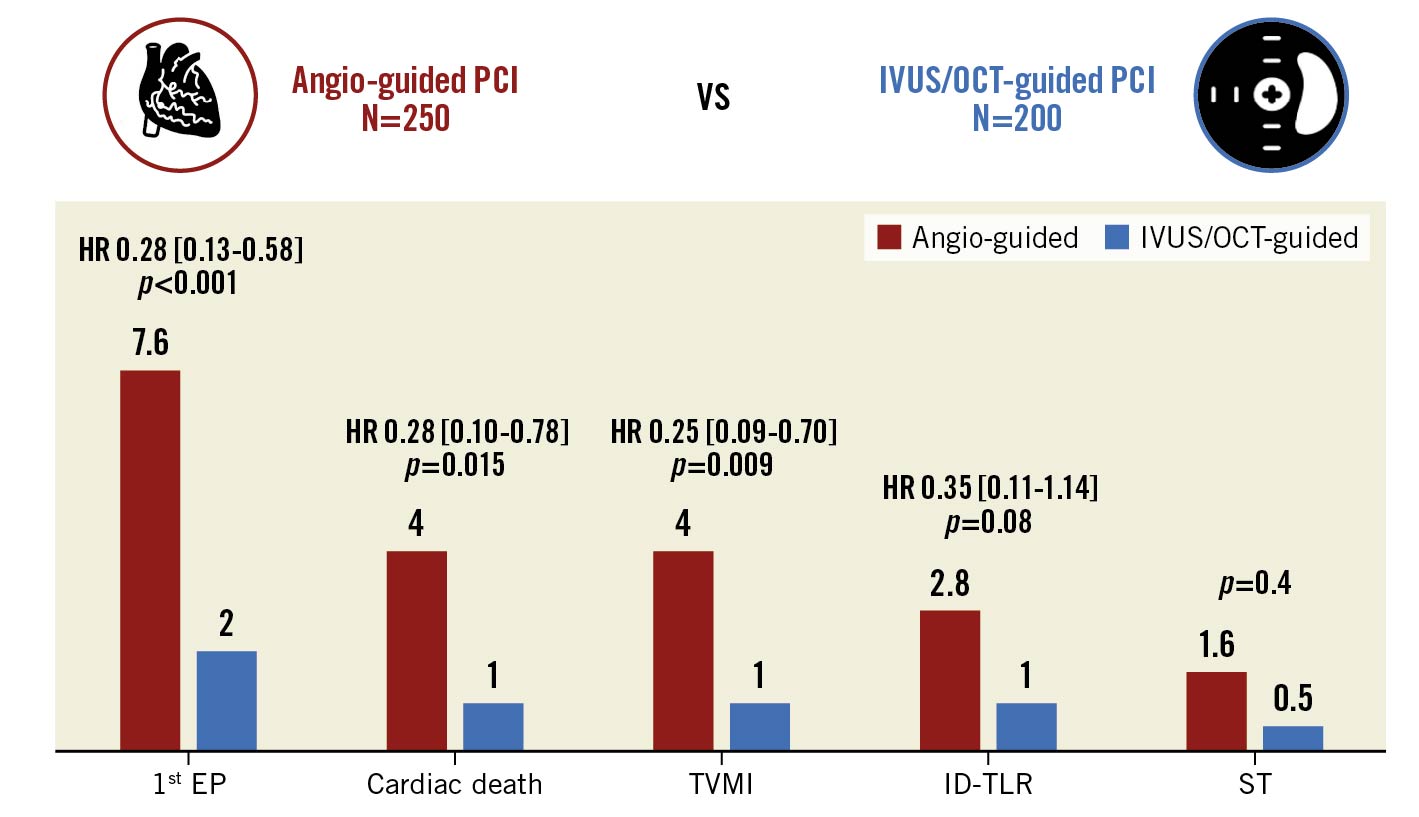
Figure 2. One-year outcomes of patients undergoing angiography-guided vs intravascular imaging-guided left main percutaneous coronary interventions. Inverse probability treatment weighted Cox regression models. Propensity score has been estimated by considering as confounding factors age, gender, diabetes, 3-vessel CAD and ACS. ACS: acute coronary syndrome; CAD: coronary artery disease; EP: endpoint; HR: hazard ratio; ID-TLR: ischaemia-driven target lesion revascularisation; IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; ST: stent thrombosis; TVMI: target vessel myocardial infarction
Limitations
The present prospective study has some limitations that should be acknowledged. It is not an all-comers registry and has some inclusion/exclusion criteria, including the type of stent used. Accordingly, the favourable results of LM PCI in our study cannot be generalised to all patients with LM CAD. In particular, they should not be extended to patients with a SYNTAX score >32, severe chronic kidney disease (CKD), severe left ventricular systolic dysfunction and those presenting with ST-elevation myocardial infarction (STEMI) or cardiogenic shock. Nevertheless, patients with low-to-intermediate anatomical complexity excluded from the ROLEX study (e.g., those with severe CKD, STEMI or severe left ventricular dysfunction, etc.) represent fewer than 7% of LM patients in all-comers registries like the DELTA 1 and 2529. Moreover, the most important baseline characteristics in the ROLEX study are very similar to the ones reported in the individual patient data meta-analysis of the EXCEL, NOBLE, SYNTAX and PRECOMBAT trials11.
The observational, non-randomised nature of the prespecified subanalyses, as well as the lower incidence of adverse events at follow-up, makes the results hypothesis-generating, with the need for confirmation in future studies. Notwithstanding, although the influence of confounders cannot be excluded, the lower incidence of the primary EP in the intravascular imaging-guided versus angio-guided LM PCI group also remained significant after adjustment for differences in age, sex, diabetes, the extent of CAD and clinical presentation by a propensity score weight analysis, and for the cluster effect within centres. Moreover, these results are consistent with those of previously published studies and reinforce current guideline recommendations1. Yet, further analysis on intravascular imaging, such as on the impact of LM distal bifurcation calcification severity on PCI strategy or on stent strut malapposition on IVUS/OCT, was not performed, as the use of intracoronary imaging was not mandatory per protocol. Notably, the results of the other 2 prespecified subanalyses should also be considered exploratory, considering the small sample size and low number of events.
One-year follow-up alone is not adequate, and we are currently progressing to 2-year follow-up. To this regard, the study steering committee has considered extending the follow-up to 5 years, in order to investigate the long-term results of LM PCI with this new-generation DES. The favourable safety and efficacy profile of LM PCI observed in our study was obtained in high-volume centres with expert interventionalists (all performing >15 LM PCI/year); operator volume is a known predictor of better outcomes of LM PCI30. Again, we attempted to mitigate this potential confounder by accounting for the cluster effect within centres. Among procedural variables highlighting the specific study environment, it should be emphasised that as many as 13% of patients received mechanical circulatory support and that this was mainly not used as a bailout. Thus, these event rates might not be reproducible in catheterisation laboratories with less experienced PCI operators or different equipment availability. Finally, the results of this study cannot be extended to LM PCI with other DES not included in this analysis. To note, another DES with similar platform sizes (SYNERGY MEGATRON; Boston Scientific) became available after the present study was conceived.
Conclusions
In this large, prospective registry on LM PCI performed with the latest-generation Resolute Onyx DES, the 1-year incidence of TLF was low, suggesting a favourable safety and efficacy profile of the procedure when a dedicated large-vessel stent platform is also available. As compared with angiographic guidance, intracoronary imaging-guided LM stenting was found to be associated with a clinically detectable benefit, and its use during LM PCI with latest-generation DES should be strongly recommended. Yet, these findings need to be confirmed by future randomised studies.
Impact on daily practice
The ROLEX study showed good safety and efficacy of LM PCI with the Resolute Onyx DES, which has dedicated extra-large vessel platforms. As the use of IVUS/OCT was found to be associated with lower rates of target vessel myocardial infarction, ischaemia-driven target lesion revascularisation, and definite/probable stent thrombosis after adjustment for potential confounders, this study supports the use of intravascular imaging guidance during LM PCI with the latest-generation dedicated large-vessel DES. Whether the Resolute Onyx DES is superior in the setting of LM PCI to other DES platforms without a dedicated large-vessel design remains to be studied in future randomised trials.
Guest editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Funding
This study was funded by an unrestricted educational and research grant from Medtronic. The company did not have any role in the design of the study, the writing of the manuscript, nor access to patient data. This registry was endorsed by the Italian Society of Interventional Cardiology.
Conflict of interest statement
G. Tarantini reports honoraria for lectures/consulting from Medtronic, Edwards Lifesciences, Boston Scientific, GADA, and Abbott. L. Nai Fovino received a research grant from Medtronic. F. Burzotta reports speakers’ fees from Abbott, Abiomed, Medtronic, and Terumo. C. Trani reports speakers’ fees from Abbott, Abiomed, Medtronic, and Terumo. M. Montorfano reports consultant fees from Abbott, Boston Scientific, and Medtronic. The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Supplementary data
To read the full content of this article, please download the PDF.

