Abstract
Background: There are limited data regarding the long-term prognosis of percutaneous coronary intervention treatment for left main (LM) ostial stenosis.
Aims: The present study sought to investigate the long-term clinical outcomes and risk factors for adverse events in LM ostial lesions following drug-eluting stent implantation (DES) in a large cohort of an LM registry database.
Methods: Patients presenting with LM coronary disease from January 2004 to December 2016 at Fuwai Hospital were included. The primary endpoint was target vessel failure (TVF), a composite endpoint of cardiac death, target vessel myocardial infarction and target vessel revascularisation. Cox proportional hazards models were constructed to identify independent predictors.
Results: Among 4,625 LM patients, 627 (13.6%) patients were identified with LM ostial lesions. There were more female patients in the ostial group (31.3%), compared with the shaft (18.1%) and bifurcation groups (19.9%) (p<0.0001). Among patients with DES implantation, 3-year TVF occurred in 44 patients (7.5%) in the ostial group, which is comparable with the other two groups. Myocardial infarction (MI) was significantly lower in the ostial group (2.0%) compared with the bifurcation group (4.2%) (p=0.02), especially for MI events originating in the LM vessel (p=0.02). For patients with ostial LM disease who received percutaneous coronary intervention (PCI) treatment, procedural complications were an independent risk factor for long-term cardiac death or MI, while a more recent PCI proved to be a protective factor.
Conclusions: PCI treatment for ostial LM lesions achieved favourable long-term outcomes, with a similar MI risk compared with the mid-shaft group but a significantly lower risk of MI compared with the distal group.
Introduction
Due to advances in interventional cardiology, percutaneous coronary intervention (PCI) treatment for patients with unprotected left main (LM) coronary artery (ULMCA) diseases has achieved safety outcomes that are equivalent to those of coronary artery bypass grafting12. According to evidence from randomised trials and meta-analyses345, PCI is recommended as an appropriate alternative to bypass grafting for LM lesions with low-to-intermediate anatomical complexity (Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery [SYNTAX] score ≤32) in recent guidelines6. Previous studies have shown that among the three sites of LM disease (ostial, mid-shaft, distal bifurcation), PCI treatment for lesions not involving distal LM stenosis had better outcomes78910. However, in those studies, investigators usually focused on distal bifurcation lesions, and LM ostial lesions were grouped into the non-bifurcation group instead of being reported separately. There are limited data that focus on LM ostial lesions, which are also believed to be associated with recurrent myocardial infarction (MI) or sudden death following PCI treatment11. The aim of the present study was to evaluate the long-term prognosis of PCI treatment for LM ostial lesions compared with mid-shaft or distal LM bifurcation lesions in a large cohort of an LM registry database.
Methods
STUDY POPULATION
A total of 4,625 patients presenting with unprotected LM disease who underwent PCI at Fuwai Hospital between January 2004 and December 2016 were consecutively enrolled in the LM registry database12. Unprotected LM disease was defined as documented myocardial ischaemia with ≥50% left main stenosis and no prior surgical revascularisation or no patent bypass graft to the left anterior descending (LAD) or left circumflex (LCx) arteries. Patient demographics, lesion characteristics and procedural information were prospectively recorded in a dedicated database. Patients were divided into 3 groups according to the location of the LM lesion. LM ostial lesions were defined as lesions located within 3 mm of the ostium with diameter stenosis over 50%. LM bifurcations were defined as >50% narrowing of the coronary artery occurring in the distal LM body and/or involving the origin of a significant LAD or LCx artery. Lesions causing >50% narrowing in both the LAD and LCx coronary arteries, in addition to the left main, were defined as true LM bifurcation lesions and classified according to the Medina classification as type 0,1,1. Patients were assigned to the bifurcation group if they had lesions that involved an LM bifurcation. Patients with both a bifurcation and other types of lesions were also put into the LM bifurcation group. Patients with both ostial and mid-shaft lesions were put into the ostial group. Additionally, baseline and residual SYNTAX scores were retrospectively assessed using standard quantitative coronary analysis methodology by an independent core laboratory (Interventional Cardiovascular Imaging Core Laboratory, National Centre for Cardiovascular Diseases, Beijing, People's Republic of China). The detailed protocol of the LM registry is listed in Supplementary Appendix 1.
Clinical follow-up via office visit or telephone contact at 1 month, 1 year, and annually thereafter up to 3 years was performed by research staff members in an independent office at Fuwai Hospital. All adverse clinical events were evaluated and adjudicated by an independent physician group which was not involved in the index PCI procedures. The study was approved by the Institutional Review Board of Fuwai Hospital. All eligible patients provided electronic informed consent by telephone interview or clinical visit during follow-up.
PROCEDURES
Coronary angioplasty and PCI procedures were performed with standard interventional techniques. Stent implantation, predilation, post-dilation or intravascular imaging utilisation were left to the discretion of the operators based on their clinical experience. Procedural complications including dissection, perforation, slow flow, no reflow, and side branch occlusion were adjudicated by operators and prospectively recorded in a dedicated database. All patients undergoing PCI were prescribed aspirin (loading dose 300 mg) plus clopidogrel (loading dose 300 mg) before the coronary intervention unless they had previously received regular anti-platelet medications (100 mg aspirin and 75 mg clopidogrel once daily for at least 6 days). After PCI, patients were maintained on aspirin (100 mg once daily) indefinitely and clopidogrel (75 mg once daily) for at least 1 year following PCI treatment; any changes to adjunctive pharmacotherapy were at the operator’s discretion.
ENDPOINTS AND DEFINITIONS
The primary endpoint of the present study was target vessel failure, which is a composite endpoint of cardiac death, target vessel MI and target vessel revascularisation (TVR). Secondary endpoints included individual components of the composite outcome, all-cause death, all MI, any revascularisation, target lesion revascularisation (TLR) and stent thrombosis as defined according to definite or probable Academic Research Consortium criteria13. Periprocedural MI was defined as creatine kinase concentration >2 times the upper limit of normal. Target vessels were defined as the entire major LM body including the upstream and downstream side branches (left anterior descending or left circumflex).
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation (SD) and were compared by the Student’s t-test. Categorical variables are presented as percentages and counts; between-group differences were compared by the chi-square test or Fisher’s exact test. Long-term adverse events rates are presented as Kaplan-Meier estimates and were compared by the log-rank test. Cox proportional hazards models were constructed to identify independent predictors for the primary and secondary endpoints. Variables associated at univariate analysis (all with a p-value of <0.1) and those judged to be of clinical importance from previously published reports were eligible for inclusion in the multivariable model-building process. The goodness-of-fit of the Cox multivariable model was assessed with the Grønnesby and Borgan test. Multivariable adjustment was used to balance the baseline difference; the variables listed in Supplementary Table 1 were included in the adjustment model. Also, pairwise testing of p-values (ostial versus mid-shaft and ostial versus distal) using the Bonferroni test was performed to reduce the occurrence of a false positive. Results are reported as hazard ratios (HR) with associated 95% confidence intervals (95% CI) and p-values. All statistical analyses were performed using SAS version 9.1.3 (SAS Institute).
Results
BASELINE CHARACTERISTICS
Among 4,625 patients with ULMCA, a total of 627 patients (13.6%) were identified with ostial LM lesions, 248 patients (5.4%) with mid-shaft LM lesions, and 3,750 patients (81.1%) with distal LM bifurcation lesions (Figure 1). Patients in the ostial group were older compared with the mid-shaft group (60.5 vs 58.8; p=0.04), although the percentage of patients over 75 years of age was similar among the 3 groups (7.3% vs 9.3% vs 9.4%, respectively; p=0.25). There were more female patients in the ostial group (ostial LM: 31.3%; mid-shaft LM: 18.1%; distal LM: 19.9%; p<0.0001). Ostial LM patients also tended to have a lower body mass index (25.3 vs 25.7; p=0.005), and lower rates of prior MI compared with the bifurcation group (17.2% vs 27.8%; p<0.0001). Left ventricular ejection fractions were higher in patients with ostial LM lesions as compared to those in both the mid-shaft (63.9 vs 62.1; p=0.001) and bifurcation groups (63.9 vs 62.9; p=0.05), although the percentage of patients with a left ventricular ejection fraction <40% was similar among the 3 groups (0.8% vs 2.0% vs 1.5%; p=0.16). There were a total of 442 (9.1%) patients who presented with acute myocardial infarction (AMI) in the present LM population, and the percentage of AMI was balanced among the three groups, while the percentage of patients who presented with ST-segment elevation myocardial infarction (STEMI) was higher in the mid-shaft and bifurcation LM groups (1.8% vs 5.2% vs 4.5%; p=0.004) (Table 1).
As shown in Table 2, compared with the mid-shaft LM group, the patients in the ostial LM group were more likely to have other vessel diseases. Lesion complexity was lower in the ostial LM group, with a lower percentage of occluded lesions (ostial LM: 2.9%; mid-shaft LM: 5.2%; distal LM: 5.4%; p=0.03) and lower SYNTAX scores (ostial LM: 18.7%; mid-shaft LM: 19.7%; distal LM: 23.8%; p<0.0001). Ostial LM lesion length was significantly lower compared with the bifurcation group (7.28 mm vs. 28.0 mm, p<0.0001), but significantly higher compared with the mid-shaft group (7.28 mm vs. 3.41 mm, p<0.0001). A transradial approach was more likely used in patients with ostial and mid-shaft LM lesions (ostial LM: 82.3%; mid-shaft LM: 88.3%; distal LM: 73.8%; p<0.0001). In the present study cohort, a total of 4,524 (97.8%) patients underwent drug-eluting stent (DES) implantation. The average number of stents used for ostial LM lesions was 1.28, which was significantly lower as compared with the mid-shaft lesion (1.41; p=0.01) and bifurcation lesion (1.74; p<0.0001) groups. A 2-stent strategy was used for bifurcation lesions in 27.2% of patients in the bifurcation group. Utilisation of an intra-aortic balloon pump was similar between the ostial and mid-shaft groups (4.1% vs 5.6%; p=0.34) but was significantly lower in the ostial group compared to the bifurcation group (4.1% vs 7.1%; p=0.005). More patients received intravascular ultrasound guidance in the ostial and bifurcation groups compared to the mid-shaft group (ostial LM: 43.5%; mid-shaft: 33.5%; bifurcation: 41.5%; p=0.02). The final lesion success rate was significantly higher in the ostial group (99.7% vs 98.0% vs 99.3%; p=0.03), with a significantly lower residual SYNTAX score (3.23 vs 4.01 vs 4.35; p<0.0001). Also, complete revascularisation, defined as a residual SYNTAX score=0, was achieved more frequently in patients with ostial LM lesions (ostial LM: 52.0%; mid-shaft: 42.7%; bifurcation: 39.8%; p<0.0001).
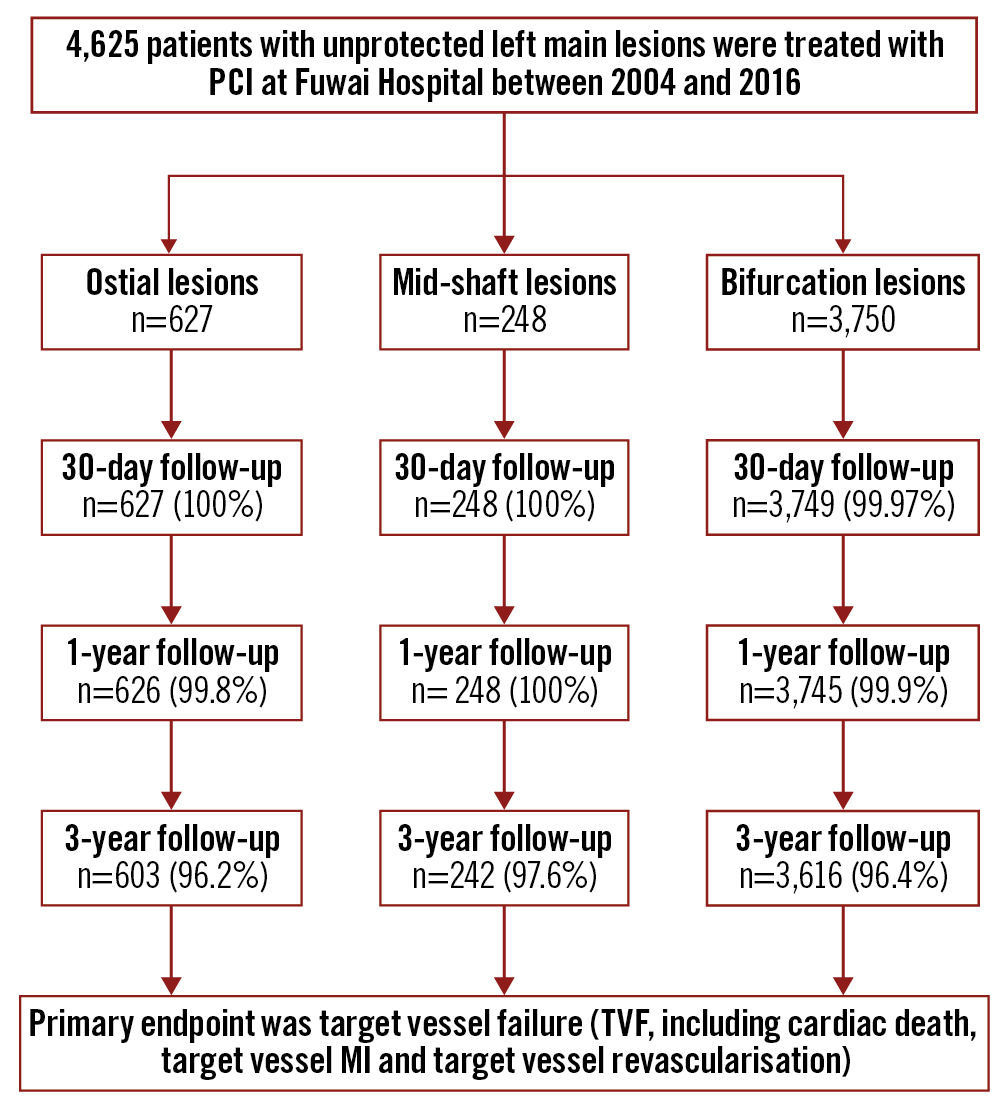
Figure 1. Study flowchart. LM: left main; MI: myocardial infarction; PCI: percutaneous coronary intervention
Table 1. Baseline demographics between patients with ostial versus mid-shaft or distal LM bifurcation lesions.
| Ostial lesion N=627 | Mid-shaft lesion N=248 | Bifurcation lesion N=3,750 | p-value (ostial vs mid-shaft) |
p-value (ostial vs distal) |
p-value (3 groups) |
|
|---|---|---|---|---|---|---|
| Age, years | 60.5±9.4 | 58.8±10.8 | 60.3±10.6 | 0.04 | 0.71 | 0.08 |
| Age ≥75 | 7.3% (46) | 9.3% (23) | 9.4% (352) | 0.34 | 0.10 | 0.25 |
| Female | 31.3% (196) | 18.1% (45) | 19.9% (747) | <0.0001 | <0.0001 | <0.0001 |
| Body mass index, kg/m2 | 25.3±3.2 | 25.6±3.1 | 25.7±3.2 | 0.29 | 0.005 | 0.02 |
| Diabetes | 28.9% (181) | 24.2% (60) | 28.3% (1,060) | 0.16 | 0.76 | 0.35 |
| Insulin-dependent | 5.3% (33) | 2.8% (7) | 5.0% (189) | 0.31 | 0.96 | 0.70 |
| Current smoker | 30.1% (189) | 37.1% (92) | 34.2% (1,281) | 0.13 | 0.03 | 0.07 |
| Hypertension | 55.8% (350) | 56.9% (141) | 58.6% (2,198) | 0.78 | 0.19 | 0.39 |
| Hyperlipidaemia | 60.9% (382) | 59.3% (147) | 59.0% (2,211) | 0.65 | 0.35 | 0.65 |
| Family history of coronary artery disease | 15.5% (97) | 17.7% (44) | 19.0% (712) | 0.41 | 0.04 | 0.11 |
| Previous percutaneous coronary intervention | 23.0% (144) | 23.8% (59) | 26.4% (989) | 0.80 | 0.07 | 0.15 |
| Prior myocardial infarction | 17.2% (108) | 19.8% (49) | 27.8% (1,041) | 0.38 | <0.0001 | <0.0001 |
| Prior stroke | 9.3% (58) | 8.5% (21) | 10.4% (391) | 0.72 | 0.37 | 0.44 |
| Peripheral arterial disease | 7.3% (46) | 5.6% (14) | 7.0% (263) | 0.37 | 0.77 | 0.67 |
| Chronic obstructive pulmonary disease | 0.3% (2) | 0.4% (1) | 0.6% (23) | 0.85 | 0.37 | 0.62 |
| Clinical presentation | 0.34 | 0.14 | 0.18 | |||
| Stable angina | 45.5% (285) | 40.7% (101) | 45.6% (1,709) | 0.20 | 0.96 | 0.33 |
| Unstable angina | 47.0% (295) | 49.6% (123) | 44.5% (1,670) | 0.50 | 0.24 | 0.18 |
| Acute myocardial infarction | 7.5% (47) | 9.7% (24) | 9.9% (371) | 0.29 | 0.06 | 0.17 |
| NSTEMI | 5.7% (36) | 4.4% (11) | 5.4% (203) | 0.44 | 0.74 | 0.74 |
| STEMI | 1.8% (11) | 5.2% (13) | 4.5% (168) | 0.004 | 0.001 | 0.004 |
| Creatinine clearance, ml/min | 91.3±26.2 | 93.5±25.8 | 92.1±28.6 | 0.28 | 0.51 | 0.59 |
| Left ventricular ejection fraction, % | 63.9±6.9 | 62.1±7.6 | 62.9±12.6 | 0.001 | 0.05 | 0.07 |
| Left ventricular ejection fraction <40% | 0.8% (5) | 2.0% (5) | 1.5% (55) | 0.13 | 0.18 | 0.16 |
| Values are mean±SD or % (n). CAD: coronary artery disease; LM: left main; NSTEMI non-ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction | ||||||
Table 2. Lesion characteristics.
| Ostial lesion N=627 | Mid-shaft lesion N=248 | Bifurcation lesion N=3,750 | p-value (ostial vs mid-shaft) | p-value (ostial vs distal) | p-value (3 groups) |
|
|---|---|---|---|---|---|---|
| Coronary artery disease extent | <0.0001 | <0.0001 | <0.0001 | |||
| Isolated LM | 22.2% (139) | 61.7% (153) | 11.4% (429) | |||
| LM+1VD | 26.5% (166) | 21.0% (52) | 31.3% (1,175) | |||
| LM+2VD | 29.5% (185) | 14.5% (36) | 37.9% (1,423) | |||
| LM+3VD | 21.9% (137) | 2.8% (7) | 19.3% (723) | |||
| Occluded lesion | 2.9% (18) | 5.2% (13) | 5.4% (203) | 0.09 | 0.007 | 0.03 |
| Calcification lesion | 10.8% (68) | 12.9% (32) | 13.5% (505) | 0.39 | 0.07 | 0.20 |
| Restenotic lesion | 1.9% (12) | 1.6% (4) | 3.2% (120) | 0.77 | 0.08 | 0.09 |
| Total lesion length (patient-level), mm | 19.9±17.8 | 22.5±17.3 | 30.1±20.2 | 0.05 | <0.0001 | <0.0001 |
| Total LM lesion length, mm | 7.28±3.61 | 3.41±0.90 | 28.0±18.6 | <0.0001 | <0.0001 | <0.0001 |
| SYNTAX score | 18.7±6.5 | 19.7±8.2 | 23.8±7.0 | 0.02 | <0.0001 | <0.0001 |
| SYNTAX score ≤22 | 73.4% (460) | 64.5% (160) | 46.6% (1,749) | |||
22 | 22.6% (142) |
28.2% (70) |
42.0% (1,574) |
|
|
|
|
| SYNTAX score >32 | 4.0% (25) | 7.3% (18) | 11.4% (427) | |||
| Transradial approach | 82.3% (516) | 88.3% (219) | 73.8% (2,767) | 0.03 | <0.0001 | <0.0001 |
| Stent type | ||||||
| Bare metal stent | 2.2% (14) | 2.4% (6) | 2.3% (86) | 0.87 | 0.93 | 0.99 |
| Drug-eluting stent | 97.4% (611) | 96.4% (239) | 98.0% (3,674) | 0.39 | 0.40 | 0.20 |
| 1st-generation DES* | 23.9% (150) | 25.8% (64) | 23.2% (869) | 0.82 | 0.92 | 0.90 |
| 2nd-generation DES | 73.8% (463) | 71.8% (178) | 74.5% (2,795) | 0.82 | 0.92 | 0.90 |
| Number of stents per patient | 1.80±1.08 | 1.94±1.07 | 2.27±1.53 | 0.10 | <0.0001 | <0.0001 |
| Total number of stents in LM | 1.28±0.68 | 1.41±0.71 | 1.74±0.81 | 0.01 | <0.0001 | <0.0001 |
| LM mean stent diameter, mm | 3.71±0.53 | 3.60±0.52 | 3.43±0.47 | 0.01 | <0.0001 | <0.0001 |
| Treatment of non-LM lesions | 0.45±0.69 | 0.47±0.70 | 0.53±0.69 | 0.81 | 0.001 | 0.002 |
| IABP | 4.1% (26) | 5.6% (14) | 7.1% (268) | 0.34 | 0.005 | 0.02 |
| 2-stent utilisation for bifurcation lesion | - | - | 27.2% (1,020) | - | - | - |
| Crush | - | - | 14.9% (689) | - | - | - |
| T-stent | - | - | 3.0% (141) | - | - | - |
| V- or kissing stent | - | - | 1.6% (76) | - | - | - |
| Culotte | - | - | 2.5% (114) | - | - | - |
| Procedural complications** | 1.9% (12) | 1.6% (4) | 2.0% (74) | 0.77 | 0.92 | 0.92 |
| Dissection | 1.3% (8) | 0.4% (1) | 1.2% (46) | 0.25 | 0.92 | 0.50 |
| Slow flow or no flow | 0.8% (5) | 0% (0) | 0.6% (21) | 0.16 | 0.47 | 0.36 |
| Major side branch occlusion | 0.2% (1) | 1.2% (3) | 0.9% (32) | 0.04 | 0.06 | 0.14 |
| Use of intravascular imaging guidance | ||||||
| IVUS | 43.5% (273) | 33.5% (83) | 41.5% (1,556) | 0.006 | 0.34 | 0.02 |
| OCT | 0 (0) | 0 (0) | 0.1% (4) | - | - | 0.63 |
| FFR | 0 (0) | 0 (0) | 0.2% (8) | - | - | 0.39 |
| Lesion success | 99.7% (625) | 98.0% (243) | 99.3% (3,724) | 0.01 | 0.28 | 0.03 |
| Residual SYNTAX score | 3.23±4.84 | 4.01±5.46 | 4.35±5.67 | 0.04 | <0.0001 | <0.0001 |
| Residual SYNTAX score=0 | 52.0% (326) | 42.7% (106) | 39.8% (1,491) | <0.0001 | <0.0001 | <0.0001 |
| Values are mean±SD or % (n). *First-generation drug-eluting stent including CYPHER (Cordis) and TAXUS (Boston Scientific), the other DES were grouped into second-generation. **Procedural complications including dissection, perforation, slow flow, no flow, side branch occlusion. DES: drug-eluting stent; FFR: fractional flow reserve; IABP: intra-aortic balloon pump; IVUS: intravascular ultrasound; LM: left main; OCT: optical coherence tomography; SYNTAX: Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery; VD: vessel disease | ||||||
THREE-YEAR CLINICAL OUTCOMES
At 3 years, 96.5% patients had completed follow-up. Among patients who had received DES, target vessel failure occurred in 44 (7.5%) patients in the ostial group, in 16 (6.8%) patients in the mid-shaft group, in 321 (9.0%) patients in the bifurcation group, with non-significant p-values of 0.15 (ostial versus mid-shaft) and 0.88 (ostial versus bifurcation) (Figure 2). However, the 3-year incidence of MI was significantly lower in the ostial group (2.0%), which is numerically lower compared with the mid-shaft group (3.0%; p=0.95), but statistically lower compared with the bifurcation group (4.2%; p=0.02). Also, target vessel MI was comparable between the ostial LM and mid-shaft groups (1.8% vs 2.1%; p=0.88), while the ostial LM lesion group had a significantly lower rate than the bifurcation lesion group (1.8% vs 3.9%; p=0.02). The incidence of patients who required repeat revascularisation was comparable between the 3 groups as were the rates of stent thrombosis (Table 3). Results in the overall population (including patients with bare metal stent implantation), sensitivity analysis excluding patients with only >50% narrowing of lesions in both the LAD and LCx coronary arteries in addition to the left main in bifurcation group, as well as the pairwise testing of p-values showed the same trend (Supplementary Table 2, Supplementary Table 3, Supplementary Table 4). Additionally, based on the sensitivity analysis comparing ostial lesions with bifurcation lesions receiving a 2-stent or provisional stent strategy, the provisional stent strategy was associated with reduced adverse events, while the main trend was unchanged in both groups (Supplementary Table 5).
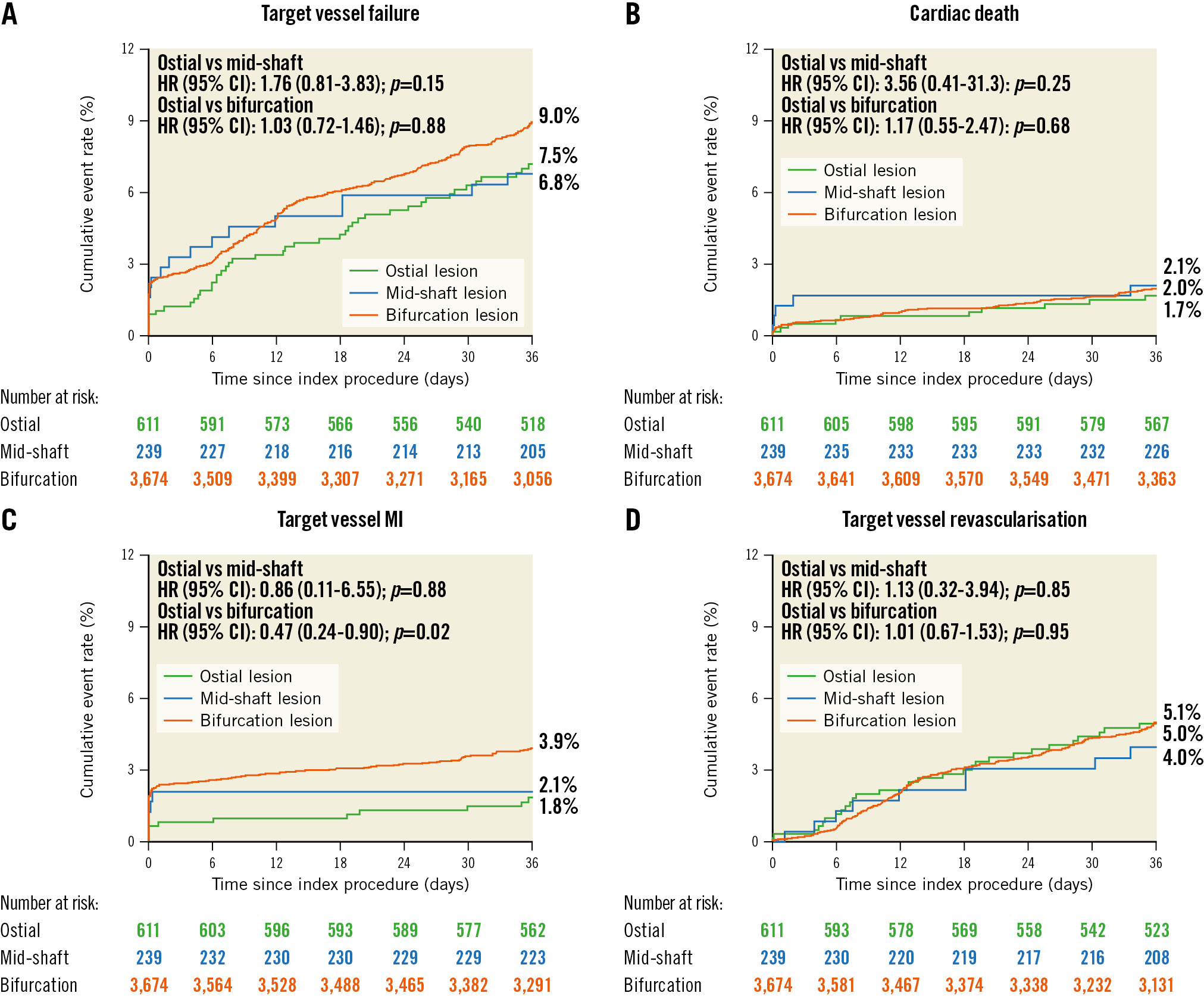
Figure 2. Time-to-event curves for 3-year clinical outcomes in patients with DES implantation. Kaplan-Meier cumulative event curves for A) target vessel failure; B) cardiac death; C) target vessel MI; D) target vessel revascularisation. Target vessel failure was defined as a composite of cardiac death, target vessel myocardial infarction, or target vessel revascularisation. CI: confidence interval; HR: hazard ratio; LM: left main; MI: myocardial infarction; TVF: target vessel failure
Table 3. Clinical outcomes in patients with only DES implantation through to 3 years.
| Adjusted by multivariable model | |||||||
|---|---|---|---|---|---|---|---|
| Ostial lesion N=611 | Mid-shaft lesion N=239 | Bifurcation lesion N=3,674 | Ostial vs mid-shaft lesionHR (95% CI) | p-value | Ostial vs bifurcation lesion HR (95% CI) | p-value | |
| 30 days | |||||||
| Target vessel failure | 1.1% (7) | 2.5% (6) | 2.5% (92) | 0.67 (0.02-22.5) | 0.82 | 0.44 (0.21-0.96) | 0.04 |
| All-cause death | 0.5% (3) | 1.3% (3) | 0.5% (20) | 0.38 (0.08-.89) | 0.24 | 0.88 (0.26-2.96) | 0.84 |
| Cardiac death | 0.3% (2) | 1.3% (3) | 0.5% (17) | 0.25 (0.04-1.52) | 0.13 | 0.69 (0.16-2.98) | 0.62 |
| Myocardial infarction | 0.8% (5) | 2.1% (5) | 2.4% (89) | 0.38 (0.11-1.31) | 0.13 | 0.33 (0.13-0.81) | 0.02 |
| Target vessel-related | 0.8% (5) | 2.1% (5) | 2.4% (88) | 0.38 (0.11-1.31) | 0.13 | 0.33 (0.14-0.82) | 0.02 |
| Stroke | 0.2% (1) | 0% (0) | 0.01% (2) | - | 0.71 | 2.93 (0.27-32.4) | 0.38 |
| Any revascularisation | 1.0% (6) | 1.3% (3) | 0.5% (20) | 0.76 (0.19-3.05) | 0.70 | 1.76 (0.71-4.39) | 0.22 |
| TVR | 0.3% (2) | 0% (0) | 0.01% (4) | - | 0.60 | 2.94 (0.54-16.0) | 0.21 |
| TLR | 0.3% (2) | 0% (0) | 0.01% (2) | - | 0.60 | 5.87 (0.83-41.7) | 0.08 |
| Definite/probable ST | 0.3% (2) | 0.8% (2) | 0.3% (11) | 0.38 (0.05-2.69) | 0.33 | 1.06 (0.24-4.80) | 0.94 |
| 1 year | |||||||
| Target vessel failure | 3.6% (22) | 5.1% (12) | 5.3% (194) | 1.58 (0.52-4.84) | 0.43 | 0.91 (0.56-1.48) | 0.71 |
| All-cause death | 1.1% (7) | 2.1% (5) | 1.6% (59) | 3.12 (0.34-28.5) | 0.31 | 0.97 (0.40-2.37) | 0.95 |
| Cardiac death | 0.8% (5) | 1.7% (4) | 1.1% (39) | 1.19 (0.01-123.2) | 0.94 | 1.00 (0.34-2.98) | 1.00 |
| Myocardial infarction | 1.1% (7) | 2.1% (5) | 3.0% (111) | 0.35 (0.004-28.7) | 0.64 | 0.37 (0.16-0.86) | 0.02 |
| Target vessel-related | 1.0% (6) | 2.1% (5) | 2.9% (107) | 0.60 (0.02-23.1) | 0.78 | 0.32 (0.13-0.80) | 0.02 |
| Stroke | 0.3% (2) | 0% (0) | 0.4% (13) | - | 1.00 | 0.86 (0.18-4.21) | 0.86 |
| Any revascularisation | 4.0% (24) | 6.4% (15) | 4.5% (164) | 0.55 (0.21-1.45) | 0.23 | 1.02 (0.63-1.62) | 0.95 |
| TVR | 2.3% (14) | 2.2% (5) | 2.4% (84) | 2.69 (0.53-13.6) | 0.23 | 1.14 (0.61-2.13) | 0.69 |
| TLR | 1.5% (9) | 1.3% (3) | 1.4% (51) | 2.00 (0.01-732.5) | 0.82 | 1.06 (0.45-2.47) | 0.90 |
| Definite/probable ST | 0.5% (3) | 0.8% (2) | 0.8% (29) | 3.08 (0.002-5,578.7) | 0.77 | 0.75 (0.17-3.33) | 0.70 |
| 3 years | |||||||
| Target vessel failure | 7.5% (44) | 6.8% (16) | 9.0% (321) | 1.76 (0.81-3.83) | 0.15 | 1.03 (0.72-1.46) | 0.88 |
| All-cause death | 2.8% (17) | 2.5% (6) | 3.6% (130) | 3.32 (0.73-15.1) | 0.12 | 1.00 (0.57-1.75) | 1.00 |
| Cardiac death | 1.7% (10) | 2.1% (5) | 2.0% (71) | 3.56 (0.41-31.3) | 0.25 | 1.17 (0.55-2.47) | 0.68 |
| Myocardial infarction | 2.0% (12) | 3.0% (7) | 4.2% (153) | 0.94 (0.12-7.52) | 0.95 | 0.47 (0.25-0.88) | 0.02 |
| Target vessel-related | 1.8% (11) | 2.1% (5) | 3.9% (142) | 0.86 (0.11-6.55) | 0.88 | 0.47 (0.24-0.90) | 0.02 |
| Stroke | 1.4% (8) | 2.2% (5) | 1.5% (54) | 0.23 (0.04-1.31) | 0.10 | 0.93 (0.42-2.08) | 0.86 |
| Any revascularisation | 8.3% (49) | 9.0% (21) | 7.9% (284) | 1.02 (0.51-2.06) | 0.95 | 1.09 (0.77-1.53) | 0.63 |
| TVR | 5.1% (30) | 4.0% (9) | 5.0% (174) | 1.13 (0.32-3.94) | 0.85 | 1.01 (0.67-1.53) | 0.95 |
| TLR | 3.5% (20) | 3.1% (7) | 3.0% (105) | 1.13 (0.16-8.04) | 0.91 | 1.16 (0.70-1.92) | 0.58 |
| Definite/probable ST | 1.0% (6) | 0.8% (2) | 1.5% (54) | 9.07 (0.19-426.2) | 0.26 | 0.71 (0.28-1.79) | 0.46 |
| Percentages are Kaplan-Meier estimates. Target vessel failure includes cardiac death, target vessel-related myocardial infarction and TVR. CI: confidence interval; DES: dug-eluting stent; HR: hazard ratio; ST: stent thrombosis; TLR: target lesion revascularisation; TVR: target vessel revascularisation | |||||||
RISK FACTORS FOR ADVERSE EVENTS IN PATIENTS WITH OSTIAL LM LESIONS
For the primary endpoint, both univariate and multivariate Cox regression analyses revealed that procedural complications were an independent risk factor in patients with ostial LM lesions (HR 4.62, 95% CI: 1.34-15.8; p=0.02), and a more recent PCI was proven to be a protective factor (HR 0.91, 95% CI: 0.83-0.99; p=0.04) (Table 4). We further divided the composite primary endpoint into safety (cardiac death or MI) and efficacy (TVR) endpoints. Multivariate Cox regression analyses showed that, for cardiac death or MI, procedural complications were an independent risk factor, while a more recent PCI was a protective factor. For the efficacy endpoints, male gender and larger stent diameter were identified as protective factors, while higher creatinine clearance was identified as a risk factor by multivariate analyses (Supplementary Table 6).
Table 4. Independent risk factors for the primary endpoint in patients with LM ostial stenosis.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Target vessel failure | ||||
| Age | 0.96 (0.94-0.99) | 0.02 | 0.97 (0.93-1.01) | 0.11 |
| Male | 1.71 (0.85-3.44) | 0.13 | 0.58 (0.27-1.23) | 0.15 |
| Body mass index | 1.00 (0.92-1.10) | 0.95 | 1.01 (0.90-1.14) | 0.85 |
| Current smoking | 1.11 (0.60-2.04) | 0.75 | - | - |
| Previous myocardial infarction | 1.30 (0.65-2.62) | 0.46 | - | - |
| Diabetes mellitus | 1.07 (0.57-1.99) | 0.84 | - | - |
| Family history of CAD | 0.96 (0.43-2.13) | 0.91 | - | - |
| Hypertension | 1.17 (0.65-2.09) | 0.61 | - | - |
| Peripheral artery disease | 0.87 (0.27-2.79) | 0.81 | - | - |
| Acute coronary syndromes | 1.04 (0.58-1.85) | 0.90 | - | - |
| Left ventricular ejection fraction | 0.98 (0.94-1.02) | 0.36 | 0.98 (0.94-1.02) | 0.36 |
| Creatinine clearance | 1.01 (1.00-1.02) | 0.09 | 1.01 (0.99-1.02) | 0.47 |
| Number of diseased vessels | 1.18 (0.90-1.54) | 0.24 | - | - |
| Moderate or heavy calcification | 0.54 (0.17-1.75) | 0.31 | 0.55 (0.13-2.37) | 0.42 |
| Number of stents per patient | 1.09 (0.86-1.39) | 0.47 | - | - |
| Total disease length (patient level) | 0.99 (0.98-1.01) | 0.52 | - | - |
| LM mean stent diameter | 0.92 (0.53-1.61) | 0.77 | - | - |
| Drug-eluting stent generation | 0.62 (0.38-1.01) | 0.05 | - | - |
| Restenotic lesion | 1.12 (0.15-8.11) | 0.91 | - | - |
| IABP insertion | 1.08 (0.26-4.45) | 0.92 | - | - |
| Treatment of non-LM lesions | 1.48 (0.83-2.63) | 0.18 | - | - |
| Procedural complication | 3.86 (1.20-12.4) | 0.02 | 4.62 (1.34-15.8) | 0.02 |
| IVUS utilisation | 0.87 (0.49-1.56) | 0.64 | 1.06 (0.56-2.00) | 0.85 |
| Residual SYNTAX score | 1.02 (0.96-1.07) | 0.58 | 1.02 (0.96-1.08) | 0.63 |
| Baseline SYNTAX score | 1.01 (0.97-1.05) | 0.66 | - | - |
| Year of percutaneous coronary intervention | 0.91 (0.84-0.99) | 0.02 | 0.91 (0.83-0.99) | 0.04 |
| Age, body mass index, left ventricular ejection fraction, creatinine clearance, number of diseased vessels, number of stents per patient, total disease length, LM mean stent diameter, residual SYNTAX score, baseline SYNTAX score, and year of percutaneous coronary intervention were included as continuous variables. CAD: coronary artery disease; CI: confidence interval; HR: hazard ratio; IABP: intra-aortic balloon pump; IVUS: intravascular ultrasound; LM: left main; SYNTAX: Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery | ||||
Discussion
In the current study, a dedicated LM PCI registry with a large number of ostial LM lesions, we demonstrated that 1) in patients with LM coronary artery disease, around 13.6% LM lesions were located in the ostium; 2) the 3-year incidence of MI was comparable between the ostial and mid-shaft LM groups, but the event rate in the ostial group was significantly lower compared to the bifurcation group, especially for MI events originating in the LM vessel; 3) in patients with ostial LM disease, procedural complications were risk factors for cardiac death or MI, while PCI performed in recent years significantly reduced those events (Central illustration).
To the best of our knowledge, our study is the first to report long-term (3 years) clinical outcomes in a large cohort of patients with LM ostial lesions who underwent PCI, based on the dedicated Fuwai LM registry database121415. In previous studies that followed LM PCI prognosis, most focused on bifurcation lesions; ostial lesions are usually combined with mid-shaft LM lesions and the two are reported as non-bifurcation groups1617. No studies have been done with a large cohort of patients addressing the long-term prognosis and risk factors of adverse events of ostial LM lesions. As we reported, around 81% of LM lesions were bifurcation lesions and ostial and mid-shaft lesions accounted for 19%, which is consistent with previous data. We also found a similar trend, as previously reported, that ostial LM lesions more often occur in females11. Female patients account for 31.3% in this ostial LM population, which is higher than previously reported in the overall LM PCI population (around 20-25%)3418, and in the LM bifurcation population (around 20%)914.
Previous studies have highlighted that PCI for ostial/mid-shaft lesions is associated with better clinical outcomes than more complex PCI for distal bifurcation lesions1920. Our current study had different findings compared with previous studies (Supplementary Table 7). The DELTA registry (Drug-Eluting Stent for Left Main Coronary Artery Disease) suggested that PCI for ostial/mid-shaft lesions was associated with lower revascularisation event rates than for distal lesions in LM disease. However, no significant differences in death or MI were observed between the 2 groups7, which is consistent with other studies that show better outcomes of ostial/mid-shaft LM lesions compared with distal bifurcation lesions due to lower revascularisation events1719. However, the same trend was not found in the present data. One reasonable explanation might be that fewer 2-stent strategies were used for bifurcation lesions in the present population. On the other hand, the event rates were relatively low, which might be related to the low-risk status of the patients involved in the present study. The different findings from the current data were also due to differing definitions of MI events. The current study used a relatively strict definition with lower thresholds of biomarker elevations, which could be induced by a jailed side branch or an occlusion triggered by the 2-stent strategy for bifurcation lesions. Complications including major side branch occlusion, major dissection or slow flow/no reflow were factors that triggered periprocedural MI. Periprocedural MI events proved to be associated with long-term cardiac death following LM PCI12 and optimised operations that avoided procedural complications during PCI might improve prognosis.
According to the current findings, procedural complications, including dissection, perforation, and blood perfusion interference are independent factors for the safety endpoint of cardiac death or MI. Stenting complications might be prevented through careful technique and, in certain cases, intravascular imaging guidance will be necessary. However, intravascular imaging should be used not only for diagnosis, but also to guide and optimise stent implantation, thus avoiding stent dislodgment and embolisation in the aorta21. It has been reported that ostial lesions carry higher restenosis rates than non-ostial lesions22; the present data found that a larger stent diameter was a protective factor for long-term restenosis. Matching the appropriate treatment strategies to the affected lesions might serve to avoid repeat revascularisation. In addition, the improved prognoses in recent years, catalysed by advanced techniques and device innovation, were observed in the present study, as the data showed that a more recent PCI was a significant protective factor for cardiac death or MI. With optimal lesion preparation and suitable device selection, as well as advanced techniques and new devices that help avoid potential complications, outcomes for patients with ostial LM stenosis undergoing PCI might be favourable in daily practice.
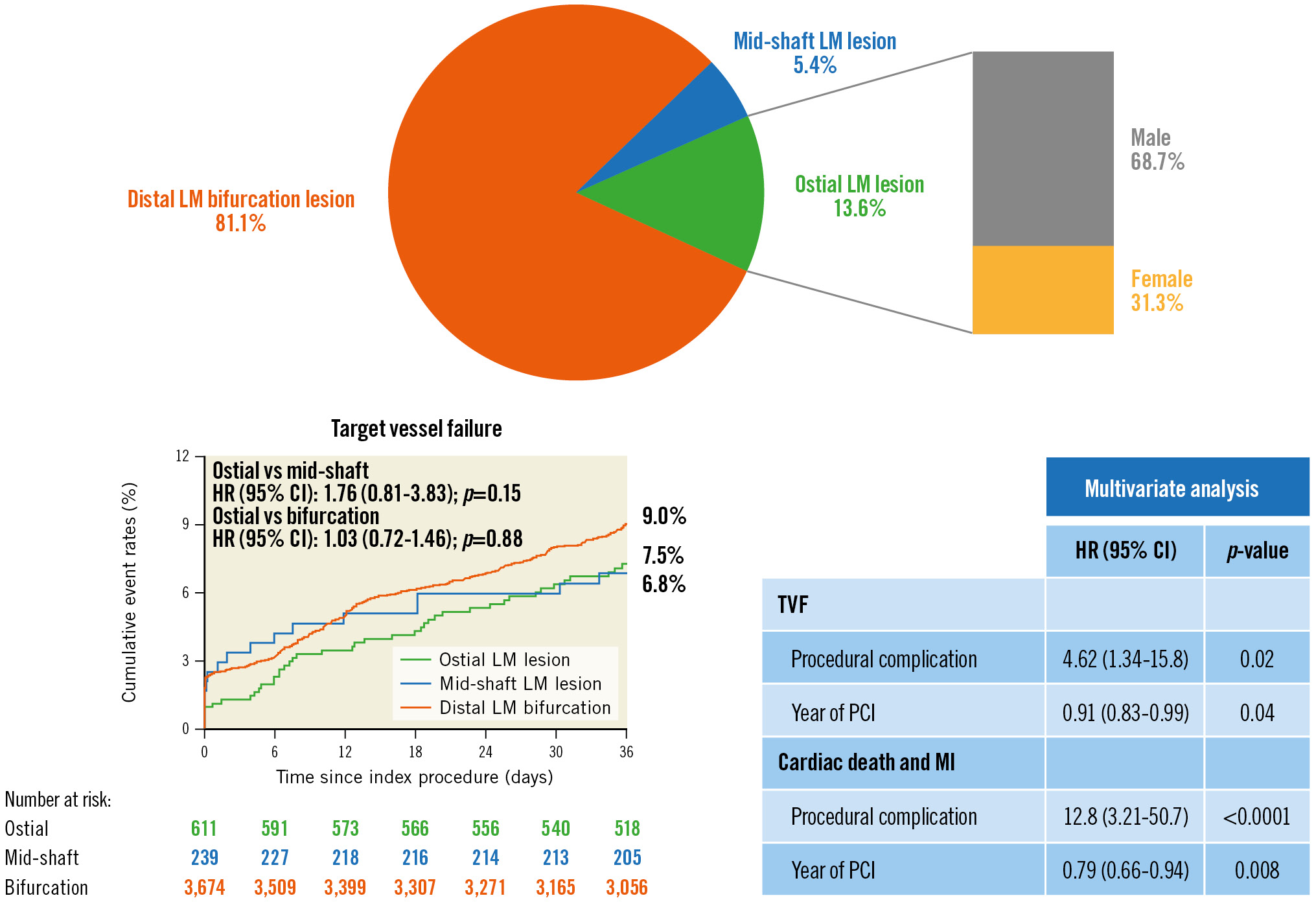
Central illustration. Target vessel failure (TVF) including cardiac death, target vessel-related myocardial infarction and target vessel revascularisation. CI: confidence interval; HR: hazard ratio; LM: left main; MI: myocardial infarction; PCI: percutaneous coronary intervention
Limitations
The present study has several limitations. First, as a retrospective study with an observational design, there might be hidden bias; however, the current study introduced a large cohort of patients with ostial LM lesions, which might provide more evidence for clinical practice. Second, the data were reported from a single centre, which might limit the external validity. Third, patient enrolment in the LM registry study began in 2004, and PCI strategies have evolved greatly since then. Fourth, event rates of cardiac death, MI, and revascularisation were relatively low, which might lead to low statistical power. Fifth, high-risk patients (older, lower ejection fraction or creatinine clearance, or three-vessel disease, etc.) were underrepresented in the current registry. Finally, periprocedural complications were reported by operators who performed the procedure, which might be associated with interobserver variability.
Conclusions
The long-term prognosis of patients with ostial LM lesions following PCI treatment is acceptable. In ostial LM patients, procedural complications were risk factors for cardiac death or MI, while improved procedures, catalysed mainly by advanced techniques and device innovation, were associated with a lower risk of adverse events.
Impact on daily practice
In the patients suffering from LM diseases, around one-tenth of LM stenoses occured at the ostium. PCI treatment for ostial LM lesions achieved favourable long-term outcomes, with a similar risk of MI compared with the mid-shaft group but significantly lower risk of MI compared with the distal bifurcation group. Based on the retrospective analysis of this LM PCI registry study, improved techniques and devices that avoid procedural complications might improve the prognosis of patients receiving PCI for ostial LM diseases.
Acknowledgements
The authors thank the staff in the Interventional Cardiovascular Imaging Core Laboratory, the National Centre for Cardiovascular Diseases, and Fuwai Hospital for their research contributions.
Funding
This study was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2022-12M-C&T-B-043).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

