
Abstract
Aims: This study sought to investigate the prognostic effect of a protocol with optimisation targets for intravascular ultrasound (IVUS)-guided left main (LM) revascularisation.
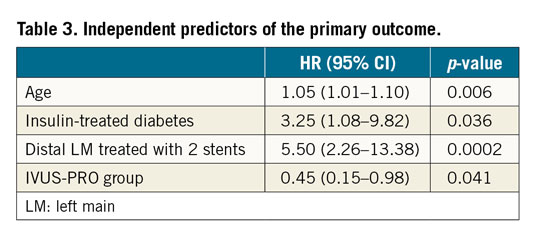
Methods and results: A protocol was prospectively applied for IVUS-guided LM revascularisation (IVUS-PRO group) including predefined optimisation targets. Using propensity score matching, we selected as control groups patients with angiography-guided PCI (ANGIO group) and IVUS-guided PCI (IVUS group) from a large multicentre registry. The primary endpoint was a composite of cardiac death, LM-related infarction and LM revascularisation at 12 months. In each group, 124 patients with comparable characteristics were included. The incidence of the primary outcome was significantly higher in the ANGIO group compared to the IVUS-PRO group (12.9% vs 4.8%, HR 0.35, 95% CI: 0.15 to 0.82, p=0.02), but not with respect to the IVUS group (12.9% vs 8%, HR 0.51, 95% CI: 0.20 to 1.22, p=0.1), driven by a lower rate of LM revascularisation (8% in the ANGIO group, 6.4% in the IVUS group and 3.2% in the IVUS-PRO group). IVUS-PRO resulted in being an independent risk predictor (HR 0.45, 95% CI: 0.15 to 0.98; p=0.041).
Conclusions: IVUS guidance of LM stenting provides prognostic benefit with respect to the use of angiography alone, particularly when following a protocol with these predefined optimisation criteria.
Introduction
Percutaneous revascularisation of the left main coronary artery (LM) is already recommended by the clinical guidelines, especially in those cases without multivessel disease1.
The use of intravascular ultrasound (IVUS) to guide PCI of the LM with drug-eluting stents (DES) has been associated with a better prognosis in several studies, though mostly retrospective registries2,3,4,5,6,7,8,9,10,11,12. In fact, the most recently released guidelines provide a class IIa recommendation for the use of IVUS in LM PCI; its use is also encouraged by recent consensus documents1,13,14.
Nonetheless, in the abovementioned studies2,3,4,5,6,7,8,9,10,11,12, what was evaluated was simply the use or non-use of IVUS to guide LM PCI, without evaluating specific protocols with predefined optimisation criteria. Thus, there is a remarkable knowledge gap in how best to utilise IVUS to guide the best outcomes in PCI of the LM.
Our group has carried out extensive research in the field of IVUS and LM. We prospectively validated a cut-off value for luminal area to defer safely the revascularisation of intermediate LM lesions15,16 and subsequently reported a large registry comparing IVUS and angiography in the guidance of LM PCI4.
In this study, we present a strategy for the use of IVUS to guide PCI of the LM, based on a protocol with clear recommendations and optimisation targets adapted to the different locations of the lesions and the morphological characteristics of the LM17. We analyse the clinical results derived from its prospective application.
Methods
POPULATION
Since January 2014 we have systematically applied an IVUS protocol for the guidance of LM PCI, including predefined optimisation targets. Patients with a clinical indication for percutaneous revascularisation of the LM as determined by the local Heart Team were eligible. Patients with cardiogenic shock at the time of PCI were excluded from the analysis.
All interventional cardiologists in the institution were urged to follow the strategy of LM PCI guided by IVUS according to the protocol previously established by consensus. However, the decision to use IVUS was ultimately left to the operator. Patients treated according to this IVUS protocol constituted the IVUS-PRO group.
PROCEDURES AND IVUS PROTOCOL
The recommendations for the use of IVUS and the optimisation criteria applied (Figure 1, Figure 2) are described in detail in Supplementary Appendix 1.
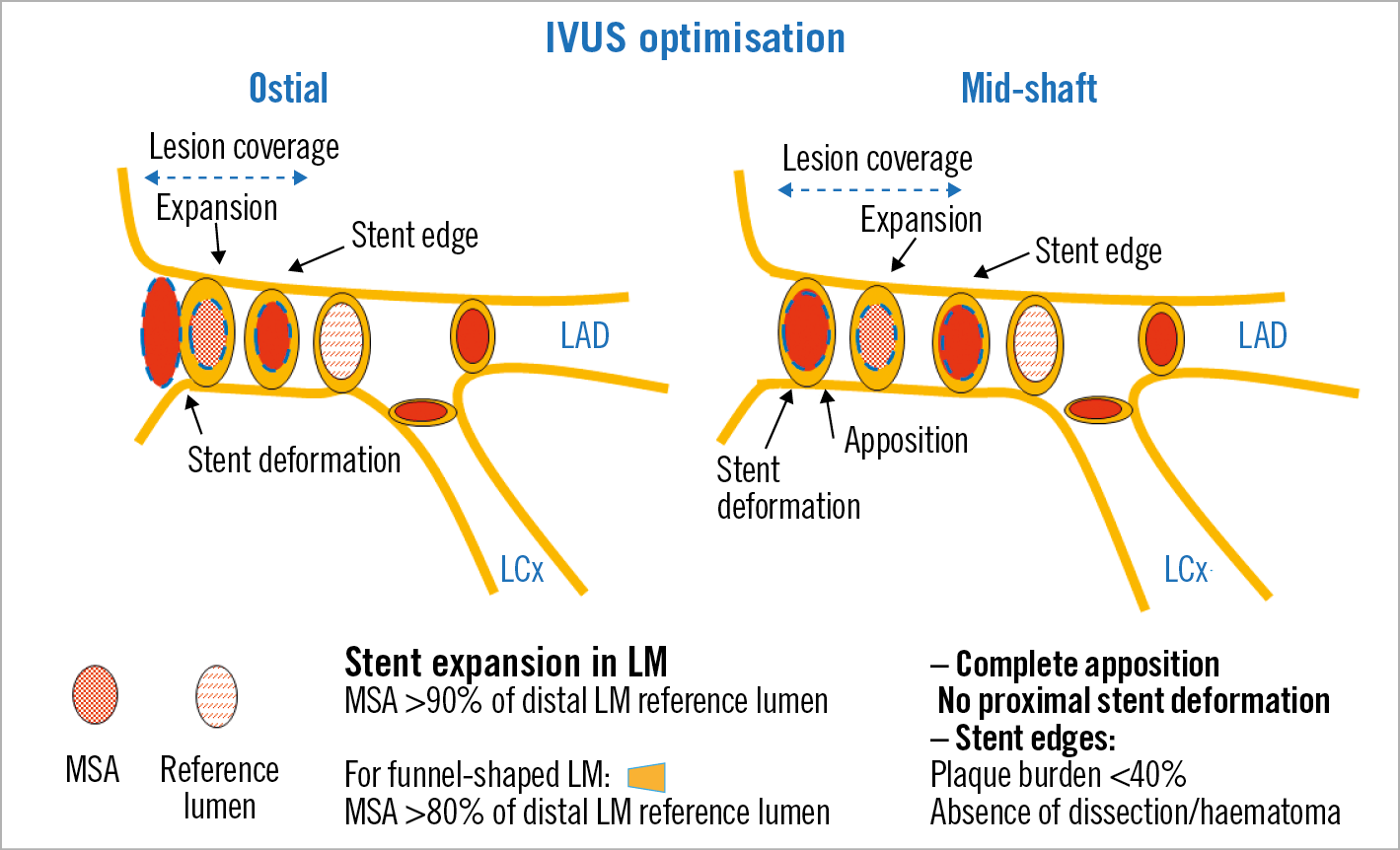
Figure 1. Optimisation targets for ostial and mid-shaft LM lesions.
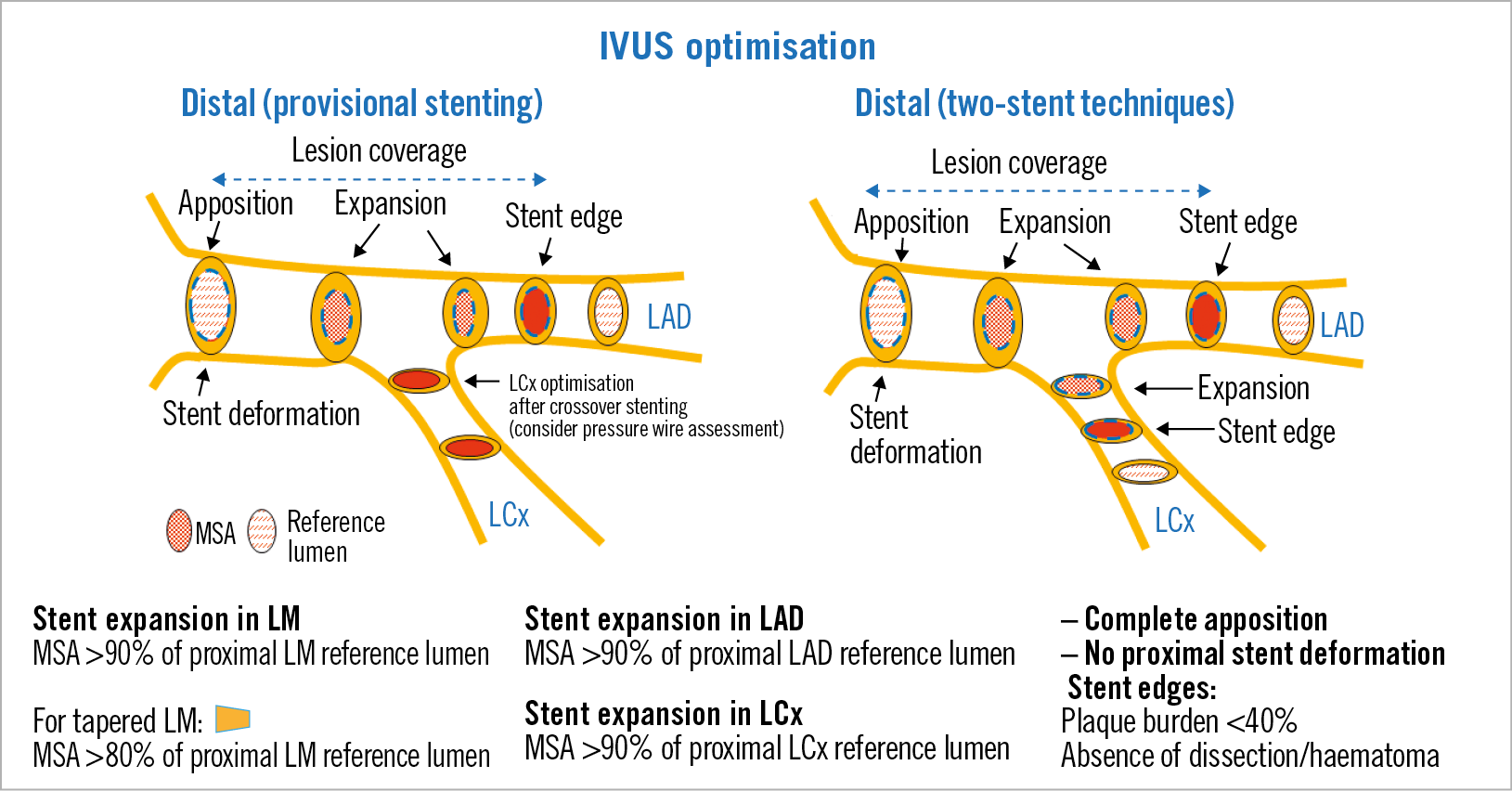
Figure 2. Optimisation targets for distal LM lesions.
A 12-month period of dual antiplatelet therapy was generally recommended during most of the study period; however, according to the more recently released clinical guidelines, the possibility of prescribing a six-month period became an alternative in the last period of the study, specifically in stable patients who did not require two stents and had a higher risk of bleeding.
There was no routine angiographic follow-up, unless clinically indicated by the referring cardiologist in the presence of symptomatic recurrence or the appearance of relevant ischaemia in non-invasive tests. For the clinical follow-up, telephone contact was made with all the patients and the data in the electronic health records for both in-hospital and out-of-hospital care were consulted.
The patients signed the specific informed consents for the procedures performed. The protocol was framed in healthcare practice and the approval of the institutional review board (IRB) was obtained for the execution of a prospective observational registry.
CONTROL GROUPS
Our multicentre research group has built a prospective database of patients with LM disease treated with DES which served as the basis for a previous publication that showed the association between IVUS guidance and better clinical outcomes4.
From this multicentre LM database, we selected as control groups those patients treated with non-first-generation DES before implementation of our IVUS protocol, using either IVUS guidance (415 patients) or only angiography (603 patients) (Figure 3). A propensity score-matching analysis was carried out to pair patients from these two groups with those of the IVUS-PRO group. With regard to the IVUS group from the multicentre database, a protocol with predefined optimisation criteria was not generally applied and in every single case all decisions were left to the operator’s judgement.
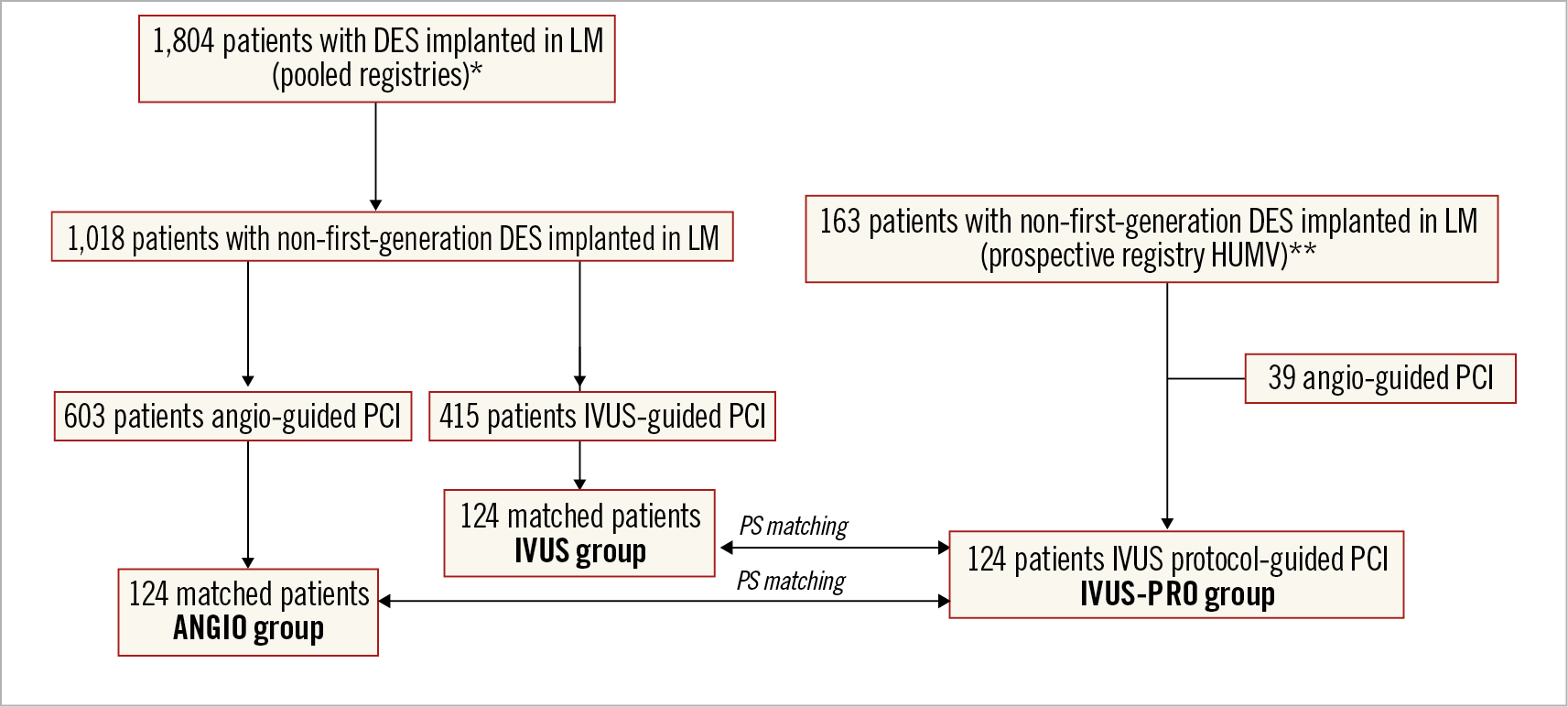
Figure 3. Flow chart of the study. * Pooled registries of patients undergoing LM PCI with DES from 2002 to 2013. ** Prospective registry in the Hospital Universitario Marques de Valdecilla (HUMV) of patients undergoing LM PCI with DES (January 2014 to March 2018).
ENDPOINTS
The primary endpoint was a composite of cardiac death, myocardial infarction (MI) (ST- or non-ST-elevation) related to the LM and target lesion revascularisation (TLR) in the LM. MI was linked to the LM lesion in either of the following circumstances: 1) LM lesion identified as culprit on angiography, based on stenosis severity/lumen morphology or intravascular imaging assessment and always considering clinical data; 2) electrocardiographic and/or echocardiographic findings suggestive of LM involvement with no confirmatory angiography available. Periprocedural MI was defined as an increase in CK-MB >10x URL, or >5x URL plus either 1) new pathological Q-waves in ≥2 contiguous leads or new left bundle branch block (LBBB), or 2) angiographically documented coronary artery occlusion or new severe stenosis with thrombosis, or 3) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality. TLR was defined as revascularisation for LM restenosis (>50%), also including proximal or distal segments (5 mm) adjacent to the stent or stents used for treatment of the lesion, and including the first 5 mm distal to the ostial circumflex artery if not stented. Any surgical revascularisation as the result of LM restenosis as previously defined was also considered a TLR. Definite or probable stent thrombosis at the LM site was considered according to the Academic Research Consortium definitions.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation or median (interquartile range) and categorical variables as percentages. Distribution was assessed for each variable with the Kolmogorov-Smirnov test. Accordingly, continuous variables were compared with the Student’s t-test if they followed a normal distribution and by Wilcoxon tests when this was not the case. The categorical variables were compared with the chi-square test or Fisher’s exact test, as required. Kaplan-Meier curves for event-free survival were obtained for each group and compared using the log-rank test and hazard ratios with 95% confidence intervals. A Cox proportional hazards multiple regression analysis was used to determine independent predictors of the primary outcome. The model included all variables that showed an association with the primary outcome in univariate analysis with a p-value <0.1. A propensity score-matching analysis was conducted (Supplementary Appendix 2) pairing patients in the three groups. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows, Version 24 (IBM Corp., Armonk, NY, USA).
Results
During the study period, from January 2014 to March 2018, a total of 124 patients underwent percutaneous revascularisation of the LM with DES guided by the IVUS protocol (IVUS-PRO group). The flow chart of the study is shown in Figure 3. These represented 76% of the patients undergoing PCI of the LM in that period, since in 39 patients IVUS was not used. These decisions were based on the operator’s judgement in each particular case and were definitely more related to the preferences of the operator than to the characteristics of the case.
By means of a propensity score matching with the IVUS-PRO group, two groups of 124 patients each were selected from the multicentre registry, the ANGIO group and the IVUS group. These groups showed clinical and angiographic baseline characteristics comparable to the IVUS-PRO group, but also between themselves, without significant differences being observed (Supplementary Table 1, Supplementary Table 2).
Regarding procedural aspects, pre-interventional IVUS was used in 87% of the cases in the IVUS-PRO group but only in 25% of the IVUS group. Among the former, in 12 cases IVUS examination was carried out after predilatation. The stents implanted were significantly larger in the two IVUS groups. Post-dilatation was more frequently used in the IVUS-PRO group and performed with larger balloons.
IVUS findings are shown in Table 1. At baseline in the IVUS group, the LM minimum lumen area (MLA) was larger and the plaque burden and the calcification arc smaller, which is explained by the fact that baseline IVUS examination was much less commonly performed in this group and mostly conducted in those cases with less angiographic severity (ambiguous stenosis). With regard to the procedural results, the LM minimum stent area (MSA) along with the LAD and LCx MSAs were significantly larger in the IVUS-PRO group. The IVUS-PRO optimisation targets were retrospectively applied in the IVUS group. All optimisation criteria, except for dissection/haematoma at the stent edges and longitudinal deformation, were more frequently met in the IVUS-PRO group.
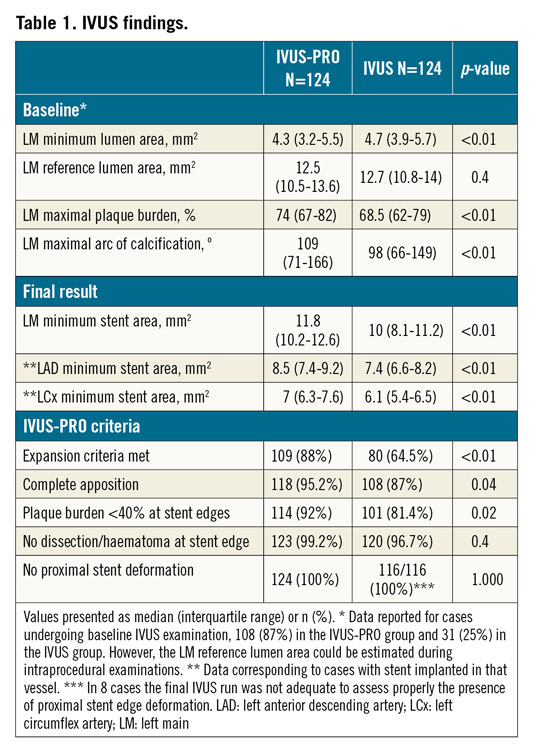
In the IVUS-PRO group, the optimisation criteria were fulfilled in the majority of patients, with the expansion having a certain lower compliance (88%), mainly due to the presence of heavily fibro-calcified lesions that limited the capacity of stent expansion either in the distal LM or in the ostium of branches. In a few cases, minor degrees of incomplete apposition were left, corresponding to distal LM lesions with a proximal landing site lumen >5.5 mm and a stent implanted from the LAD to the LM with a nominal diameter <4 mm. Overexpansion of these stents >2 mm over the nominal size was considered inappropriate. Only one case was left with a short (<2 mm) intimal dissection extending <45º. Stent deformation was detected during intraprocedural IVUS examinations in seven cases (5.6%) and was finally solved in all cases.
No patients were lost to follow-up. Survival curves for the composite primary endpoint are shown in Figure 4. There were significant differences between the IVUS-PRO and ANGIO groups but not between the IVUS and ANGIO groups. Survival free of TLR is shown in Figure 5, demonstrating a strong but not significant trend favouring the IVUS-PRO group. The reported adverse cardiovascular events are listed in Table 2. None of the periprocedural myocardial infarctions was related to the LM lesion but to lesions in other locations; these were caused by transient or permanent side branch occlusion, non-reflow phenomenon or distal thrombus embolisation.
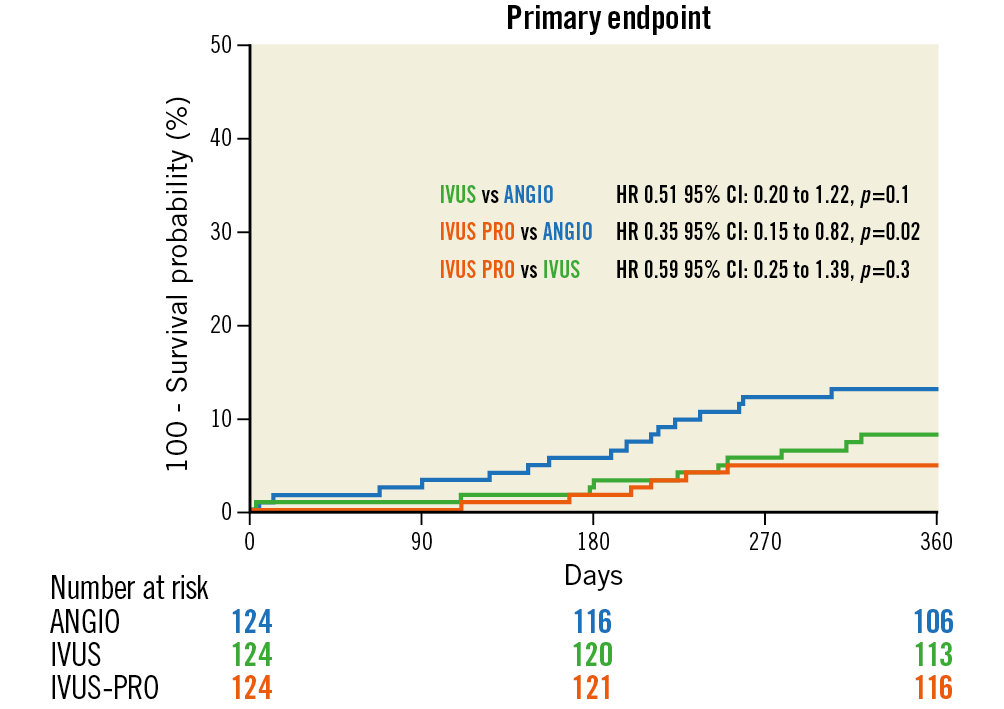
Figure 4. Outcomes of the study groups. Primary endpoint-free survival curves (composite of cardiac death, ST- or non-ST-elevation myocardial infarction related to the LM lesion and target lesion revascularisation in the LM).
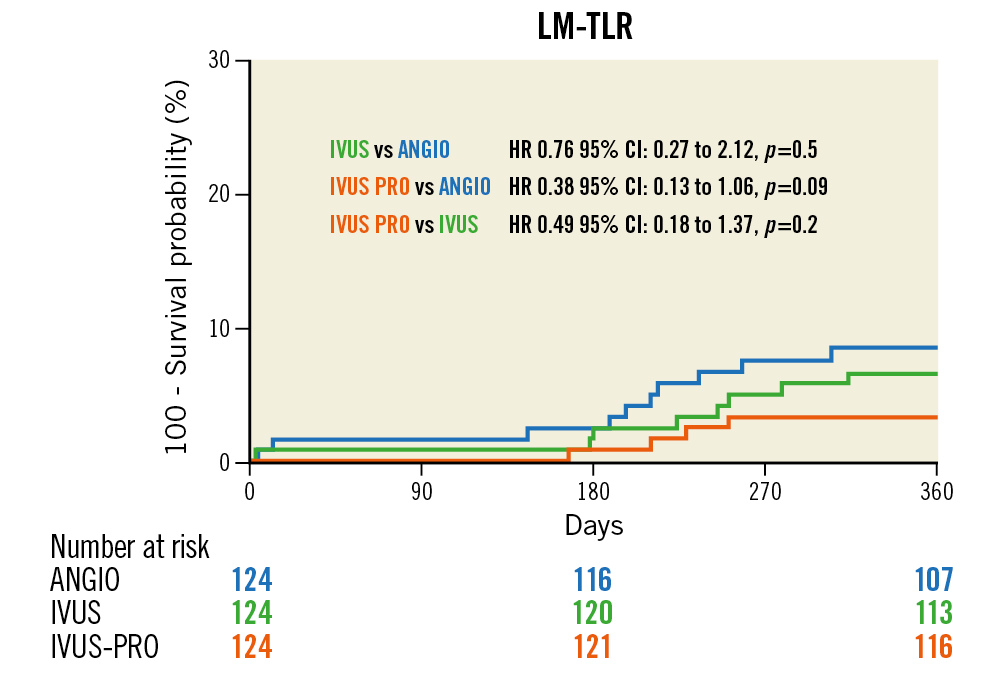
Figure 5. Outcomes of the study groups. LM lesion revascularisation-free survival curves.
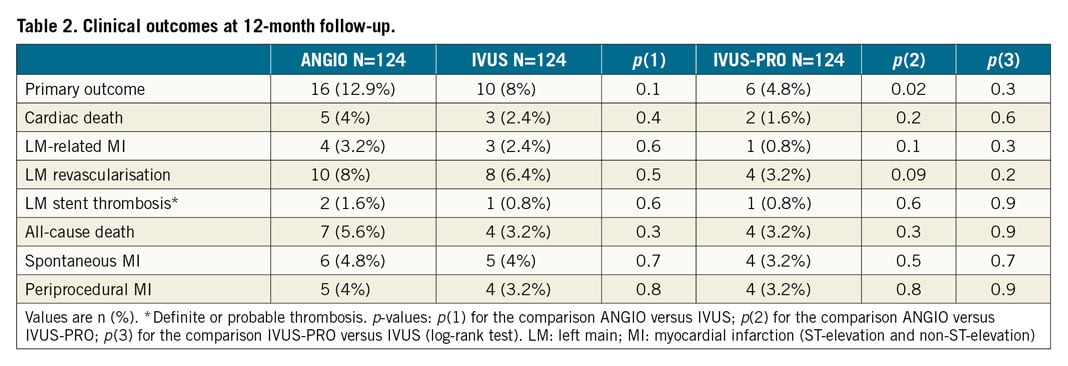
Among the four cases requiring TLR in the IVUS-PRO group, two were reported in the subgroup of 15 patients with suboptimal expansion (13.3%) and the other two in the optimal expansion subgroup (1.8%). LM revascularisation was performed in the ANGIO group in 10 cases and, among these, 6 presented effort angina (3 positive stress echo, 2 positive treadmill test and 1 no test conducted) and 4 an ACS. In the IVUS group, TLR was carried out in 8 cases and, among these, 5 presented effort angina (3 positive stress echo, 1 positive treadmill test, 1 nuclear stress test) and 3 an ACS. Finally, in the IVUS-PRO group, TLR was required in 4 cases and, among these, 3 presented effort angina (3 positive stress echo) and 1 an ACS.
Independent predictors for the primary outcome are listed in Table 3. The IVUS-PRO group resulted in being an independent predictor for a lower risk.
Discussion
The main findings of this study can be summarised as follows. 1) The application of a detailed protocol for the use of IVUS with predefined optimisation targets to guide PCI of the LM with new-generation DES is associated with better outcomes compared to the use of angiography alone. 2) The systematic use of a protocol with well-defined targets seems to provide an additional clinical benefit with respect to a non-protocolised use of IVUS.
Differences in the primary endpoint were primarily driven by differences in the TLR. This finding makes sense, since IVUS guidance is associated with better stent sizing and subsequent higher expansion rates, providing a larger in-stent lumen. Thus, the clinical event most sensitive to the procedural advantage linked to IVUS guidance is TLR, with infarction or death rates being affected to a lesser degree.
The use of IVUS to guide DES implantation in the LM provides a significant clinical advantage according to the multiple studies (mostly registries) conducted so far2,3,4,5,6,7,8,9,10,11,12. However, none of these previous studies prospectively evaluated a specific IVUS protocol with a set of PCI optimisation criteria.
Our protocol recommended the use of IVUS before PCI, so the planning of the optimal PCI strategy was facilitated, starting with adequate plaque modification techniques and correct stent sizing. Targets for stent expansion were established in the protocol. Stent underexpansion is a well-known major predictor of stent failure but there are no uniform criteria regarding recommended targets for PCI optimisation in clinical practice for the LM. The optimal IVUS MSA values for preventing in-stent restenosis in the LM were retrospectively assessed in 403 patients undergoing DES implantation18. These values were 5.0 mm2 for the LCx ostium, 6.3 mm2 for the LAD ostium, 7.2 mm2 for the distal LM, and 8.2 mm2 for the proximal LM. However, these cut-off values for the MSA, derived from studies carried out in an Asian population, are conditioned by the size of the coronary vasculature which, in turn, has ethnic and anthropomorphic determinants19,20. Therefore, the absolute values of MSA have a limited value as optimisation targets. With respect to the relative values of stent expansion, different targets for stent optimisation in overall coronary lesions have been proposed13. In the particular case of the LM, given its short length and clinical relevance, we thought it would be appropriate to choose a 90% expansion cut-off, which was modified (80%) according to the particular anatomy of the LM in each case17.
In our study 88% of cases met the expansion criteria. Accordingly, the LM MSA in the IVUS-PRO group was significantly larger than in the IVUS group. It is noteworthy that the average MSA values achieved in our study were higher than those reported in the aforementioned study18.
No clear link exists between acute malapposition (in the absence of underexpansion) and subsequent stent failure; however, in the case of the LM there are aspects that encouraged us to recommend correcting as far as possible the incomplete strut apposition. In our registry, very few cases were left with minor incomplete apposition. These were limited to those with a large disproportion of size between the implanted stent from the LAD to the LM and the size of the proximal LM (notable tapering). However, the magnitude of incomplete apposition was minor (<0.5 mm axial distance and <2 mm long) and considered to be benign21.
Regarding stent edges, avoidance of stent landing sites with plaque burden >40% appears to be clinically important, as this has been linked to subsequent stent edge restenosis following new-generation DES implantation22. Large edge dissections by IVUS have been reported as correlates of early stent thrombosis23, whereas minor edge dissections (only intimal, <45º and <2 mm in length) are unlikely to be clinically significant and possibly do not require correction24. Finally, the longitudinal deformation of the stent could be more frequent in LM procedures and may increase the risk of events and hinder future reinterventions on the left coronary artery25,26. Therefore, we took great care to recognise and, when required, tackle this phenomenon.
Limitations
This is an observational comparative registry with baseline differences between groups. Notwithstanding the use of a propensity score matching, it still remains possible that some unmeasured confounders could have had an effect on clinical outcome.
It is clear that a randomised trial would be the most appropriate design, but the optimisation criteria to apply in such an eventual trial (really complex and expensive to carry out) should be based on a previous prospective experience such as the one described here. In the meantime, the use of a protocol like this could be very helpful to the community of interventional cardiologists.
The sample size was limited and the study was underpowered for certain clinical outcomes. Nonetheless, significant prognostic differences emerged in favour of the IVUS-PRO group with the remarkable differences in post-procedural IVUS findings providing a rationale for these clinical differences.
The registry from which the IVUS group was extracted was not originally designed to assess the influence of different IVUS optimisation criteria on final clinical outcomes. Therefore, the IVUS criteria for stent sizing or identification and treatment of malapposition or underexpansion were mostly unknown. The decisions taken after IVUS examination in this control group were left to the operator’s judgement. Data regarding procedure duration, contrast medium volume and radiation were not available in all groups.
Conclusions
IVUS guidance of LM stenting provides a prognostic benefit with respect to the use of angiography alone, particularly when using a detailed IVUS protocol comprising a set of predefined optimisation criteria such as those considered in this study. These findings should be evaluated further in randomised controlled trials.
|
Impact on daily practice The use of IVUS may improve the prognosis of patients undergoing left main percutaneous revascularisation but there are no well-established criteria for optimisation. The application of an IVUS protocol with the predefined optimisation targets considered in this study for left main coronary artery revascularisation appears to improve outcomes with respect to the use of angiography alone or even with respect to the use of IVUS outside this protocol. |
Conflict of interest statement
J.M. de la Torre Hernandez reports receipt of grants/research support from Abbott, Biosensors, Bristol Myers Squibb, and Amgen, and receipt of honoraria or consultation fees from Boston Scientific, Medtronic, Biotronik, AstraZeneca, and Daiichi Sankyo. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

