
Abstract
Aims: The aim of this study was to compare the outcomes between multivessel and infarct-related artery (IRA)-only percutaneous coronary intervention (PCI) in patients with acute myocardial infarction (AMI), multivessel disease (MVD), and severe renal dysfunction (RD) using the nationwide AMI registry.
Methods and results: Among 13,104 patients, 537 diagnosed with AMI and MVD who had severe RD at presentation (estimated glomerular filtration rate [GFR] <30 mL/min/1.73 m2, mean: 19.1±7.5 mL/min/1.73 m2) and underwent PCI during index hospitalisation were selected. The patients were classified according to treatment strategy, i.e., multivessel PCI (49.0%) or IRA-only PCI. The primary endpoint was major adverse cardiac events (MACE), a composite of all-cause death, myocardial reinfarction, re-hospitalisation for heart failure, and any repeat revascularisation at one year. The safety outcome was the worsening of renal function (WRF), defined as a 30% reduction in estimated GFR from baseline to 12-month follow-up. The adjusted MACE risks were similar in groups after Cox regression (41.8% vs 39.8%, hazard ratio [HR] 1.008 [0.743-1.367]) and propensity score-matching analysis (HR 0.974 [0.651-1.377]). Multivessel PCI showed a significant tendency of higher rates of WRF (24.8% vs 11.1%, adjusted odds ratio 2.134 [0.976-4.668]).
Conclusions: Multivessel PCI was associated with similar outcomes compared to IRA-only PCI in patients with AMI, MVD, and severe RD.
Introduction
In contemporary practice, among patients with ST-segment elevation acute myocardial infarction (STE-AMI) in need of primary percutaneous coronary intervention (PCI), between 40% and 65% have concurrent multivessel disease (MVD), which has been associated with worse clinical outcomes1. Although the findings suggesting a beneficial effect of multivessel PCI in recently published randomised trials have led to changes in major cardiology societies’ guidelines, now supporting multivessel intervention (Class IIa, level of evidence A)2, patients with severe renal dysfunction (RD) were excluded in the majority of the trials reported to date3,4,5,6. These patients with severe RD exhibit highly complex and different pathogenic processes of coronary arterial luminal narrowing compared with individuals with normal renal function; some treatments have no definite clinical benefit, similar to statins in individuals with end-stage RD7. Thus, whether multivessel PCI should be conducted during index hospitalisation in patients with AMI, MVD, and severe RD remains controversial.
Therefore, we aimed to compare the clinical outcomes between multivessel PCI and infarct-related artery (IRA)-only PCI in patients with AMI who had MVD accompanied by severe RD using a large-scale, nationwide, multicentre, dedicated registry for AMI.
Methods
STUDY PROTOCOL AND POPULATION SELECTION
The study population was derived from the nationwide, multicentre, prospective Korean Acute Myocardial Infarction Registry-National Institutes of Health (KAMIR-NIH). The KAMIR-NIH is a dedicated prospective, web-based observational cohort study that consecutively enrolled patients diagnosed with AMI who were eligible for primary PCI at 20 tertiary university hospitals from November 2011 to December 2015 without any exclusion criteria8. The protocol of the KAMIR-NIH was approved by the ethics committee at each participating centre and all patients provided written informed consent upon enrolment. Among 13,104 patients who were enrolled in the KAMIR-NIH, those with AMI and MVD who also presented with severe RD and underwent PCI were selected. Patients were excluded from the analysis if they underwent thrombolysis before PCI, presented in cardiogenic shock, had single-vessel disease, or were lost to follow-up before one year. Finally, 537 patients were selected for this analysis. These patients were then classified according to treatment strategy (i.e., multivessel PCI or IRA-only PCI) (Figure 1). Patients who underwent non-IRA PCI at the time of primary PCI or within index hospitalisation were included in the multivessel PCI group.
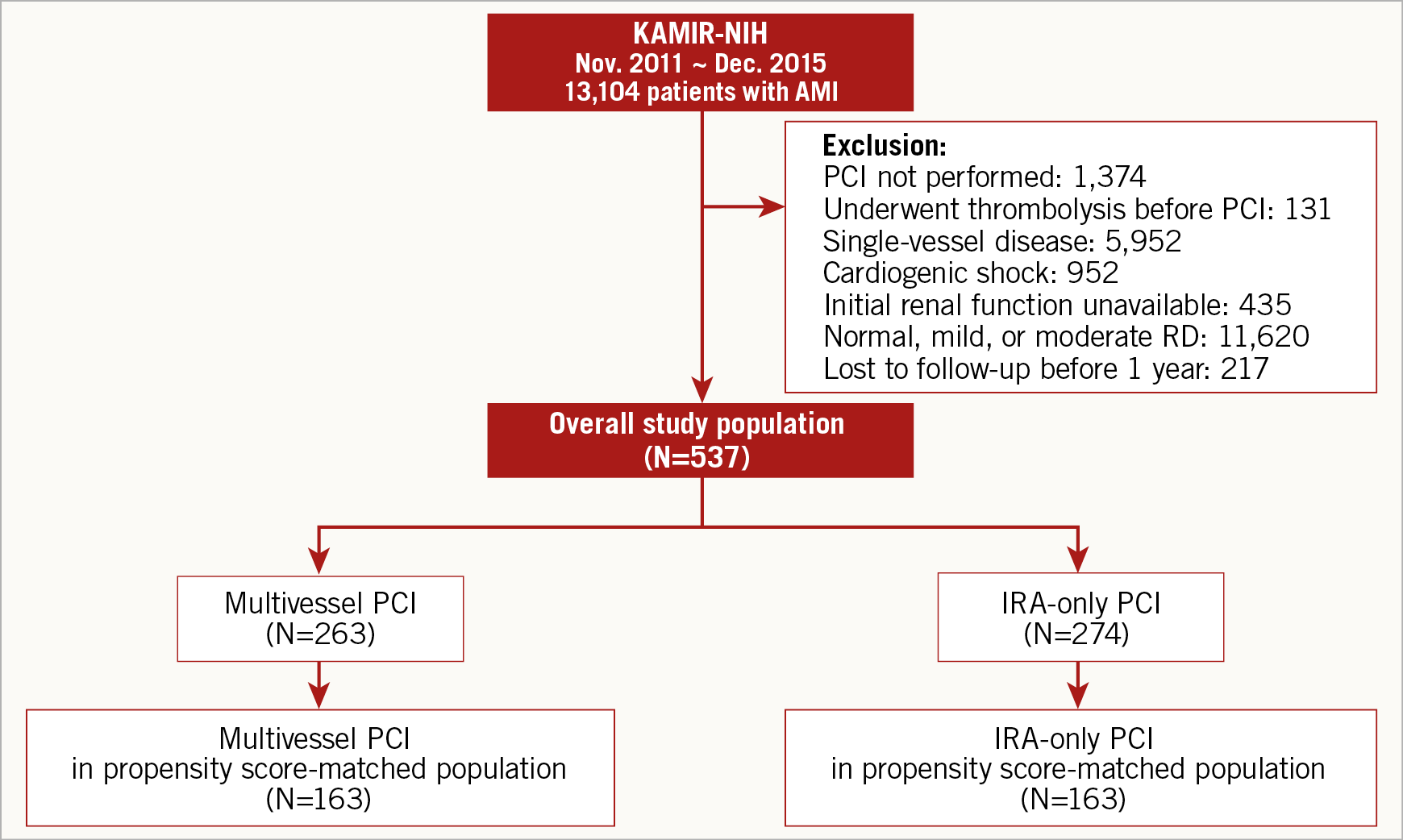
Figure 1. Study flow. AMI: acute myocardial infarction; IRA: infarct-related artery; KAMIR-NIH: Korean Acute Myocardial Infarction Registry-National Institutes of Health; PCI: percutaneous coronary intervention; RD: renal dysfunction
DEFINITIONS
The specific definition of AMI is presented in Supplementary Appendix 1. The glomerular filtration rate (GFR) was used to determine renal function. Under steady-state conditions, the GFR can be estimated from the serum creatinine using the Cockcroft-Gault formula. Severe RD was defined as an estimated GFR of <30 (mL/min/1.73 m2)9. MVD was defined as ≥50% diameter stenosis in at least one major non-IRA or in the left main coronary artery, as in previous trials10. PCI was considered successful if the final residual stenosis was <30% with Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow.
PATIENT MANAGEMENT, DATA COLLECTION, AND FOLLOW-UP
Patient management was performed in accordance with the current standard AMI guidelines. The patients who underwent PCI received 300 mg aspirin and 600 mg clopidogrel, 180 mg ticagrelor, or 60 mg prasugrel before PCI. Unfractionated heparin (50 to 70 U/kg) was administered before or during PCI to maintain an activated clotting time of 250 to 300 seconds. Unless there was an acknowledged reason for discontinuing dual antiplatelet therapy, all patients were recommended to take 100 to 300 mg aspirin indefinitely in addition to 75 mg clopidogrel or other potent antiplatelet agents, such as 10 mg prasugrel once daily or 90 mg ticagrelor twice daily, for ≥1 year. The choice of the prescribed P2Y12 inhibitor was left to the operator’s discretion according to the guidelines and the patients’ bleeding risk. Medications, including renin-angiotensin-aldosterone system blockers, beta-blockers, and statins, were also recommended in accordance with the practice guidelines. All data were collected by independent clinical research coordinators using a web-based case report form in the internet-based Clinical Research and Trial (iCReaT) management system, a data management system established by the Centers for Disease Control and Prevention, Ministry of Health and Welfare, Republic of Korea (iCReaT Study No. C110016). Clinical events that occurred during hospitalisation and within the one-year follow-up were examined.
STUDY ENDPOINTS
The primary study endpoint was major adverse cardiac events (MACE) within 12 months, a composite of all-cause death, myocardial reinfarction, re-hospitalisation for heart failure, and any repeat revascularisation at one year. The secondary endpoints were the individual components of MACE, non-IRA repeat revascularisation, and definite or probable stent thrombosis at one year. All clinical outcomes were defined in accordance with the Academic Research Consortium11. In addition, the safety outcome was the worsening of renal function (WRF), defined as a 30% reduction in estimated GFR from baseline to 12-month post-AMI follow-up.
STATISTICAL ANALYSIS
Cox proportional hazard regression in a propensity score-matched population was performed. A multivariable logistic regression model was used to generate the propensity scores, which indicate the probability of the patients being treated with the multivessel PCI strategy. All available covariates were included in this model, following the recommendations for analysis using the propensity score exactly12. For the propensity score matching, a 1:1 matching process without replacements was performed using a greedy algorithm with a calliper width of 0.2 standard deviations, yielding 163 patients in the multivessel PCI group matched with 163 controls in the IRA-only PCI group. Balance between the two groups after propensity score matching was assessed by calculating the percent standardised mean differences in the covariates used in the propensity score generation. The percent standardised mean differences after propensity score matching were within ±10% across all matched covariates, demonstrating successful balance achievement between the groups (Supplementary Table 1). An extended description of the statistical analysis is presented in Supplementary Appendix 1.
Results
Of the total 13,104 patients who were enrolled in the KAMIR-NIH, 7,152 subjects had multivessel disease. Among them, 537 subjects were selected for the study: 263 (49.0%) underwent multivessel PCI, and 274 underwent IRA-only PCI. The baseline clinical characteristics, including medications at discharge, are summarised in Supplementary Table 2. The multivessel PCI group had a higher body mass index and a lower prevalence of previous cerebrovascular disease. The proportion of patients with non-STE-AMI at presentation was higher; the left ventricular ejection fraction (%) on echocardiography was lower in the multivessel PCI group than in the IRA-only PCI group. The baseline estimated GFR was 19.1±7.5 mL/min/1.73 m2; there was no difference in the baseline estimated GFR between the groups. Also, the groups did not differ in terms of the levels of evidence-based medications taken to treat AMI (antiplatelets, beta-blockers, renin-angiotensin-aldosterone system blockers, and statins).
Supplementary Table 3 shows the angiographic and procedural features of all patients. The left main artery as the culprit vessel was more prevalent in the multivessel PCI group. Among the 263 patients in the multivessel PCI group, 205 (77.9%) underwent immediate non-IRA PCI, and 58 (22.1%) underwent staged non-IRA PCI during the same hospitalisation. The patients of the multivessel PCI group underwent less thrombus aspiration and received fewer stents. Based on the angiographic assessment findings, 56.7% of the multivessel PCI cases were classified as complete revascularisation without residual stenosis, and the remaining 43.3% were classified as incomplete revascularisation.
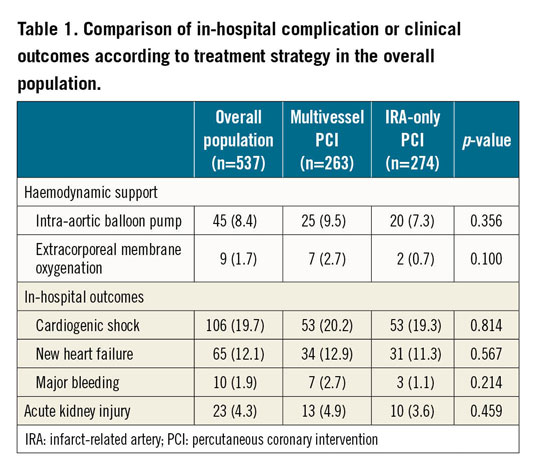
We found no significant between-group differences in the incidence of in-hospital complications (Table 1), although the multivessel PCI group patients received more haemodynamic support.
The primary endpoint was achieved in a total of 219 (40.8%) patients at a median follow-up of 362 days (interquartile range, 286-383 days). A comparison of the clinical outcomes between the multivessel PCI and IRA-only PCI groups is presented in Table 2. The incidence of MACE and all-cause death was comparable between the groups (Figure 2). Further, myocardial reinfarction, all-cause death or myocardial reinfarction, re-hospitalisation for heart failure, any repeat revascularisation, non-IRA repeat revascularisation, and definite or probable stent thrombosis did not differ significantly between the multivessel PCI group and the IRA-only PCI group (Figure 3).
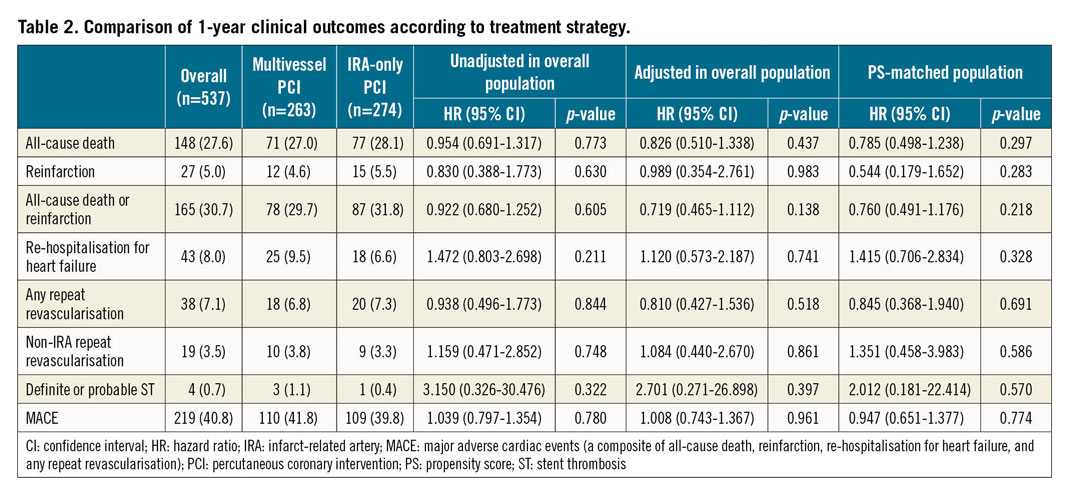
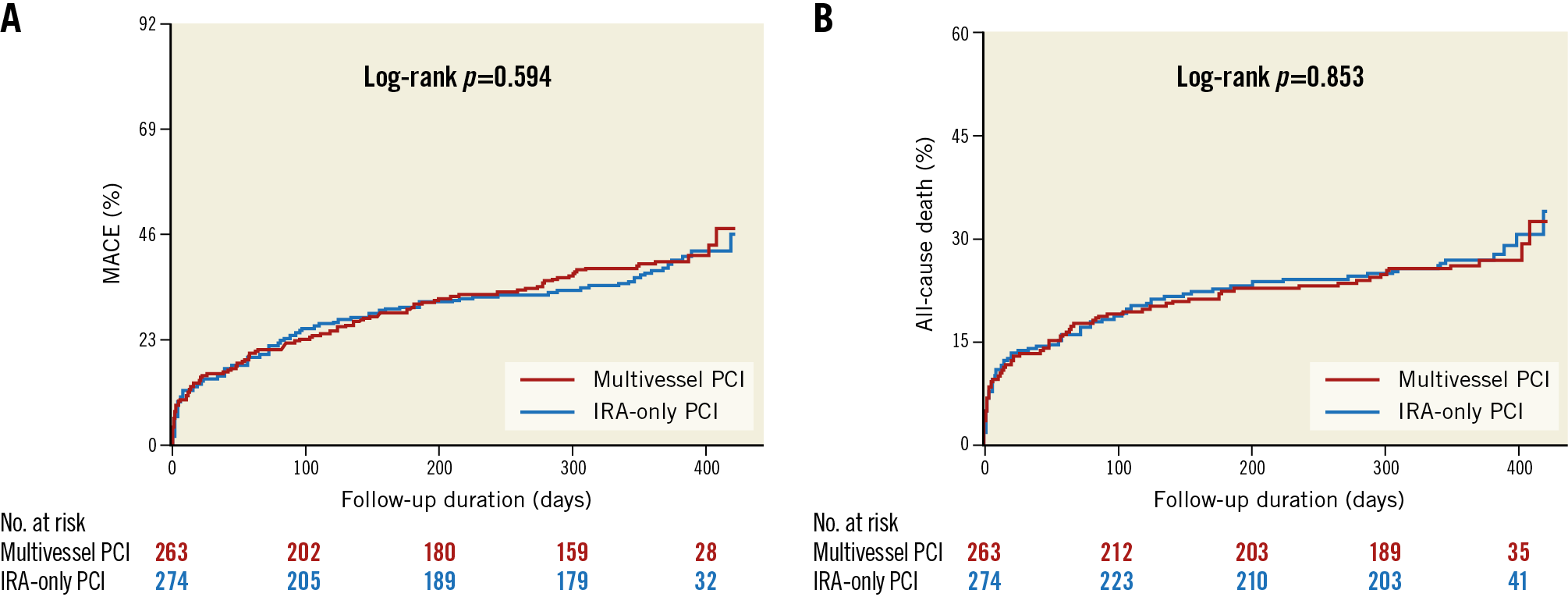
Figure 2. Cumulative incidence of MACE and all-cause death. Kaplan-Meier curves with cumulative hazards of (A) MACE and (B) all-cause death compared according to the PCI strategy. IRA: infarct-related artery; MACE: major adverse cardiac events; PCI: percutaneous coronary intervention
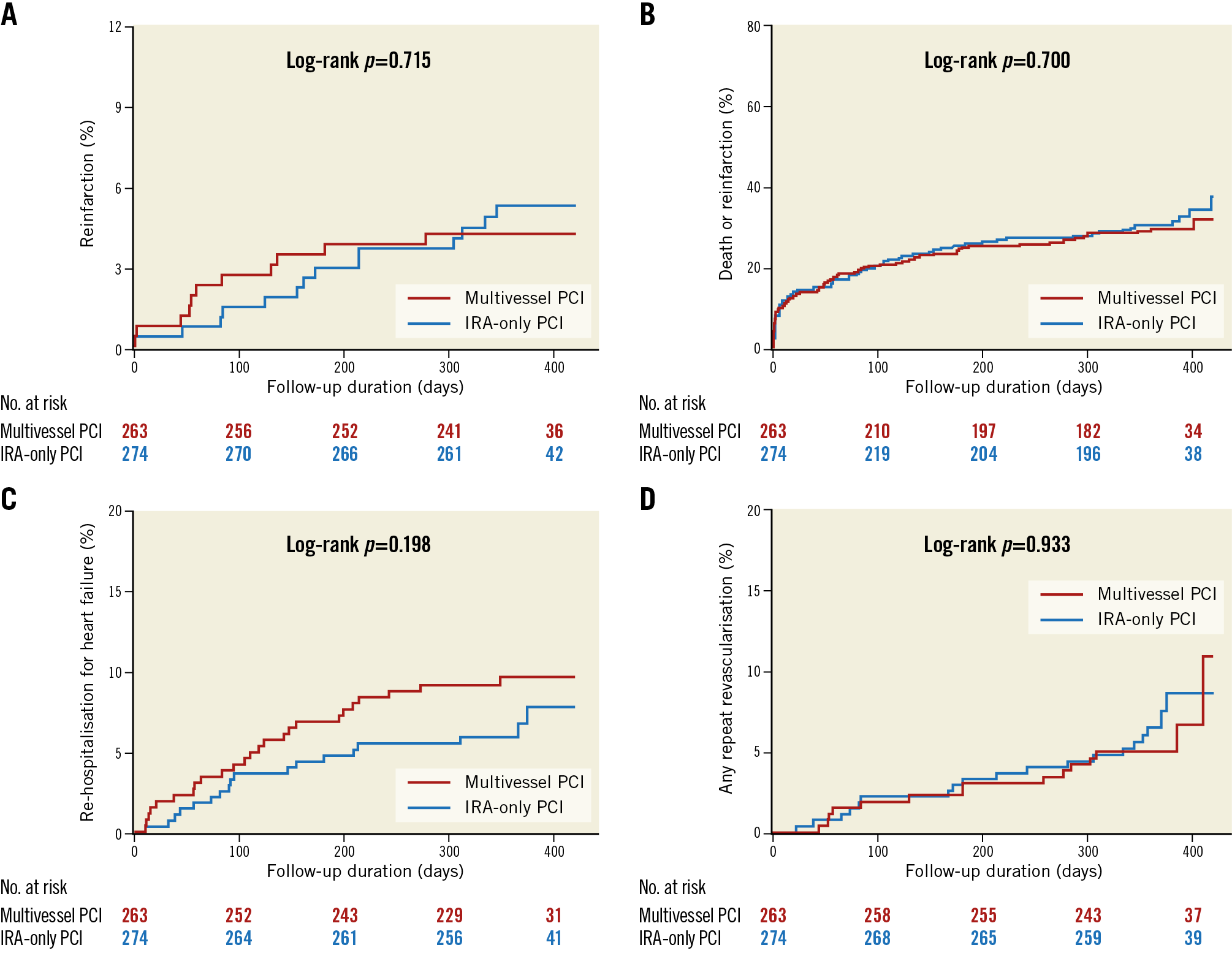
Figure 3. Cumulative incidence of individual clinical outcomes. Cumulative incidence of individual clinical outcomes including components of the composite outcomes (A) reinfarction, (B) all-cause death or reinfarction, (C) re-hospitalisation for heart failure, and (D) any repeat revascularisation. IRA: infarct-related artery; MACE: major adverse cardiac events; PCI: percutaneous coronary intervention
Multivariable Cox regression analysis (Table 2) revealed that the MACE were similar regardless of the initial PCI strategies. The adjusted risks for all-cause death, myocardial reinfarction, all-cause death or myocardial reinfarction, re-hospitalisation for heart failure, any repeat revascularisation, non-IRA repeat revascularisation, and definite or probable stent thrombosis were also similar in the two groups, irrespective of the initial PCI strategy.
After 1:1 propensity score matching, 163 patients were generated in each group. The C-statistic value for the propensity score model was 0.789 (95% confidence interval [CI]: 0.751-0.827), indicating good discrimination. There were no significant differences in the baseline clinical, angiographic, or procedural characteristics of the propensity score-matched cohort (Supplementary Table 1). A total of 124 (38.0%) MACE occurred during follow-up in the matched population (Supplementary Table 4). Sensitivity analyses using propensity score-matching adjustment consistently showed that the risks of all clinical outcomes were comparable between the multivessel PCI and IRA-only PCI groups (Table 2).
Figure 4 presents the prognostic impact of multivessel PCI among the various subgroups. In the subgroup analysis, the similar risk of MACE between the multivessel PCI group and IRA-only PCI group was consistent across all subgroups without significant interaction p-values (elderly patients aged >70 years, women, patients with diabetes mellitus, patients with STE-AMI, patients with left ventricular ejection fractions of <40%, left main or left anterior descending artery as the culprit vessel, procedure for complex lesions [American College of Cardiology/American Heart Association (ACC/AHA) lesion classification type B2/C], and patients with three-vessel disease). Next, we examined whether the two different types of AMI, STE-AMI versus non-STE-AMI, were independently affected according to PCI strategy regarding clinical outcomes (Supplementary Table 5, Supplementary Table 6).
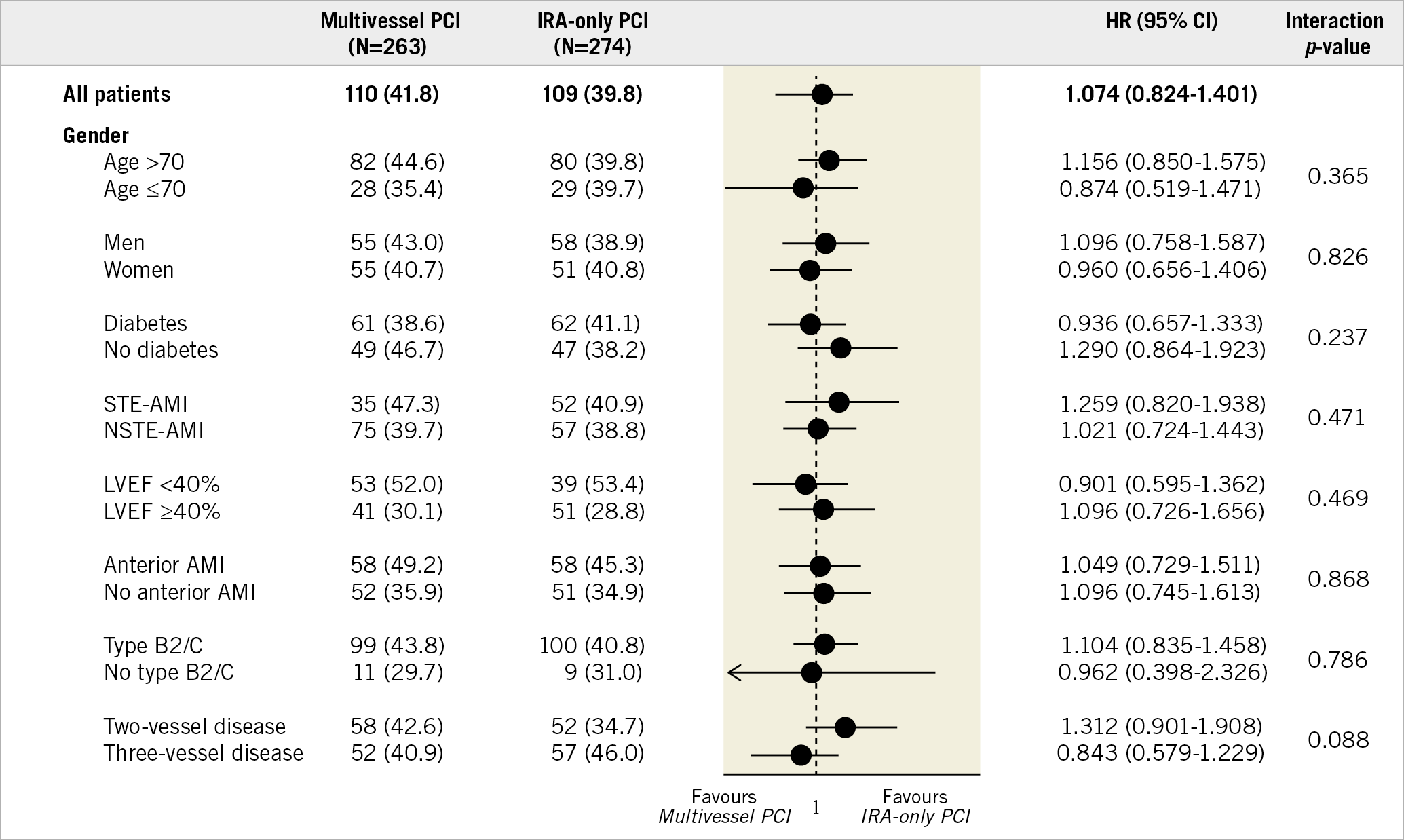
Figure 4. Exploratory subgroup analysis for MACE. AMI: acute myocardial infarction; CI: confidence interval; HR: hazard ratio; IRA: infarct-related artery; LVEF: left ventricular ejection fraction; MACE: major adverse cardiac events; NSTE-AMI: non-ST-segment elevation acute myocardial infarction; PCI: percutaneous coronary intervention; STE-AMI: ST-segment elevation acute myocardial infarction
The multivariable Cox proportional hazards models identified the independent predictors of MACE and all-cause death (Supplementary Table 7). Multivessel PCI was not independently associated with a decreased risk of MACE (adjusted hazard ratio [HR] 1.008, 95% CI: 0.743-1.367; p=0.961) and all-cause death (adjusted HR 0.826, 95% CI: 0.510-1.338; p=0.437) at one year.
Among the 537 patients analysed in the study, 213 had complete estimated GFR data sets at both baseline and the 12-month follow-up. The mean estimated GFR increased by 4.47 mg/dL (95% CI: 2.55-6.40; p<0.001). As regards the safety outcome, the overall incidence of WRF was 17.8% (38/213); the multivessel PCI group showed a significant tendency of having higher event rates of WRF compared with the IRA-only PCI group, even after adjustment for age, body mass index, diabetes, left ventricular ejection fraction, acute kidney injury during index hospitalisation, and type of treatment (multivessel PCI versus IRA-only) (24.8% vs 11.1%, adjusted odds ratio [OR] 2.134, 95% CI: 0.976-4.668; p=0.058).
Discussion
In the present study, we analysed 537 patients with AMI who had MVD and severe RD and underwent PCI in order to evaluate the differential prognostic impact between multivessel PCI and IRA-only PCI. The main findings of this study were as follows: there was no difference of outcomes between multivessel and IRA-only PCI among the population with severe RD at a median follow-up of 12 months. In addition, this finding was consistent even in the multiple sensitivity analyses, including the Cox proportional hazards regression in the propensity score-matched cohort, and even across the various exploratory subgroup analyses.
Based on the results of recent randomised controlled trials3,4,5,6, the Task Force on Myocardial Revascularisation of the European Society of Cardiology issued guidelines stating that one-stage multivessel PCI during STE-AMI without cardiogenic shock should be considered in patients in the presence of multiple, critical stenoses or highly unstable lesions2. However, evidence-based studies on the benefits of multivessel PCI in patients with AMI and severe RD are lacking because randomised trials often exclude those with severe RD. Therefore, the findings from these randomised clinical trials cannot be applied to patients with severe RD. As the guideline recommendations already vary according to the presence or absence of cardiogenic shock2,10,13, further individualisation of the treatment strategy may require adaptation in certain clinical circumstances, especially in patients with high-risk features, such as severe RD.
Theoretically, treatment of the non-IRA may not only lower the risk of further repeat revascularisation, but also improve the clinical outcomes by assisting the recovery of myocardial perfusion and ventricular function. However, our study was not able to determine the better treatment strategy between multivessel PCI and IRA-only PCI for patients with severe RD accompanied by AMI and MVD. The lack of difference between the two treatment strategies in this study might be due to several reasons. Firstly, the higher dose of contrast medium in the multivessel PCI group may have led to acute left ventricular volume overload with a negative effect on myocardial function and recovery. Secondly, the prolonged duration of the multivessel PCI procedure may be especially hazardous at a time when the patient is haemodynamically compromised, leading to a potentially higher incidence of bleeding and inflammation. Thirdly, additional myocardial damage may have been induced by PCI in stable lesions. Finally, we speculate that the lack of difference between the multivessel and IRA-only PCI might be related to the significantly higher dose of contrast material that was used in the multivessel PCI group and the consequent decline in renal function. Although estimated GFR data at the 12-month follow-up were available only in ~40% of the patients, the overall incidence of WRF tended to be higher in the multivessel PCI group. This may be explained by the potential risk of contrast-induced nephropathy after a greater contrast load in those undergoing multivessel PCI. The CULPRIT-SHOCK trial also showed that, among patients with AMI and cardiogenic shock, the rate of renal replacement therapy tended to be lower in the culprit lesion-only PCI group than in the multivessel PCI group13. Because of these hazards, it is possible that the additional benefits of the multivessel intervention at the time of index hospitalisation in these patients might be offset.
Considering the higher incidence of multiple comorbidities and the sequentially higher death rate in the population with RD, however, a high proportion of patients might die earlier before the potential benefit of multivessel PCI is realised. Therefore, further evaluation using biomarkers of renal function, inflammation, and myocardial damage from the central core laboratory, as well as detailed angiographic analyses, could be performed to elucidate the potential underlying mechanisms for these clinical outcomes. Compared to the general population, patients with RD are susceptible to an increased risk of periprocedural complications and are also more likely to have diffuse coronary artery disease that may be challenging for revascularisation. These facts may cause clinicians to consider a conservative management approach instead of revascularisation in patients with RD. Previous data suggest that aggressive therapy is underutilised in patients undergoing dialysis who experience AMI14.
Study limitations
The present study provides novel insight because it considered exclusively patients with AMI and severe RD using the outcomes of revascularisation performed by a variety of cardiologists in major PCI centres around Korea. However, our results should be viewed in the context of several important limitations. Firstly, although we used various analytical methods, including propensity score matching, to address possible confounding factors, this study has all the limitations of a registry, and residual confounding cannot be excluded. In addition, these data did not capture physician rationale for the selection between multivessel and IRA-only PCI, specifically the multivessel intervention in patients with STE-AMI in an era when multivessel PCI for STE-AMI was assigned a class III recommendation by the ACC/AHA guidelines during the KAMIR-NIH study period. Thus, we cannot draw definitive conclusions. The findings of this study should be considered as hypothesis-generating and warrant prospective evaluation in adequately powered and randomised controlled trials. Secondly, patients who underwent a planned staged revascularisation after discharge could not be identified from the database and, in this study, such patients would be classified into the IRA-only PCI group followed by a repeat revascularisation event. Thirdly, we assessed the lesion severity of the non-IRA patients using angiographic assessment alone. As shown in the DANAMI-3—PRIMULTI and COMPARE-ACUTE trials, nearly one half of visually significant non-IRA lesions were physiologically insignificant, with fractional flow reserve values of >0.805,6. Also, we do not have data on the SYNTAX score or the severity of the non-IRA diameter stenosis, which would be important for the clinical outcomes following complete revascularisation, as shown in the previous study15. Fourth, clinical events were not centrally adjudicated in this registry. Fifth, procedure-related risk factors, such as procedure time, total radiation dose, and amount of contrast dye used, were not evaluated. If data regarding the elapsed time and dose of contrast media used during PCI were available, we might be able to understand the relationship between these parameters and the incidence of complications better; however, this information was unavailable. Finally, definition of the type of lesion (culprit versus non-culprit) did not follow a protocol, and it is therefore likely that this decision varied according to how the interventional cardiologist interpreted the angiographic results at the time of coronary angiography. Given how difficult it can be to identify the culprit artery in patients with non-STE-AMI and MVD, it cannot be ruled out that incorrect identification may have influenced the results.
Conclusions
In the present study, there was no difference in the clinical outcomes between multivessel and IRA-only PCI among the population with AMI and severe RD at a median follow-up of 12 months. Rather, multivessel PCI might be related to an increased incidence of renal complications during follow-up. Additional data may be required. Until the results are available, interventional cardiologists have to make their decisions based on the findings of their clinical evaluation of the patients, and taking into account clinical characteristics, disease severity, and lesion complexity.
|
Impact on daily practice In the present study, multivessel PCI at the time of primary PCI or within the index hospitalisation showed no difference in clinical outcomes compared with IRA-only revascularisation in patients with AMI and severe RD. Additional data may be required. Until the results are available, interventional cardiologists have to make their decision based on the findings of their clinical evaluation of the patients, and taking into account clinical characteristics, disease severity, and lesion complexity. |
Funding
This research was supported by a fund (code 2016-ER6304-01) for research from the Korea Centres for Disease Control and Prevention.
Conflict of interest statement
The authors have no conflicts of interest to declare.

