Abstract
A significant proportion of patients presenting with acute coronary syndromes (ACS) have multivessel disease (MVD). Despite the abundance of clinical trials in this area, several questions regarding the procedure of complete coronary revascularisation remain unanswered. This state-of-the-art review summarises the latest evidence on complete revascularisation (CR) in this subset of patients and critically appraises clinical decision making based on non-culprit lesion (NCL) assessment. Future areas of research are put into perspective.
Introduction
In a substantial proportion of patients with acute coronary syndromes (ACS), the pathophysiological process of coronary artery disease (CAD) is not limited to one single vessel, and multivessel disease (MVD) can be found in 50% of the cases1,2.
Complete revascularisation (CR) has been associated with decreased risk of composite outcomes driven mainly by reduced subsequent revascularisations, with recent studies supporting the benefit of non-culprit lesion (NCL) percutaneous coronary intervention (PCI) on hard clinical endpoints3,4,5,6,7. The optimal timing for the treatment of NCLs and how to identify the amenable lesions are still matters of debate. Furthermore, whether the goal of CR should be the treatment of ischaemia-related lesions or vulnerable plaques prone to thrombosis has yet to be determined. Despite the evidence supporting the safety of deferred PCI in patients with ACS on the basis of pressure-derived measurements, the reliability of physiological assessment of NCLs in the acute phase of ACS has not yet been defined5,6,8,9,10,11. The presence of chronic total occlusions (CTO) among NCLs may dramatically impact on the prognosis of those patients and imposes a tailored decision-making strategy12. For cardiogenic shock (CS) patients presenting with MVD (nearly 80%), the available data are highly controversial and the treatment strategy remains extremely challenging13.
Overall, this review provides a comprehensive assessment of the latest results of randomised controlled rials (RCTs), highlights the current guideline recommendations and sheds light on future directions for the treatment of patients with ACS and MVD.
Multivessel disease in patients with ACS presenting with ST elevation
The primary objective of PCI in ST-elevation myocardial infarction (STEMI) patients is to restore epicardial flow in the culprit vessel and myocardial perfusion14.
Despite robust data favouring CR, the optimal timing for NCL revascularisation has still to be determined14.
In the PRAMI trial, angiography-guided CR yielded a 65% reduction in the composite primary endpoint when compared to culprit-only revascularisation during the index procedure3. The CvLPRIT trial randomised STEMI patients with MVD to a culprit-only strategy or to an angiography-guided CR approach. CR, performed either at the time of index procedure (64%) or before hospital discharge (36%), resulted in a 55% reduction of the primary composite endpoint of all-cause mortality, recurrent myocardial infarction (MI), heart failure, and ischaemia-driven revascularisation at 12 months of follow-up4.
A fractional flow reserve (FFR)-guided CR approach versus culprit lesion-only treatment was investigated in DANAMI-3-PRIMULTI and in the COMPARE-ACUTE trial. In both trials, CR was found to be associated with a benefit in terms of the composite primary endpoint, driven largely by the need for repeat revascularisation5,6.
The COMPLETE trial is by far the largest and the only powered study to address hard endpoints7. More than 4,000 patients were randomised. Angiography-guided CR (with FFR performed in 50 to 69% stenosis) proved to reduce the composite outcome of cardiovascular (CV) death and MI at a median of 36 months of follow-up (7.8% in the CR group vs 10.5% in the culprit-only group – hazard ratio [HR] 0.74, 95% confidence interval [CI]: 0.60 to 0.91; p=0.004)7. Data from these five trials are summarised in Supplementary Table 1.
The benefit of CR in terms of CV mortality was confirmed in a recent meta-analysis based on 10 RCTs and 7,030 patients. A reduced CV death and MI rate (both as combined and as individual endpoints) was found, regardless of whether immediate or staged CR was performed. The results were consistent whether an FFR-guided or an angiography-guided approach was performed15.
KEY MESSAGE
RCTs and meta-analyses support the benefit of CR in STEMI patients with MVD, regardless of the mode of selection and the timing of NCL treatment (Figure 1).
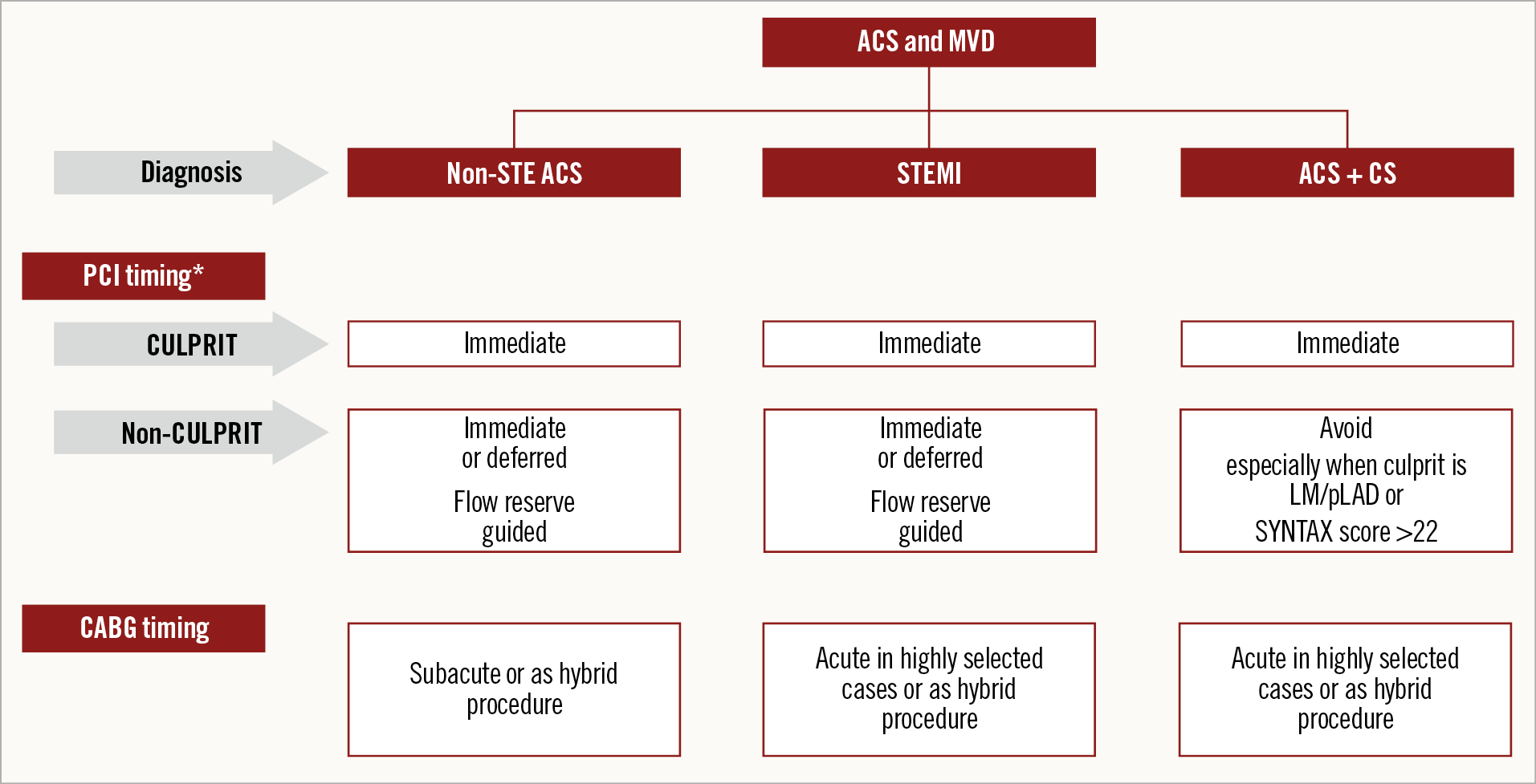
Figure 1. Schematic representation of proposed revascularisation strategies in patients with ACS and MVD. *PCI timing is relative to the time of coronary angiography. ACS: acute coronary syndromes; CS: cardiogenic shock; MVD: multivessel disease
Multivessel disease in patients with ACS presenting without ST elevation
Contrary to the case for STEMI where reperfusion of the infarct-related artery was established as a therapeutic goal in the 1980s, the treatment of patients presenting with NSTEMI was more conservative, with antithrombotic therapies and optimal medical therapy. Early studies probably classified a large proportion of patients as having unstable angina because (high-) sensitive or even cardiac-specific biomarkers were not available. When an early invasive strategy for NSTEMI was established as being superior, the comparator was non-invasive or watchful waiting with crossover permitted. It is important to recognise that NSTEMI presenters, compared to STEMI patients, are older, more often diabetics and present with more diffuse coronary disease, including more prior revascularisations without a clear culprit lesion16. As a result, the ruling principle in these early studies was CR engaging each treatable lesion. In the pivotal FRISC II trial, equipoise almost existed between the number of patients having PCI versus coronary artery bypass graft (CABG), 44% versus 38%, respectively, most within 10 days of the index event17. This Scandinavian study was the first to demonstrate a significant difference in 12-month mortality, favouring an invasive strategy with intended CR (2.2% vs 3.9%), recommending CABG for those with left main stenosis (LMS) and/or triple vessel disease. Later studies on this topic and their pooled individual patient-level data confirmed the benefit of an early invasive strategy on recurrent MI with a trend towards lower mortality18.
Indirect evidence for the benefit of an early and CR strategy in NSTEMI patients is the high crossover rate in the early studies comparing invasive versus conservative management, reaching almost 50% in long-term follow-up18,19. Recently published propensity-matched data from a UK registry of NSTEMI patients demonstrated a reduced all-cause mortality for complete versus incomplete revascularisation19. The timing of the intervention itself, when occurring within the first week, seems of minor importance in haemodynamically stable patients20,21,22. Contemporary all-comers studies in NSTEMI patients suggest that the rate of CABG might be declining (closer to 7-12%), possibly due to better stent technology, staged PCI and increased experience with CTO techniques. Consequently, increased rates of CR in patients with NSTEMI and MVD undergoing PCI have been observed over the past decade, leading to improved survival16,21,23 (Supplementary Figure 1). Centre preferences and expertise might explain the wide span in completeness of revascularisation in NSTEMI patients across centres, varying from 38% to 79% in one study20.
KEY MESSAGE
Indirect evidence supports the benefit of CR in NSTEMI patients with MVD with decreased mortality and readmission for MI and repeat revascularisation (Figure 1).
Completeness of revascularisation in patients with ACS presenting with or developing cardiogenic shock
PREVALENCE OF MVD
The worst outcomes among ACS patients are observed in those 5-10% who present with or develop CS, reaching 80-90% mortality among patients with mechanical complications24. In ACS accompanied by CS, the mortality has remained rather constant for the past three decades at almost 50% within the first month25,26. Acute angiography is recommended in this high-risk population where three quarters present with MVD and/or left main (LM) involvement14,27.
REVASCULARISATION STRATEGY BEFORE CULPRIT-SHOCK TRIAL
For almost two decades the guiding principle for treatment in shock patients was CR based on data from the SHOCK trial which compared revascularisation to conservative management with 38% in the revascularisation arm receiving CABG and 55% treated with PCI25. This strategy has been recommended by both American and European guidelines28,29. An aggressive strategy with CR, regardless of whether PCI or CABG is performed, in those individuals with a high risk of PCI failure does seem to incur an early hazard; however, this is, to some extent, mitigated by the long-term effects25,30,31. Registry data recently supported the hypothesis that complete or multivessel PCI in CS would lead to myocardial protection, relieve the global ischaemic burden and result in improved long-term survival32.
REVASCULARISATION STRATEGY AFTER THE CULPRIT-SHOCK TRIAL
Eventually, a prospective randomised study on MVD versus culprit-only PCI in patients with CS was published. The CULPRIT-SHOCK trial, with a composite endpoint of death and renal replacement, actually demonstrated harm from immediate multivessel PCI compared to culprit-only PCI after 30 days and one year (55.4% vs 45.9% and 59.5% vs 52%, respectively)26,27. However, the post hoc landmark analysis revealed that the mortality difference was confined to the first month, with no difference in total mortality or CV mortality thereafter. Actually, there were numerically fewer deaths in the multivessel PCI arm at 12 months (5.3% vs 6.7%)27. Undoubtedly, immediate multivessel PCI leads to longer procedures, which in earlier studies have been associated with more complications including stroke, but also excess of contrast loads, i.e., 60 ml in the CULPRIT-SHOCK trial.
Deeper dives into the same trial have demonstrated that a higher baseline SYNTAX score is associated with more depressed LV function, a higher need for mechanical ventricular support, longer procedure times and a lower success rate when treating the culprit artery. Importantly, higher SYNTAX scores independently predicted 30-day and one-year outcomes with no interaction between the SYNTAX score and the revascularisation strategy33. Patients with a culprit lesion in the LM or proximal left anterior descending (LAD) are particularly vulnerable to further multivessel PCI, with an absolute excess in one-year mortality of almost 20% (69.9% vs 50%)34.
KEY MESSAGES
– Immediate multivessel PCI in acute CS has been associated with higher rates of death and renal failure as compared to culprit-only PCI.
– More complex CAD and the presence of the culprit lesion in the LM or proximal LAD are associated with worse outcomes when a multivessel PCI is performed (Figure 1).
Assessment of non-culprit lesions
STEMI PATIENTS
The optimal time and the preferred modality to assess the NCLs in STEMI patients remain a real diagnostic challenge. Most of the observational and randomised studies so far have used angiographic diameter stenosis severity to determine the NCLs requiring further intervention. In the acute STEMI setting, coronary vasoconstriction due to alpha-adrenergic stimulation may lead to angiographic overestimation of NCL stenosis degree by approximately 10% and to unnecessary overtreatment35.
PHYSIOLOGICAL ASSESSMENT OF NCLs
FFR measurement of NCLs has been extensively and safely used in large RCTs to guide CR in the STEMI setting, resulting in lower acute and repeat revascularisation rates compared to angio-guided revascularisation5,6. However, the diminished sensitivity of the purinergic adenosine receptors associated with an increased level of endothelin-1, higher left ventricular (LV) end-diastolic pressure and myocardial oedema may all contribute to a decreased hyperaemic flow in NCLs and a consequent underestimation of their FFR significance in the acute STEMI setting36.
A recent substudy of the REDUCE-MVI trial showed a mean decrease of 0.03 of FFR values between the index procedure and one-month follow-up, more evident in larger infarcts37.
On the other hand, the instantaneous wave-free ratio (iFR) measurement is affected by an increased resting flow in NCLs in the acute setting, resulting in an overestimation of lesion significance. The iSTEMI substudy found a mean discrepancy of 0.03 in iFR values between the index procedure and a staged procedure performed at a median of 16 days (IQR: 5-32)38.
NSTEMI PATIENTS
PHYSIOLOGICAL ASSESSMENT OF NCLs
The evaluation of NCLs is even more challenging in the NSTEMI setting. At least two of the angiographic criteria (intraluminal filling defect, plaque ulceration, plaque irregularity, dissection, or impaired flow) may be found in more than one lesion in up to 40% of NSTEMI patients39,40,41,42.
Both the FAMOUS-NSTEMI trial (n=350) and the ACS subgroup of the FAME trial (n=328) were not powered to assess the superiority of FFR-guided over angiography-guided revascularisation in terms of clinical outcomes. They included physiology assessment of both culprit and NCLs, resulting in less robust evidence than for STEMI patients43,44.
Limited data are available for non-hyperaemic indices in ACS patients. The pooled analysis of the DEFINE-FLAIR and iFR-SWEDEHEART randomised trials showed similar safety in deferring revascularisation with both iFR and FFR, despite the higher rate of deferral with iFR45.
IMAGING ASSESSMENT
The haemodynamic paradigm alone, based on physiological indices, may not provide all the information needed to guide a safe revascularisation of NCLs. Moreover, both iFR and FFR are continuous variables being reported in RCTs and considered for decision making in a dichotomous fashion.
Both STEMI and NSTEMI patients may have underlying ruptured or eroded plaques also in NCLs. The PROSPECT study demonstrated that non-angiographic significant NCLs with a large plaque burden, a small luminal area, or a combination of both features detected by intravascular ultrasonography (IVUS) imaging were associated with the same major adverse cardiac event (MACE) rates of culprit lesions at three-year follow-up46. Both systemic effects and local inflammation of NCLs in ACS patients might contribute to plaque instability. The presence of a minimum lumen area (MLA) <3.5 mm2, fibrous cap thickness <75 mm, lipid arc circumferential extension >180°, and optical coherence tomography (OCT)-defined macrophage infiltration yielded a higher rate of MACE in the 1,003 patients (53.4% ACS) of the CLIMA study47. Interestingly, a sub-analysis from the COMPLETE trial showed that nearly half of the patients undergoing OCT had an obstructive non-culprit lesion (>70% visual diameter stenosis) with vulnerable plaque. These findings may explain the reduced MI rates associated with a strategy of routine PCI of obstructive NCLs48.
KEY MESSAGES
– Physiological assessment of NCLs in ACS patients is safe and results in reduced repeat revascularisation rates.
– Transient physiological changes in the acute setting may impact on NCL severity assessment in the acute STEMI setting, with potentially underestimated and overestimated severity by FFR and iFR, respectively. This should be kept in mind when obtaining borderline values for significance (Figure 2).
– Intravascular imaging could provide further insights for the evaluation of the plaque morphology and high-risk plaque features. However, the role of these techniques in guiding clinical decision making has yet to be determined (Figure 2).
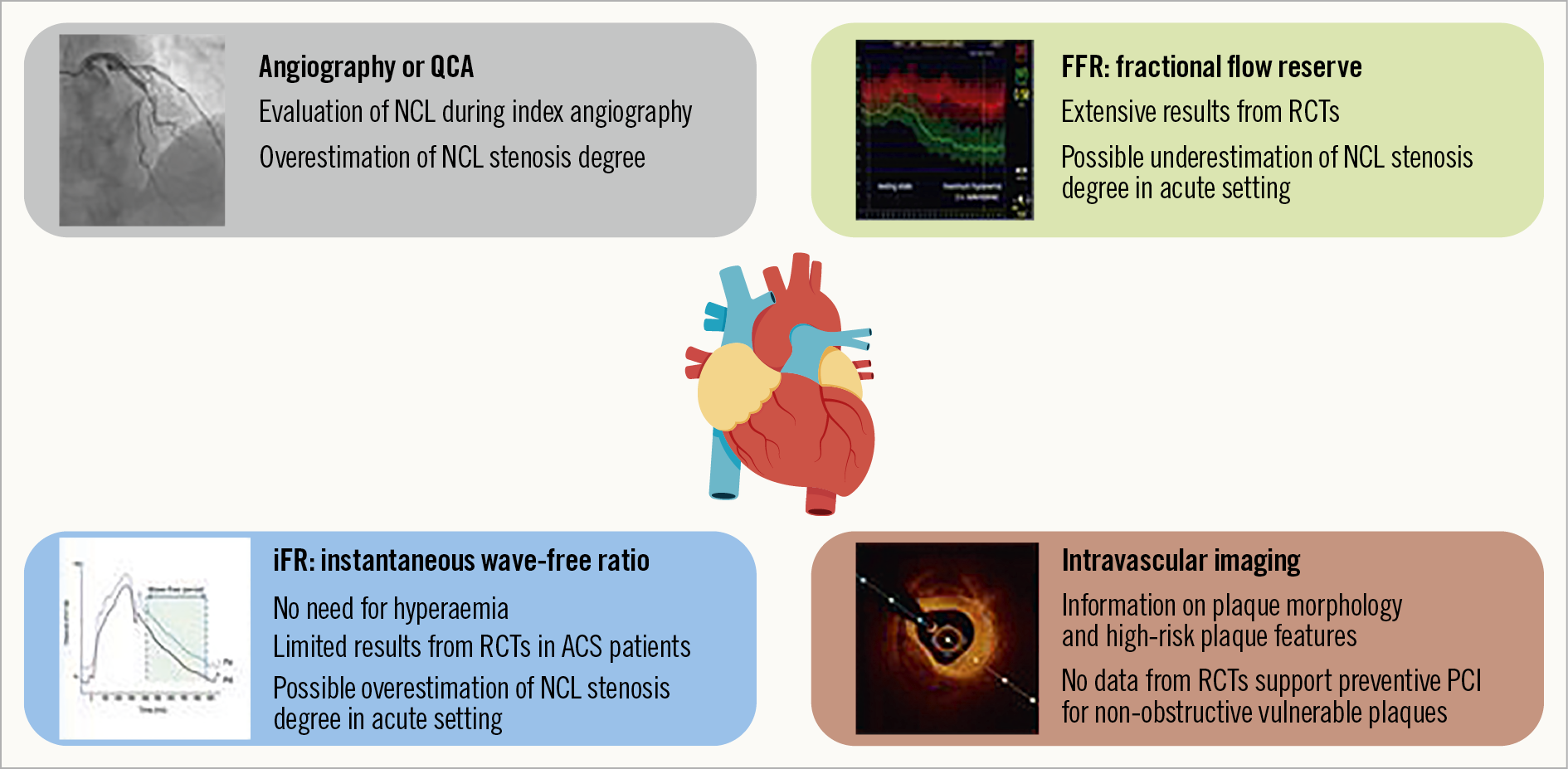
Figure 2. Schematic overview of modes of NCL assessment.
Complex non-culprit lesions
CHRONIC TOTAL OCCLUSION LESIONS
The presence of a concurrent chronic total occlusion (CTO) in NCLs in the setting of ACS is frequent and is a substantial determinant of the prognosis of patients.
A CTO is found in approximately every tenth patient with STEMI49,50 and, despite the scarce data, its prevalence in NSTEMI patients shows comparable rates51. In patients with ACS complicated by CS, a CTO lesion is even more frequent and present in every fourth to every fifth patient49,50,52.
The mortality rate for ACS patients with a concurrent CTO is almost twice as high after 3-5 years compared to ACS patients without a concurrent CTO, even after adjustment for age, comorbidities and Killip class49,52. The main mechanism might be the additional extent of myocardium at risk due to acute blockade of the infarct-related artery which supplies not only its own myocardial target area but also (via collaterals) remote myocardium in the area of the CTO artery49,53.
Data about revascularisation of a bystander CTO in ACS patients are extremely limited since the vast majority of RCTs investigating culprit-only PCI versus multivessel PCI excluded patients with a concomitant CTO, and retrospective analyses addressing this question imply the risk of a relevant selection bias4,5. Inclusion of CTO in NCLs in the recent COMPLETE trial was extremely selected and limited to a non-representative subgroup of only 2% of the study population7. Although not powered to detect differences in MACE rates, the EXPLORE trial was the only RCT evaluating the benefit of an early PCI of a concurrent CTO in ACS patients54. The primary outcomes of LV ejection fraction and LV end-diastolic volume measured by cardiac magnetic resonance (CMR) imaging after four months were not different between the early CTO-PCI arm (N=150) and the conservative treatment arm (N=154). However, there was a significant interaction regarding location of the CTO, with a favourable outcome for the revascularisation strategy in patients with a CTO located in the LAD. Both this specific subgroup and the entire study population had comparable MACE rates between the treatment arms up to a median follow-up of 3.9 years. Moreover, the quality-of-life benefit, in terms of a significantly lower rate of freedom from angina in the CTO-PCI arm at one year, faded away in the subsequent years of follow-up55. Potential biases of these findings, such as lack of any viability test prior to CTO PCI and a relatively low success rate of 73% according to the core lab adjudication, might have weakened the effect of CTO PCI.
LEFT MAIN STENOSIS (LMS)
NCL LMS is rare in patients with ACS (Supplementary Figure 2). A registry-based study from Korea revealed an LMS as the NCL in only 99 of 7,655 patients with MI (1.3%)56. Focusing on the location of NCLs in STEMI patients, a large meta-analysis of eight STEMI trials found significant LMS in 807 out of 12,710 patients (6.4%)57. Most of the RCTs investigating culprit-only versus multivessel PCI in different ACS settings excluded patients with an indication for urgent CABG affecting the majority of patients with an NCL LMS3,5,6,25,58. COMPLETE and CvLPRIT included only ≤10 ACS patients with LMS as the NCL, not allowing the generation of any evidence for this specific subgroup4,7. Unless there is a critical degree of stenosis and any sign of an unstable morphology, e.g., thrombotic lesion, ulceration or plaque rupture related to the NCL LMS, a deferred revascularisation approach is recommended for most patients with CS and patients with STEMI after primary PCI of the culprit lesion.
KEY MESSAGES
– In general, there are no data to recommend PCI of a bystander CTO in patients with ACS in the acute phase. A CTO PCI may be considered in a few cases based on symptoms, ischaemia, and viability after stabilisation and optimal medical therapy, as recommended for stable CAD.
– The decision to pursue NCL LMS PCI remains an individual decision, taking into account the clinical setting, lesion morphology and complexity, and the operator`s experience, preferably after Heart Team discussion.
Current guidelines, grey areas and future perspectives
Current European guidelines, giving a class IIA recommendation to routine revascularisation of NCLs before hospital discharge, were developed before the publication of the COMPLETE trial wherein the benefit of CR was observed regardless of whether NCL PCI was performed during the index hospitalisation or after discharge7,14,28. These results were achieved in the first RCT powered to determine a meaningful reduction in the risk of the clinically important outcome of CV death or MI and are likely to influence future guidelines on this topic (Figure 3).
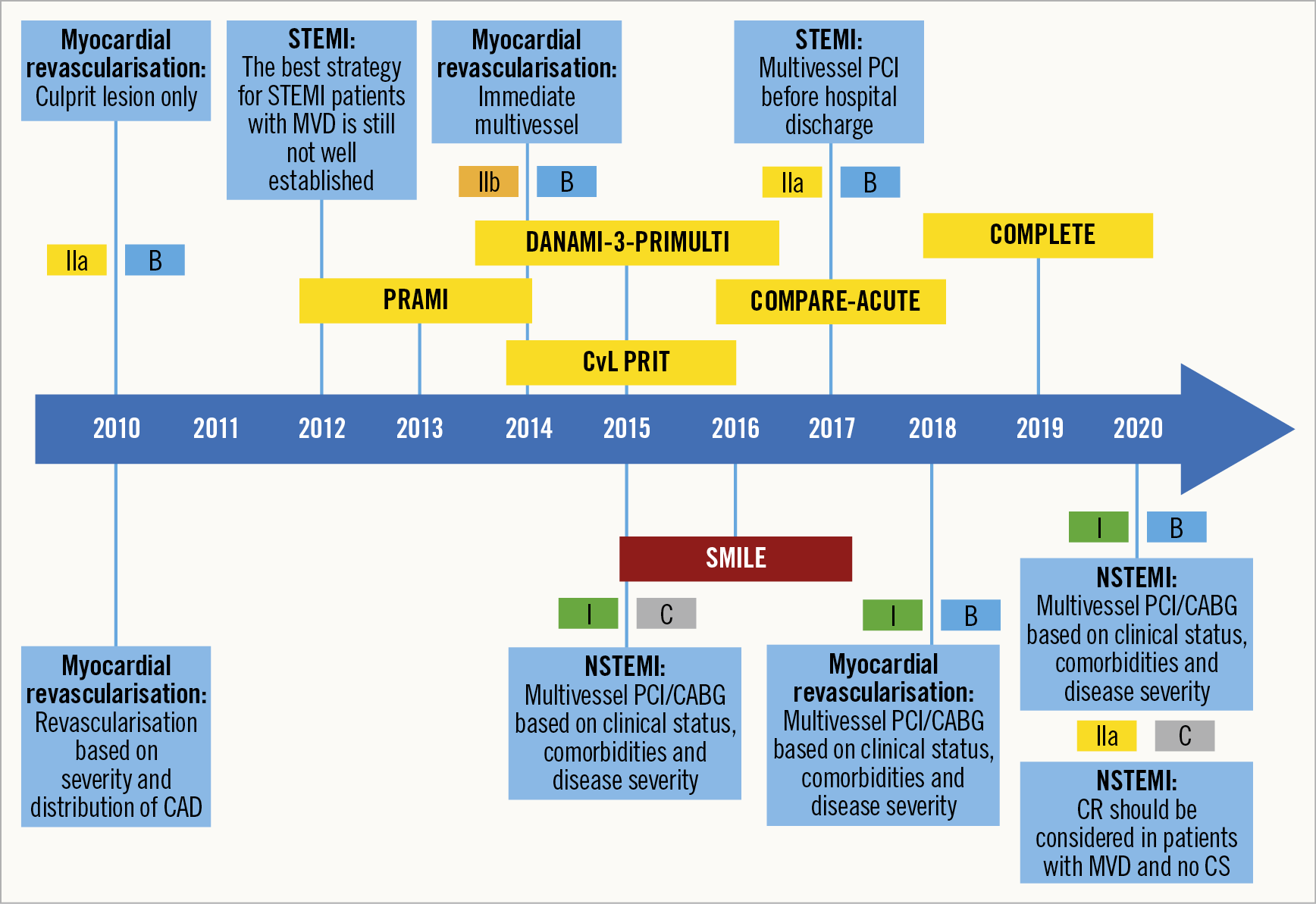
Figure 3. Revascularisation strategy for NSTEMI/STEMI with MVD. Evolution of guidelines and RCTs.
Whether an immediate CR approach is non-inferior to staged CR (within six weeks after the procedure) will be investigated in the BIOVASC trial (NCT03621501). The MULTISTARS AMI trial will compare index procedure CR to staged revascularisation of NCLs within 19-45 days (NCT03135275).
An immediate versus staged FFR-guided NCL PCI strategy was planned to be investigated in 4,052 STEMI patients enrolled in the FULL-REVASC trial. This study was designed to detect differences in one-year all-cause mortality and MI (NCT02862119). However, the trial has halted recruitment in the light of the COMPLETE trial results.
The clinical relevance of FFR-guided NCL revascularisation has recently been assessed in the FLOWER-MI trial where STEMI patients were randomised after successful primary PCI in a 1:1 fashion to either FFR-guided or angio-guided CR during the index procedure or a staged procedure before discharge (≤5-day) (NCT02943954). The primary outcome of all deaths, MI, or unplanned hospitalisation leading to urgent revascularisation at 12 months occurred in 5.5% of the FFR-guided group compared with 4.2% of the angiography-guided group. The hypothesis of superiority of an FFR-guided versus an angiography-guided complete revascularisation within the same hospitalisation was not met in this trial (HR 1.32, 95% confidence interval: 0.78 to 2.23; p=0.31). However, the lower than expected incidence of events and the wide confidence intervals for the estimate of effect do not allow a conclusive interpretation58.
Finally, the iMODERN trial will compare an iFR-guided approach of NCLs during the acute setting with a deferred stress perfusion CMR-guided strategy during the outpatient follow-up in a cohort of 1,146 STEMI patients with MVD (NCT03298659).
In contrast to the STEMI setting, the optimal timing for CR in NSTEMI patients has so far been investigated in only one RCT. The complete single-stage strategy yielded less MACE than staged multivessel PCI, driven mainly by repeat revascularisation (HR 0.55, 95% CI: 0.36-0.83, p=0.004)59. However, exclusion of patients with an estimated glomerular filtration rate (eGFR) <60 ml/min in this trial greatly limits the transferability to a real-world NSTEMI population.
Despite the lack of RCTs, the vast majority of available data favour a CR strategy in patients presenting with NSTEMI. Our interpretation of the data to pursue CR is in line with the recently published ESC guidelines (Class IIa/level of evidence C)60. Regarding the optimal timing, the guidelines recommend that revascularisation of NCLs may be considered during the index procedure (Class IIb, B) (Figure 3). However, we would propose an individual assessment based on procedural aspects of culprit lesions and NCLs, operator experience, and patient characteristics.
Conclusions
All ACS patients, except for those presenting with CS, should be offered CR. This means PCI of the culprit lesion as well as NCLs during the index procedure, before discharge or at least within the first month, when the coronary anatomy is suitable. In daily practice, an individualised approach is preferred according to patient factors, anatomic findings and institutional experience. PCI, despite its dominance in ACS, cannot be offered to all, and the treatment spectrum in ACS ranges from medical management to CABG; complex cases should always be evaluated by the Heart Team.
Tribute to Professor Anthony Gershlick
All the authors of this manuscript would like to pay tribute to a pioneering interventional cardiologist, Professor Anthony Gershlick, a leading expert in this field.
Acknowledgements
The authors would like to thank Carlo Di Mario for his input to our endeavour. Many thanks are due to Laura Hoyer, RN, for preparation of the Figures and Tables.
Conflict of interest statement
P. Clemmensen has received honoraria/reports personal fees from Abbott, Acarix, AstraZeneca, Aventis, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Evolva, Fibrex, Janssen, Merck, Myogen, Medtronic, Mitsubishi Pharma, The Medicines Company, Nycomed, Organon, Pfizer, Pharmacia, Regado, Sanofi, Searle and Servier. P.C. Smits has received institutional research grants from Abbott, St. Jude Medical, MicroPort and SMT, and has received speaking/consulting fees from Abbott, St. Jude Medical, MicroPort, AstraZeneca and Terumo. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

