
Abstract
Aims: The Compare-Acute trial showed superiority of fractional flow reserve (FFR)-guided acute complete revascularisation compared to culprit-only treatment in patients with ST-segment elevation myocardial infarction (STEMI) and multivessel disease (MVD) at one year. The aim of this study was to investigate the outcome at three years, together with cost analysis of this strategy.
Methods and results: After primary percutaneous coronary intervention (PCI), 885 patients with STEMI and MVD were randomised (1:2 ratio) to FFR-guided complete revascularisation (295 patients) or infarct-related artery (IRA)-only treatment (590 patients). After 36 months, the primary endpoint (composite of death, myocardial infarction, revascularisation, stroke) occurred significantly less frequently in the FFR-guided complete revascularisation group: 46/295 patients (15.6%) versus 178/590 patients (30.2%) (HR 0.46, 95% CI: 0.33-0.64; p<0.001). This benefit was driven mainly by the reduction of revascularisations in the follow-up (12.5% vs 25.2%; HR 0.45, 95% CI: 0.31-0.64; p<0.001). Cost analysis shows benefit of the FFR-guided complete revascularisation strategy, which can reduce the cost per patient by up to 21% at one year (8,150€ vs 10,319€) and by 22% at three years (8,653€ vs 11,100€).
Conclusions: In patients with STEMI and MVD, FFR-guided complete revascularisation is more beneficial in terms of outcome and healthcare costs compared to IRA-only revascularisation at 36 months.
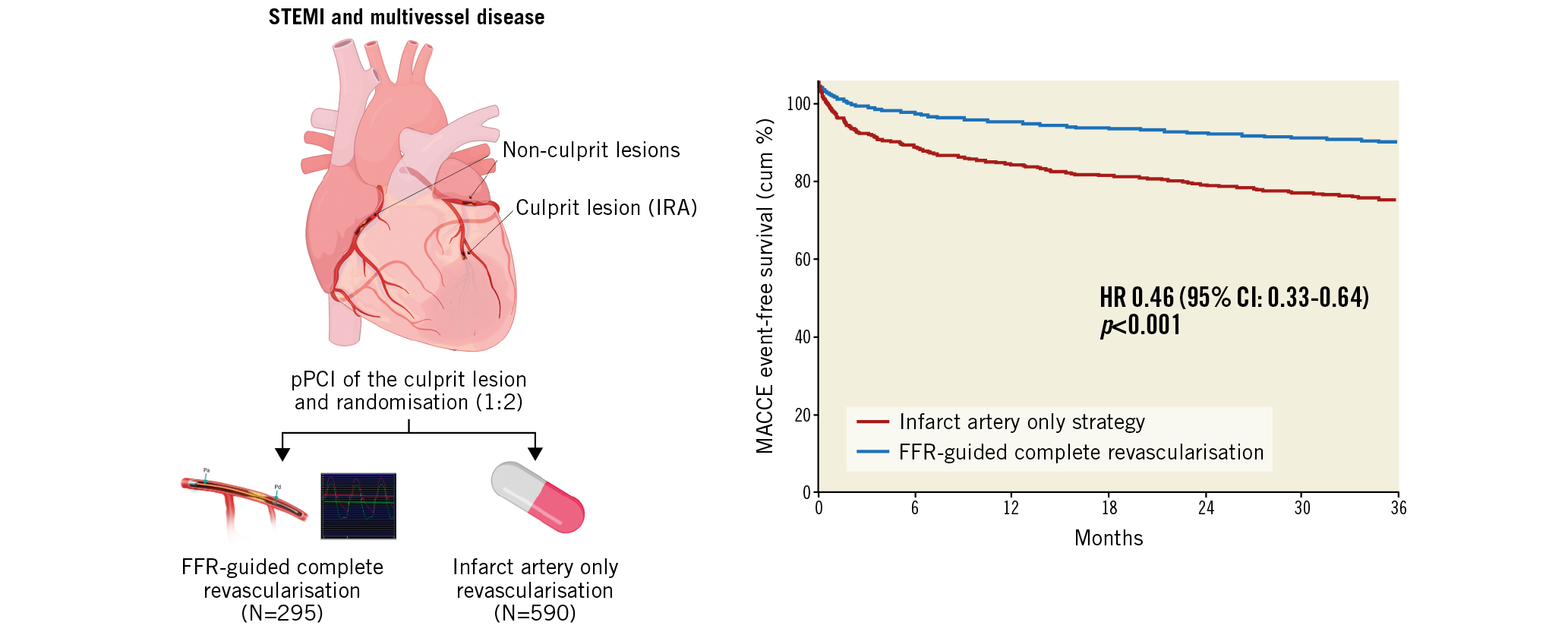
Visual summary. Three-year follow-up of FFR-guided complete revascularisation versus IRA-only strategy in patients with STEMI and multivessel disease.
Introduction
Primary percutaneous coronary intervention (PCI) of the infarct-related artery (IRA) is the mainstay of treatment of patients with ST-segment elevation myocardial infarction (STEMI), provided it is performed in a timely manner and by an experienced team1. Around half of the patients presenting with STEMI exhibit multivessel coronary artery disease (MVD). This subpopulation is known to have worse short- and long-term prognosis2. The correct therapeutic strategy for non-culprit lesions has been extensively debated.
Five recent randomised trials have shown that an angiography-guided strategy (PRAMI3, CvLPRIT4, COMPLETE5) and a fractional flow reserve (FFR)-guided strategy (DANAMI-3-PRIMULTI6 and Compare-Acute7) of complete revascularisation are beneficial compared to IRA-only revascularisation. The recently published COMPLETE trial5, having randomised more than 4,000 STEMI patients with multivessel disease, showed that angiography-guided complete revascularisation provides a significant reduction in the composite outcome of cardiovascular death and myocardial infarction (MI) at a median follow-up of three years.
Pressure-derived FFR provides information about the functional severity of coronary stenosis and is currently the standard of care in guiding revascularisation in the presence of intermediate stenosis, both in patients with single-vessel and in those with multivessel disease. FFR ≤0.80 defines haemodynamically significant coronary stenosis that warrants revascularisation8.
The role of FFR in the acute setting is less established. The DANAMI-3-PRIMULTI study randomised 627 patients with STEMI and MVD to PCI of the IRA only versus FFR-guided complete revascularisation in a staged strategy. At a median follow-up of 27 months, the primary endpoint (a composite of all-cause mortality, non-fatal reinfarction, and ischaemia-driven revascularisation) occurred more frequently in the group that underwent PCI of the IRA only6.
Compare-Acute (ClinicalTrials.gov identifier: NCT01399736) was an investigator-initiated, prospective, multicentre, randomised trial that involved 24 sites, in which 885 patients with STEMI were randomised 1:2 to FFR-guided complete revascularisation or IRA-only revascularisation, with the aim of demonstrating that FFR-guided complete revascularisation in the acute setting of STEMI treatment was superior to IRA-only revascularisation9.
After one year of follow-up, patients randomised to complete revascularisation showed a lower incidence of the primary endpoint, a composite of all-cause mortality, non-fatal MI, any revascularisation and cerebrovascular events (MACCE), compared to the IRA-only revascularisation arm. This difference was driven mainly by an increased number of revascularisations in the IRA-only revascularisation arm during the 12 months of follow-up7.
The long-term outcome and the cost benefit of this strategy are unknown. We hereby present the results of the three-year outcome of the Compare-Acute trial and its cost analysis.
Methods
STUDY DESIGN AND POPULATION
The design of the Compare-Acute study has been described previously9: 885 patients from 24 centres in Europe and Asia, with STEMI and MVD, were enrolled and randomised in a 1:2 ratio (295 patients vs 590 patients) to FFR-guided complete revascularisation or to IRA-only revascularisation.
A full list of inclusion and exclusion criteria has been published previously7.
The study was approved by the ethics committee of each participating site and informed consent was obtained before the procedure according to the International Conference on Harmonisation Good Clinical Practice guidelines.
FFR MEASUREMENT, TREATMENT AND FOLLOW-UP
After successful primary PCI of the IRA, eligible patients underwent randomisation (1:2 ratio). FFR measurement was performed in both groups, but the result was blinded to the patient and treating physician in the case of patients randomised to IRA-only revascularisation. FFR measurement, treatment and follow-up strategy have been described previously7. Figure 1 shows the randomisation and follow-up of patients.
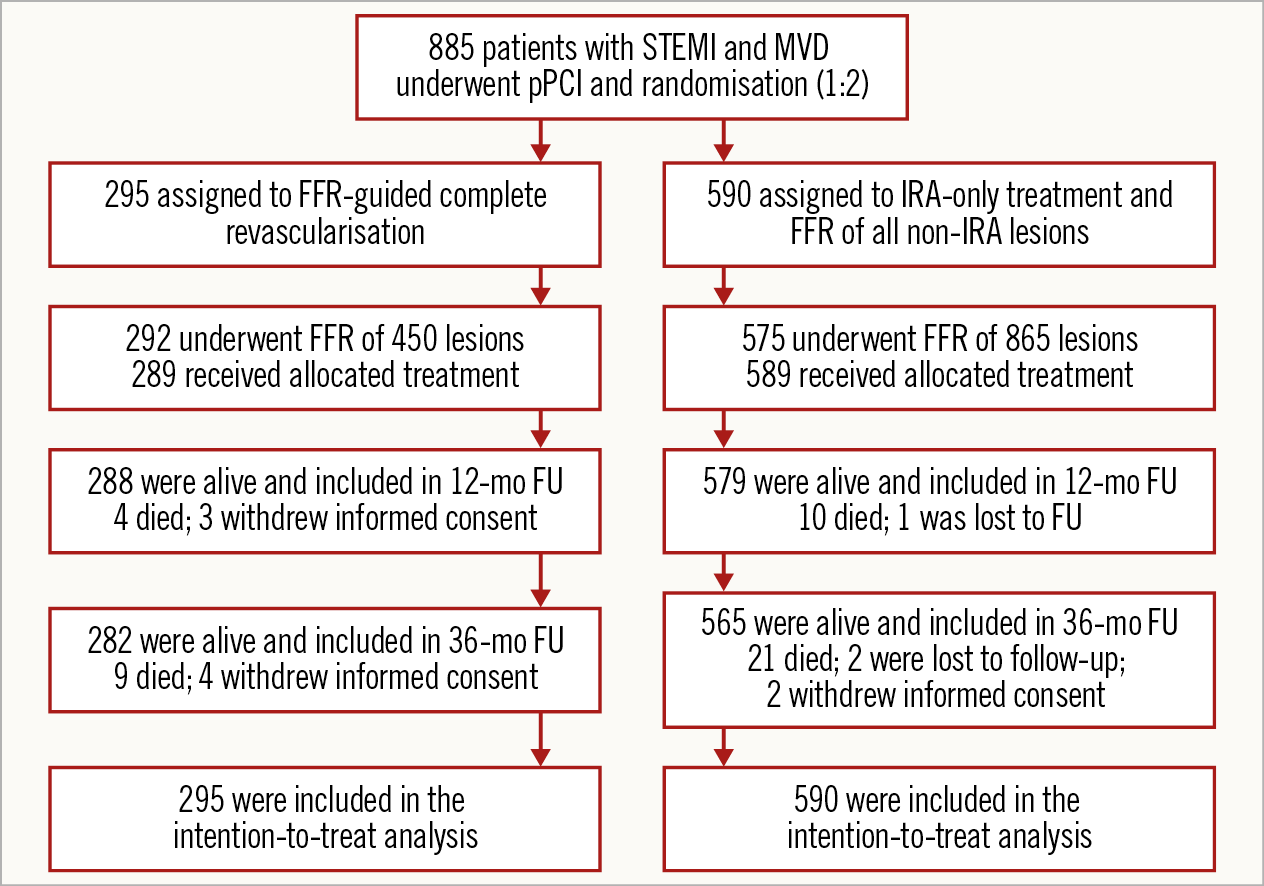
Figure 1. Randomisation, treatment and follow-up. FU: follow-up; MVD: multivessel disease; STEMI: ST-elevation myocardial infarction
STUDY ENDPOINTS
The primary endpoint was defined as the composite of all-cause mortality, non-fatal MI, any revascularisation and cerebrovascular events (MACCE) at one year.
Secondary endpoints included the primary endpoint at 36 months, each component of the primary endpoint, the composite of all-cause mortality and MI; the composite of cardiac death, MI, any revascularisation, stroke, and major bleeding (NACE); the composite of hospitalisation for heart failure, unstable angina, chest pain, NSTEMI and STEMI; stent thrombosis; treatment costs. A revascularisation was defined as “urgent” by the following definition: any revascularisation for recurrent ischaemia that in the investigator’s opinion cannot be delayed for more than 24 hours and/or is defined by the investigator as a non-elective procedure.
Moreover, three subgroup analyses were pre-specified: a comparison of patients in both groups with treated lesions with FFR ≤0.80 versus patients with untreated lesions with FFR ≤0.80; a comparison of acute versus staged treatment for lesions with FFR ≤0.80; a comparison of patients with treated versus untreated lesions with FFR >0.80.
COST ANALYSIS
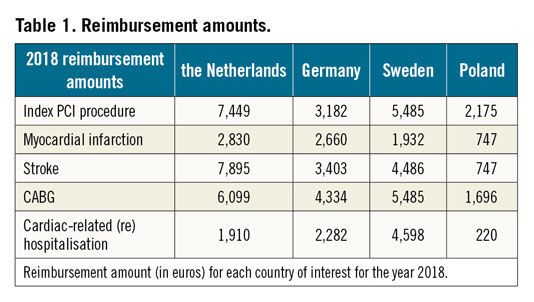
Cost analysis was performed with a healthcare payer perspective, meaning that unit costs (each patient’s costs) will be the reimbursement prices according to the healthcare system. Diagnosis-related group systems (DRGs) is a form of reimbursement used in many healthcare systems, in which any procedure has a fixed reimbursement cost that covers all expenses. The reimbursement is different in each country participating in the Compare-Acute trial; thus, the cost analysis was performed using DRGs from different countries – the Netherlands, Germany, Sweden and Poland.
Procedure costs (including events in the follow-up) were collected in the respective countries of interest as the reimbursement furnished by the healthcare system, and account for the year 2018 (Table 1). Average costs per strategy arm were calculated by multiplying the respective procedure or event cost with the number of procedures or events, divided by the total number of patients (average cost = {Σ costs of index procedures + Σ (costs per event*number of events)}/number of patients).
STATISTICAL ANALYSIS
Statistical analysis and sample sizing have been described previously elsewhere9. All analyses were performed on an intention-to-treat basis. A post hoc, per-protocol analysis including all patients according to the received treatment was also performed. Continuous data are presented as means with standard deviations for normally distributed variables and as medians with minimum and maximum values for variables that were not normally distributed. Differences between both groups for continuous data were assessed with the use of an unpaired t-test when data were normally distributed and with the Mann-Whitney U test when not. Categorical data were analysed with the use of the chi-square test (or Fisher’s exact test if a cell contained a number <5). A two-sided p-value of ≤0.05 was considered to indicate significance. Kaplan-Meier time-to-event plots were constructed for clinical events, and treatment groups were compared with the use of the log-rank test. Cox proportional hazards models were fitted to estimate hazard ratios with 95% confidence intervals (CIs) for treatment comparisons. Statistical analysis was performed with the use of SPSS software, Version 25.0 (IBM Corp., Armonk, NY, USA).
Results
BASELINE DATA
Baseline characteristics, angiographic and procedural data are shown in Table 2 and Table 3.
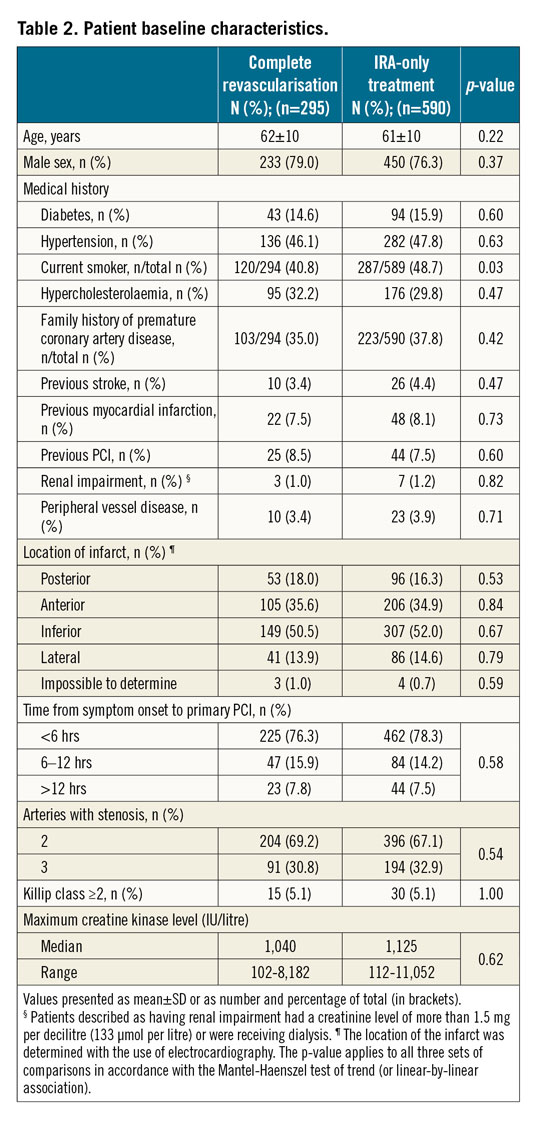
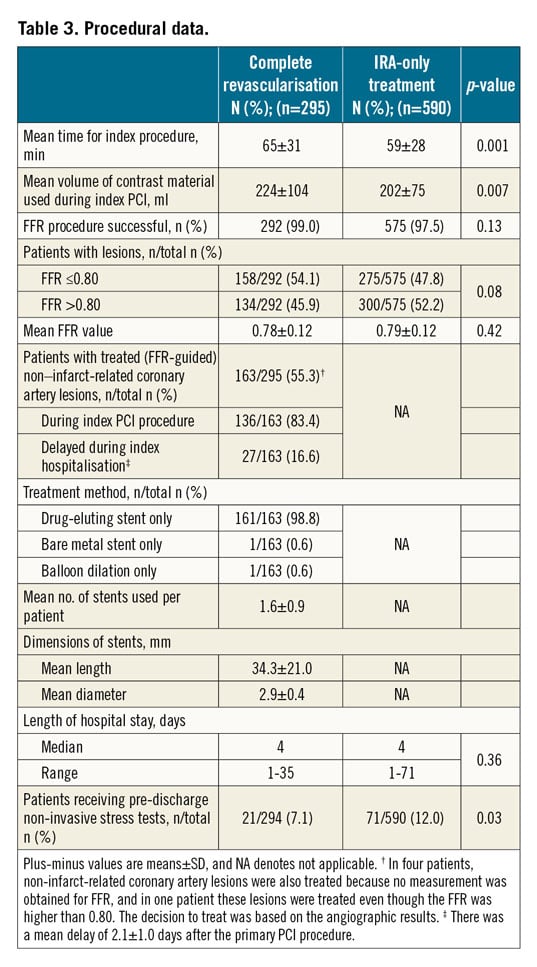
In the complete revascularisation group, 158 of 292 patients (54.1%) had positive FFR (FFR ≤0.80) and thus underwent complete revascularisation. Five additional patients had PCI of non-IRA lesions that were not based on FFR values. Most PCIs of the non-IRAs were performed during the index procedure (136/163, 83.4%); the remainder of patients (27/163, 16.6%) underwent complete revascularisation during the same hospital stay (mean waiting time, 2.1 days).
Two hundred and seventy-five of 575 patients in the IRA-only group (47.8%) had positive FFR lesions at baseline. In this arm, 59 patients underwent staged revascularisation within 45 days (not considered as an event, as per protocol) and, among these, 44 patients had one or more non-IRAs with positive FFR. Median hospital stay was not different in the two groups (4 days vs 4 days; p=0.36).
ENDPOINTS
After three years, eight patients (1.1%), four in each group, were lost to follow-up or withdrew consent (Figure 1).
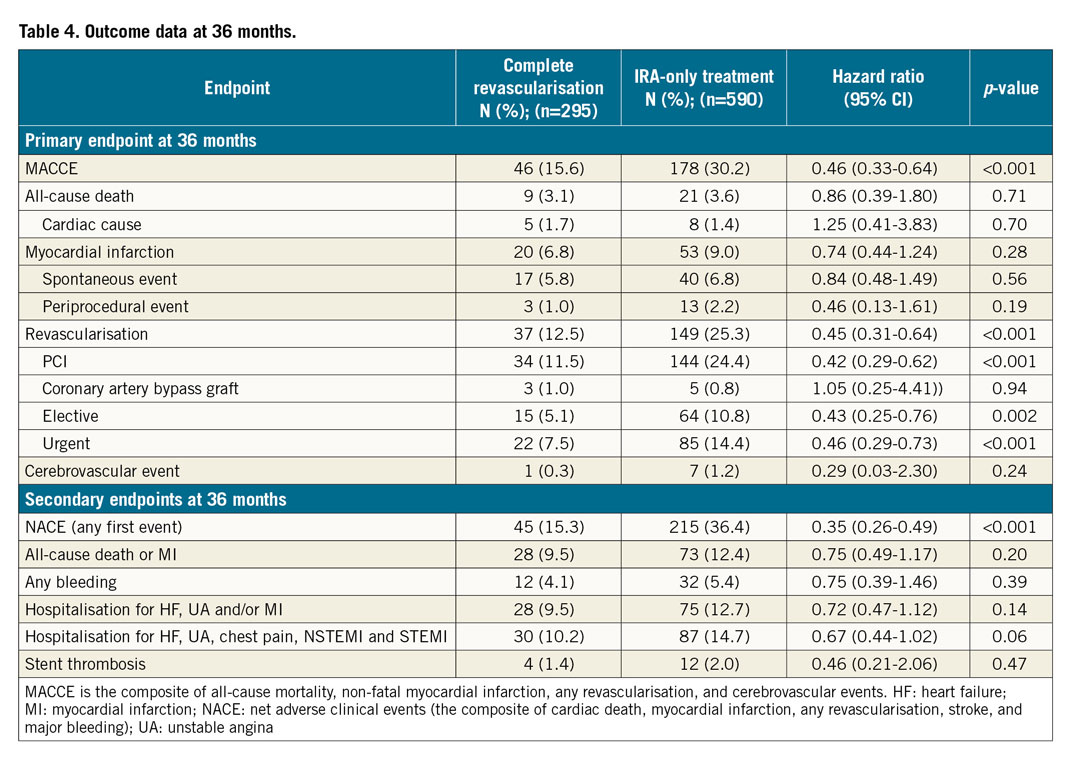
After 36 months, the primary endpoint MACCE occurred in 15.6% of patients (46/295) in the complete revascularisation group versus 30.2% (178/590) in the IRA-only group (hazard ratio [HR] 0.46, 95% CI: 0.33-0.64; p<0.001) (Figure 2).
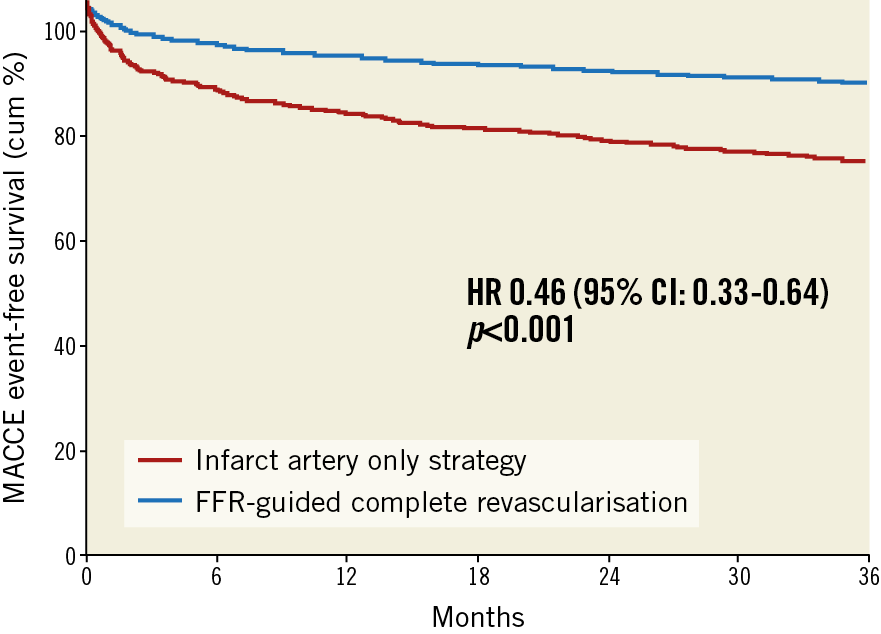
Figure 2. Kaplan-Meier event curves of the combined primary outcome at 36 months. MACCE is a composite of all-cause mortality, non-fatal myocardial infarction, any revascularisation, and cerebrovascular events.
The incidence of all-cause mortality (9 patients vs 21 patients; 3.1% vs 3.6%; HR 0.86, 95% CI: 0.39-1.8; p=0.71), MI (20 patients vs 53 patients, 6.8% vs 9.0%; HR 0.74, 95% CI: 0.44-1.24; p=0.28) and cerebrovascular events (1 patient vs 7 patients; 0.3% vs 1.2%; HR 0.29, 95% CI: 0.03-2.3; p=0.24) remained numerically lower in the FFR-guided complete revascularisation group, but did not reach statistical significance. However, the incidence of revascularisation (PCI or coronary artery bypass grafting [CABG]) was significantly lower in the FFR-guided complete revascularisation group compared to the IRA-only revascularisation group (37 patients vs 149 patients; 12.5% vs 25.2%; HR 0.45, 95% CI: 0.31-0.64; p<0.001).
The majority of these revascularisations had an urgent indication and occurred more frequently in the IRA-only revascularisation group (22 patients vs 85 patients; 7.5% vs 14.4%; HR 0.46, 95% CI: 0.29-0.73; p<0.001).
The composite of all-cause death and MI was slightly less frequent in the complete revascularisation group: 28 patients vs 73 patients in the IRA-only group, without reaching statistical significance (9.5% vs 12.4%; HR 0.75, 95% CI: 0.49-1.17; p=0.20).
The secondary endpoint of NACE (composite of cardiac death, MI, any revascularisation, stroke and major bleeding) occurred in 15.3% of patients (45/295) in the complete revascularisation group versus 36.4% of patients (215/590) in the IRA-only group (HR 0.35, 95% CI: 0.26-0.49; p<0.001).
Finally, hospitalisations for heart failure, unstable angina, atypical chest pain, typical angina, NSTEMI and STEMI were lower in the FFR-guided complete revascularisation group, despite not reaching statistical significance (30 patients vs 87 patients; 10.2% vs 14.7%; HR 0.67, 95% CI: 0.44-1.02; p=0.06). Outcome data are summarised in Table 4.
The results of the three pre-specified subgroup analyses, the post hoc per-protocol analysis, and the post hoc one-year landmark analysis are shown in Supplementary Appendix 1, Supplementary Appendix 2 and Supplementary Figure 1.
COST ANALYSIS
According to the Dutch system, and using the data derived from the one-year follow-up study, the average cost per patient was 8,150€ in the FFR-guided complete revascularisation group, and 10,319€ in the IRA-only revascularisation group, accounting for a reduction of 21% of the per-patient cost. An FFR-guided complete revascularisation strategy is cost-saving also after three years of follow-up: the average cost per patient was 8,653€ in the complete revascularisation group and 11,100€ in the IRA-only revascularisation group (22% cost reduction).
The same three-year data were confirmed using DRG analysis according to the German system (complete revascularisation 4,887€ vs IRA-only 5,200€; 6.0% cost reduction) and the Swedish system (complete revascularisation 6,205€ vs IRA-only 8,133€; 24% cost reduction). An FFR-guided complete revascularisation strategy was not cost beneficial according to the Polish system (complete revascularisation 3,704€ vs IRA-only 3,685€; 0.5% cost increase). All data are shown in Figure 3.
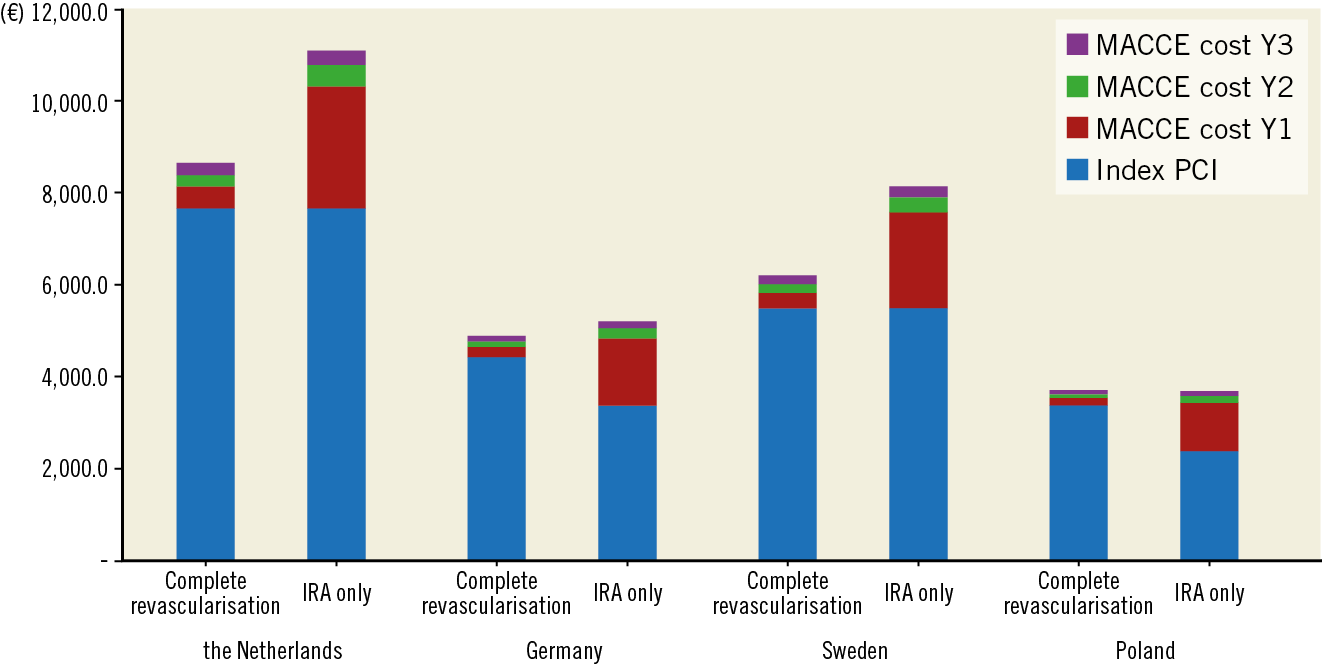
Figure 3. Governmental average cost per patient at 36 months. The Figure shows the cumulative per-patient average cost in four different countries using a diagnosis-related group (DRG) system of reimbursement.
Discussion
The 36-month follow-up of the Compare-Acute trial demonstrates the benefit of an FFR-guided complete revascularisation strategy in patients with STEMI and multivessel disease in reducing a composite endpoint of all-cause mortality, non-fatal MI, any revascularisation and cerebrovascular events. This benefit was driven mainly by an increased incidence of any revascularisation in patients treated with an IRA-only strategy. Moreover, the FFR-guided complete revascularisation strategy in the acute setting of STEMI is also cost-saving from a payer perspective.
Although almost half of the patients presenting with STEMI have multivessel disease, the decision as to whether to perform complete revascularisation is still debated. To date, five randomised trials including Compare-Acute have addressed this question. All of them have provided positive results in favour of complete revascularisation. Despite extensive questioning about the reliability of FFR measurement in the acute setting, we believe that adding functional evaluation of non-culprit coronary lesions will provide additional information about those stenoses that will actually benefit from revascularisation and also reduce the number of unnecessary PCIs. In the DANAMI-3-PRIMULTI study, 69% of intermediate lesions proved functionally significant (FFR ≤0.80), whereas around 50% of lesions in the Compare-Acute population were FFR positive6.
Our study provides additional evidence on the importance of physiological evaluation of non-IRA lesions in the setting of MI, not only for the idea that FFR-positive lesions warrant revascularisation, but also for the safety of deferring revascularisation of FFR-negative lesions even in the acute setting (Supplementary Figure 2, Supplementary Figure 3). Importantly, in our subgroup analysis comparing patients with FFR-positive lesions who underwent PCI and patients with FFR-positive lesions who received only medical treatment, we found that the number not only of revascularisations, but also of MIs was increased at follow-up in the group of patients treated medically (Supplementary Appendix 1, Subgroup analysis 1), confirming what is known from FFR data in stable angina patients (DEFER trial and FAME 2) and adding extra evidence for FFR in the subset of acute coronary syndrome patients10,11. Despite the increased incidence of MACCE in the IRA-only group being driven mainly by an increased number of revascularisations, we found that most revascularisations in the follow-up were clinically urgent and more frequent in the IRA-only group.
The recently published COMPLETE trial5 randomised patients with STEMI and multivessel disease to angiography-guided complete revascularisation or IRA-only treatment. Its results strengthen the evidence of the Compare-Acute trial, showing that complete revascularisation reduces not only the number of revascularisations in the follow-up but also the composite outcome of death and MI.
Another common question is whether revascularisation of non-culprit lesions should be performed during the primary PCI or in a staged procedure. Although our trial was not designed for this analysis, our data (Supplementary Appendix 1, Subgroup analysis 2) suggest that there is no substantial difference in choosing either of those strategies. In line with our findings, a recent sub-analysis of the COMPLETE trial demonstrated that the benefit of complete revascularisation emerges mainly at longer follow-up and is maintained irrespective of the timing of complete revascularisation12.
Finally, not only does complete revascularisation give benefit in terms of outcome, but it can also reduce healthcare-related costs: by reducing the total number of procedures and adverse events, this approach can save up to 22% of the per-patient cost.
Limitations
Some limitations of the trial must be considered. Since it was designed as open-label, there might have been some bias by the referring physicians of patients in the IRA-only group towards revascularisation. However, most of the staged and non-staged revascularisations in this group were performed in FFR-positive lesions. Furthermore, although clinical hard endpoints, such as death and MI were numerically lower in the FFR-guided complete revascularisation group, this trial was not sufficiently powered to detect statistical significance for these low-frequency events. Other than the COMPLETE trial5, a meta-analysis on individual patient-level data of FAME 2, DANAMI-3-PRIMULTI and Compare-Acute shows that FFR guidance for complete revascularisation can result in a combined cardiac death and MI benefit compared to an optimal medical strategy13.
Finally, we performed cost analysis on bundle payment (DRGs) from a payer perspective, but no detailed cost-effectiveness analysis, because we obtained no information on quality of life and actual healthcare consumption costs.
Conclusions
The three-year follow-up of the Compare-Acute trial demonstrates the benefit of an FFR-guided complete revascularisation strategy in reducing the incidence of a composite outcome of death, myocardial infarction, cerebrovascular events and revascularisation in patients with STEMI and multivessel disease. The benefit of this strategy is driven mainly by the reduction in revascularisations (the majority of which are urgent) during follow-up and can result in reduction of healthcare costs.
|
Impact on daily practice The results of the three-year follow-up of the Compare-Acute trial, in line with recent findings, demonstrate the benefit of a complete revascularisation strategy in patients with STEMI and multivessel disease in terms of outcome. Moreover, this strategy appears more cost-effective. |
Acknowledgements
We acknowledge the help of Mrs Ria van Vliet for assistance with the conduct of the trial, and Mrs Hanneke Fischer, M.Sc, for assistance with the cost analysis.
Funding
This work was supported by Maasstad Cardiovascular Research BV, which received research grants from Abbott Vascular and St. Jude Medical.
Appendix. Study collaborators
Paul J. Ong, MD; Department of Cardiology, Tan Tock Seng Hospital, Singapore. Rainer Hambrecht, MD; Department of Cardiology, Klinikum Links der Weser, Bremen, Germany. Oskar Angeras, MD; Department of Cardiology, Gothenburg University Hospital, Gothenburg, Sweden.
Conflict of interest statement
P.C. Smits reports grants from Abbott Vascular and St. Jude Medical during the conduct of the study. F-J. Neumann reports grants from Abbott Vascular, Medtronic, GlaxoSmithKline and St. Jude Medical during the conduct of the study, personal fees from Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Ferrer, The Medicines Company and Novartis, and grants and personal fees from Pfizer, Boston Scientific, Biotronik, Edwards Lifesciences and Bayer Healthcare, outside the submitted work. O. Angerås reports personal fees from Abbott Medical and Boston Scientific, outside the submitted work. G.W. Frederix received a fee from Abbott Vascular to perform the cost analysis. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

