Abstract
Aims: The aim of this article is to review what is currently known about fractional flow reserve (FFR) and related coronary physiological indices in patients with acute coronary syndrome (ACS) including non-ST-elevation (NSTEMI) and ST-elevation myocardial infarction (STEMI) with a view to making recommendations for daily practice.
Methods and results: We explored all relevant publications to date including literature reviews, clinical trials and registries. We identified sufficient data on FFR in the setting of NSTEMI to confirm it to be a reliable and useful tool for lesion-level decision making with certain pitfalls as outlined below. There was limited published literature on FFR in STEMI. However, there is some evidence that, in patients who are stable after culprit lesion intervention, FFR may be of value for assessing the functional significance of non-culprit lesions. When measured in the culprit artery of patients with STEMI, the index of myocardial resistance (IMR) predicts long-term clinical outcomes.
Conclusions: In patients with ACS, there is an increasing evidence base to support the role of FFR to guide revascularisation and of IMR to predict outcome.
Introduction
Fractional flow reserve (FFR) is a well-validated technique to guide coronary intervention by identification of lesion-level ischaemia; however, studies to date have mainly involved stable patients outside of ST-elevation myocardial infarction (STEMI)1,2. The use of FFR to assess culprit and/or non-culprit lesions in the setting of acute ischaemia represents a controversial area due to the potential impact of acute pathophysiological disturbances in the microvasculature on the ability to induce maximal hyperaemia. Furthermore, there may be cases where plaque instability necessitates intervention in the absence of flow limitation, and these lesions may be undertreated if an exclusively FFR-guided strategy is adopted. We will review the published literature in this area and make recommendations on the use of FFR in patients with ACS based on the available data.
Multivessel disease
Multivessel coronary disease (MVD) is observed in approximately 30-50% of patients presenting with acute ST-elevation myocardial infarction (STEMI) and is associated with a worse prognosis3,4. In the recently published PRAMI trial, 54% of STEMI cases had MVD as defined by a stenosis of 50% or more in one or more coronary arteries other than the infarct-related artery3. Likewise, in patients with non-ST-elevation myocardial infarction (NSTEMI), 30-59% of patients have MVD5-7. In patients with STEMI and MVD, the culprit artery is generally obvious but the functional significance of non-culprit lesions may be difficult to determine. The ability to assess accurately the functional significance of non-culprit stenoses at the time of primary PCI for STEMI would potentially facilitate a strategy of complete revascularisation during the index procedure with consequent health and economic benefits. In patients with NSTEMI and MVD, the same scenario applies, but often with the additional difficulty of correctly identifying the culprit itself, an issue that may also be addressed by functional testing in the cathlab.
Revascularisation strategies in patients with ACS and multivessel disease
The European Society of Cardiology (ESC) guidelines suggest basing the revascularisation strategy in UA/NSTEMI (either culprit-only PCI or multivessel PCI or CABG) on clinical status and disease severity according to local Heart Team policy (class 1/level of evidence C)8. In patients with STEMI, it is recommended that primary PCI (PPCI) should be limited to the culprit vessel with the exception of cardiogenic shock and persistent ischaemia after PCI of the supposed culprit (class 2a/level of evidence B). If staged PCI to non-culprit vessels is being considered, non-invasive stress testing (myocardial perfusion scintigraphy, stress echocardiography, PET or MRI) should be used for ischaemia and viability testing prior to a decision to proceed with PCI9
These latter recommendations were based on observational data suggesting that PCI of a non-culprit artery at the time of PPCI in patients with STEMI was associated with increased mortality at 90 days10,11. Specifically, in a cohort analysis from the HORIZONS-AMI trial, multivessel PCI at the time of PPCI was associated with a higher one-year mortality (9.2% vs. 2.3%) and stent thrombosis rate (5.7% vs. 2.3%) than staged PCI11. Controversy in this area due to conflicting evidence has led to numerous clinical trials, which are in progress at present, as well as the recently published PRAMI trial. This study demonstrated that, in a group of 234 STEMI patients randomised to preventive PCI in non-culprit arteries versus 231 randomised to culprit-only PCI, there was an absolute 14% risk reduction in the primary outcome (a composite of death from cardiac causes, non-fatal MI, or refractory angina) in favour of preventive PCI (HR=0.35; 95% CI: 0.21 to 0.58; p<0.001)12. PRAMI used conventional angiographic indices of lesion severity (diameter stenosis >50%) to identify non-culprit targets for PCI. It is highly likely that some of these lesions were not functionally significant. As such, FFR guidance has the potential to identify the group of patients/lesions which may benefit from immediate multivessel PCI.
Identification of the culprit and non-culprit vessels in patients with ACS
Whilst this is frequently straightforward in cases of acute STEMI, it can often be difficult to identify the infarct-related artery in cases of UA/NSTEMI, especially when no localising ECG changes have been observed and no regional wall motion abnormalities are notable on echo. Angiographic features suggestive of acuity include haziness, irregularity, eccentricity, ulceration, filling defects, thrombus, flow disturbances and subtotal occlusion. Invasive imaging, such as optical coherence tomography (OCT), intravascular ultrasound (IVUS) and near-infrared spectroscopy, may enable identification of a ruptured plaque or dissection in difficult cases13. While FFR has the ability to identify the vessel with physiologically restricted coronary flow, this vessel/lesion may not necessarily be the culprit in patients with ACS in whom plaque rupture and atherothrombotic embolisation may be present without flow limitation. Nevertheless, due to the impact of physical forces on plaque behaviour, haemodynamic severity is a biomarker for instability.
Anatomical and functional lesion assessment in patients with ACS
There are well-founded concerns that the angiographic severity of non-culprit lesions may be overestimated in STEMI due to diffuse vasoconstriction poorly responsive to conventional vasodilators. A study evaluating lesion severity in non-culprit vessels in 48 patients imaged within nine months post STEMI demonstrated that lesion severity decreases with time (presumably as thrombus is resorbed and vascular tone normalises), with minimal lumen diameter on QCA improving from 1.53±0.51 mm to 1.78±0.65 mm, (p<0.001) and diameter stenosis from 49.3±14.5% to 40.4 ±16.6%, (p<0.0001)14.
The physiological principles underlying FFR are critically dependent on the ability to achieve maximal hyperaemia, which then allows an assumption of a linear relationship between pressure and flow. In patients with acute myocardial infarction, there are multiple contributors to changes in microcirculatory function, which may impair the ability to achieve maximal hyperaemia (minimal myocardial resistance), and thereby compromise the accuracy of FFR assessments in non-culprit vessels. These include neuro-hormonal activation of resistance vessels, increased LV diastolic pressure, impaired LV systolic function and hypoxic vascular stunning with secondary effects on vasodilatory mechanisms. Outside the context of ACS, it has previously been demonstrated that maximal myocardial blood flow measured using positron emission tomography in regions remote from the area supplied by a significantly stenosed epicardial vessel is reduced (2.89 ±0.93 ml/min/gm vs. 3.67± 0.94 ml/min/gm in a normal control group, p<0.05), suggesting a remote effect on flow in non-infarct-related myocardium, which again raises theoretical concerns over the validity of FFR in MVD15. However, in a study measuring Doppler-derived coronary flow reserve (CFR) in patients with and without MVD, CFR was preserved in non-infarct-related arteries, even in the presence of previous remote myocardial infarction, and there was no significant difference compared to a control group without coronary disease (3.08±0.61 vs. 3.03±0.69, p=NS)16.
In the ACS setting, FFR values tend to be higher in lesions with Thrombolysis In Myocardial Infarction (TIMI) flow grade 2 versus TIMI flow grade 3, and flow has been shown to be reduced in non-culprit vessels in a previous clinical study. In a prospective observational study FFR was evaluated in primary PCI patients post PCI and two groups with TIMI 2 and TIMI 3 flow were compared. There were no significant differences on intravascular ultrasound, but FFR values were notably divergent (0.98±0.02 vs. 0.93±0.05; p=0.017). This is explained by microvascular dysfunction exerting a negative effect on coronary flow with resultant lower TIMI flow grade and a higher FFR. Furthermore, although PCI in the culprit vessel has been demonstrated to increase flow in the non-culprit vessels, flow may remain below normal values17,18. All of this suggests that patients who have microvascular dysfunction may have falsely elevated FFRs and, for these and other reasons, the PRAMI trial did not use FFR to guide PCI in the patients randomised to preventive intervention.
Another important concept to consider when interpreting any FFR result, but particularly in ACS, is the mass of viable myocardium being perfused by the artery in question. It has previously been demonstrated that the FFR value is inversely proportional to the ejection fraction. This means that if there is a large area of infarction with less viable myocardium, higher FFR readings can be expected for the same degree of stenosis. The reverse also holds true so, when there is a large territory of viable myocardium supplied by a vessel with the same angiographic severity, the FFR will be lower due to the large vascular bed and higher flow state giving rise to a greater hyperaemic transstenotic pressure gradient19.
Clinical studies of FFR and IMR in culprit and non-culprit vessels
In an all-comer ACS population including NSTEMI and STEMI (>24 hours post MI), 201 consecutive patients with non-flow-limiting lesions (FFR ≥0.75) had an event-free survival at 11 months of 90% with 7.5% suffering cardiac events related to the deferred coronary lesion, including one cardiac death and a 2% incidence of MI. In addition, most patients were free from angina20.
In a separate study of 48 patients, the ability of FFR to predict perfusion defects on radioisotope perfusion imaging performed at a mean of 3.7±1.3 days post NSTEMI and STEMI was shown to be around 90% at an optimal FFR cut-off of 0.7821.
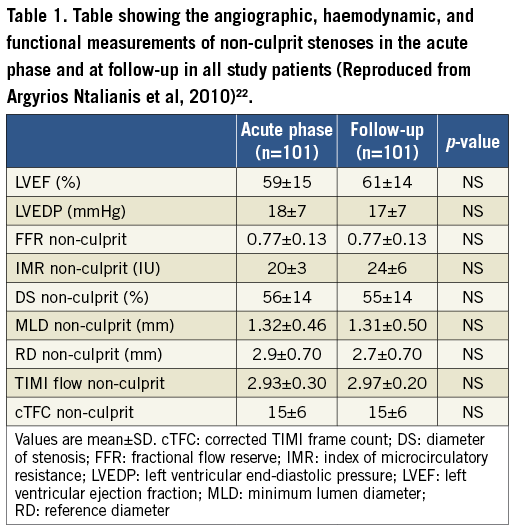
The most notable study to date was from Ntalianis et al who studied 75 acute STEMI patients and 26 NSTEMI patients (<72 hrs post onset). They measured FFR in the non-culprit stenoses immediately following PCI of the culprit vessel and then repeated the FFR at 35±4 days post initial procedure. There was no change in percentage stenosis or MLA despite a demonstrable improvement in LV ejection fraction. FFR remained unchanged between the acute and follow-up phases in patients with STEMI (0.78±0.10 vs. 0.76± 0.10, p=NS) and NSTEMI (0.77±0.10 vs. 0.77±0.20, p=NS) (Figure 1, Figure 2, Table 1). In only two lesions did an initial FFR >0.80 subsequently decrease to <0.75. In a small subgroup of 14 patients there was also no change in the index of microcirculatory resistance22.
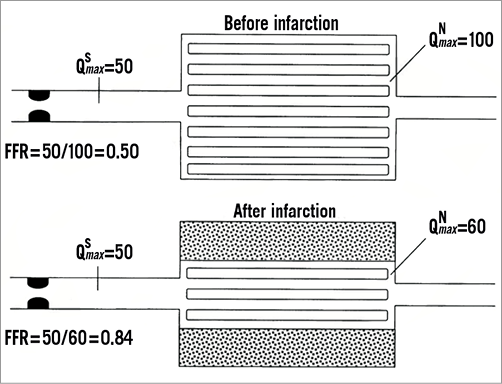
Figure 1. Schematic of coronary stenosis and its dependent myocardium before and after MI. FFR is defined as the ratio of maximal myocardial blood flow in the presence of epicardial stenosis (QSmax) to maximal myocardial blood flow in the absence of epicardial stenosis (QNmax). In clinical practice, FFR can be calculated by the ratio of distal coronary pressure to aortic pressure during hyperaemia. After MI, the amount of viable myocardium distal to stenosis is smaller than before, associated with a decrease in absolute hyperaemic blood flow. Therefore, in a hypothetical case, epicardial stenosis remains unchanged, hyperaemic pressure gradient decreased, and FFR increased. Thus, despite unchanged anatomic severity of stenosis, its functional severity has decreased because of the smaller amount of viable tissue to be supplied. (Reproduced with permission19).
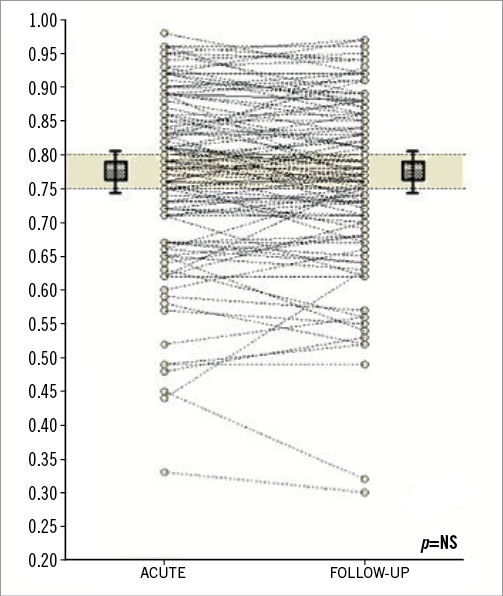
Figure 2. Plot of FFR values (Y-axis) of non-culprit coronary artery stenoses during the acute phase and follow-up (X-axis). (Reproduced with permission22).
A second study has examined the reproducibility of non-culprit lesion FFR in STEMI patients who had 55 non-culprit stenoses with at least a 50% diameter stenosis by visual angiographic assessment. FFR was performed at the time of primary PCI and repeated at 42±10 days. There was a small decrease in FFR over time (0.84±0.08 vs. 0.82±0.08, p=0.025) with a good correlation between the paired FFR measurements (Pearson’s coefficient R=0.85, p<0.001). In only three patients did the second FFR value lead to a reclassification of functional significance based on an FFR cut-off of ≤0.8018.
The resistance reserve ratio (RRR) is a measure of the ability to achieve maximal hyperaemia. It is the ratio of basal resistance (BR) to the index of hyperaemic microvascular resistance (IMR). Whereas IMR is a measure at peak hyperaemia and reflects structure, RRR quantifies the vasodilatory response of the coronary microcirculation to a hyperaemic stimulus (adenosine). Emerging data suggest this ratio has discriminatory value in patients with stable and unstable coronary disease (Figure 3).
Figure 3. Resistance reserve ratio formula. (Reproduced with permission from Layland J. et al, 2013)23.
In a prospective study by our group analysing RRR in 50 patients with stable angina, 40 patients with acute STEMI and 50 patients within one to four days of NSTEMI showed no significant difference between non-culprit vessels in stable angina (2.9 [2.3-3.9]) and either culprit vessels in stable angina (2.8 [1.7-4.8], p=0.75) or culprit vessels in NSTEMI (2.46 [1.6- 3.9]; p=0.61) (Table 2).
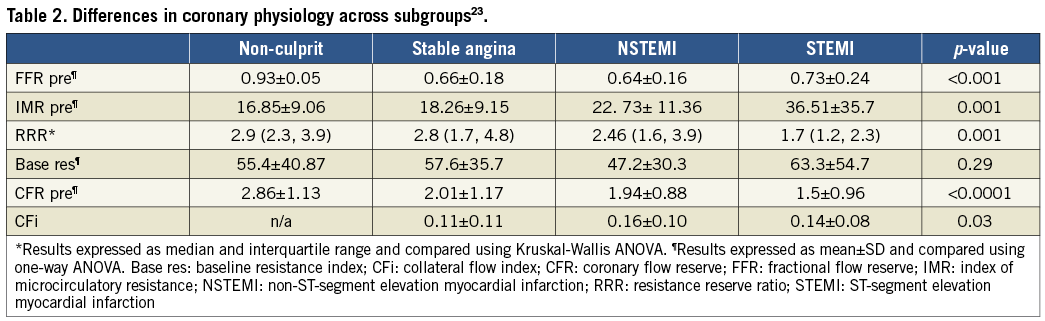
RRR was significantly lower in the STEMI patients (1.7 [1.2-2.3]; p<0.0001).There was no difference in IMR in patients with SA in the non-culprit vs. culprit vessel (16.8±9.1 vs.18.3 ±9.2; p=0.44). However, as expected, IMR was higher in NSTEMI and STEMI compared with the non-culprit SA vessel (NSTEMI, 22.7±11.3; p=0.015, STEMI, 36.5±35.8; p<0.0001) (Table 2)23.
Clearly, although the resistance indices can be higher in NSTEMI it appears that the microcirculation can dilate sufficiently in selected patients to enable maximal hyperaemia and allow valid FFR measurements.
IMR is a very important prognostic tool that can be measured during pressure wire studies in patients undergoing primary PCI. In a landmark recent study, 253 of these patients with IMR >40 had a higher rate of the primary endpoint of death or rehospitalisation for heart failure at one year than patients with an IMR ≤40 (17.1% versus 6.6%; p=0.027)24. This marker has the potential to identify those patients who may require closer follow-up and more aggressive medical management to avoid poorer outcome.
Whether in the setting of stable CHD or ACS and with both FFR and perfusion imaging one can never be certain that maximal hyperaemia has been achieved with adenosine or any other vasodilator drug. In both clinical settings there may be microvascular dysfunction, with the added possibility of microvascular “stunning” in ACS. Whilst, theoretically, this may result in false negative FFRs, the available data do not indicate that this compromises the ability of an FFR-guided revascularisation strategy to improve clinical outcomes. Microvascular dysfunction/“stunning” will also impair resting myocardial blood flow and thereby may also cause false negative results for any of the non-hyperaemic indices of stenosis severity such as Pd/Pa and iFR. In addition, whilst these indices have advantages in terms of cost, time and patient comfort, recently published studies have confirmed that, compared to FFR, they have equivalent reduced diagnostic accuracy and misclassify between 10-20% of lesions.
The time-dependent changes in microcirculatory function necessitating caution in acute coronary physiological assessment post primary PCI were well demonstrated in a study involving 44 STEMI patients who underwent physiological assessment immediately post primary PCI and a day later divided into low ejection fraction (EF) group (group 1, n=15), and high EF group (group 2, n=29). In the high EF group, IMR was 37 after primary PCI and 23 after 24 hrs (p=0.003). After primary PCI, median IMR did not change in the low EF group. CFR was significantly improved in the high EF group after primary PCI, median (interquartile range) CFR was 1.8 (1.1-2.4) and after 24 hrs CFR was 2.6 (2.1-3.2), p=0.002. In the low EF group, after primary PCI, median CFR was not significantly improved, at a median of 1.4 immediately post PCI and 1.9 after 24 hrs25. Clearly, a dynamic state in the microcirculation will mean that reproducibility in FFR may be potentially compromised, particularly in large viable revascularised territories.
FFR-guided decision making in patients with UA/NSTEMI
The FAME study showed that in patients with multivessel disease there was a 30% reduction in adverse cardiac events (death, MI, target vessel revascularisation) with an absolute risk reduction of 5% in the group undergoing FFR-guided PCI compared to those undergoing angiographically guided PCI26. A secondary analysis in 2011 clarified that the benefit observed in the overall trial population was also seen in the UA/NSTEMI group. Overall, FAME included 328 patients with UA/NSTEMI, of whom 178 were randomised to angiographically guided PCI and 150 to FFR-guided PCI. Patients who had had a myocardial infarction with ST-segment elevation could be included if the infarction had occurred at least five days before PCI. Patients who had had a myocardial infarction without ST-segment elevation could be included earlier than five days after the infarction if the peak creatine kinase level was less than 1,000 U per litre.
An absolute reduction in adverse cardiac events of 5.1% was observed in the FFR-guided group as well as less contrast usage and on average one stent less per patient (1.9±1.5 vs. 2.9±1.1, p<0.01)27. Overall, the absolute risk reduction was slightly higher in the ACS patients compared to the rest of the study patients while the relative risk reduction was similar, implying that, although there are theoretical concerns regarding global microvascular dysfunction extending beyond the culprit vessel territory, practically this does not appear to be a clinical issue affecting the benefit of FFR-guided PCI in ACS patients (Figure 4).
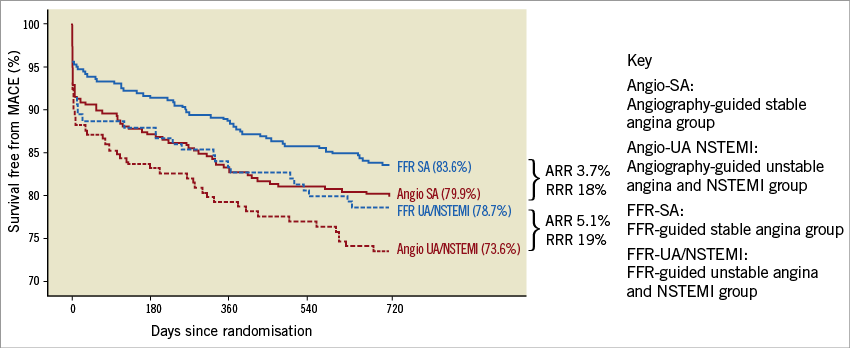
Figure 4. Kaplan-Meier curves for survival free from MACE at two years stratified to diagnosis and treatment strategy. Also indicated are absolute risk reduction (AAR) and relative risk reduction (RRR) of MACE by fractional flow reserve (FFR)-guided percutaneous coronary intervention in patients with unstable angina (UA) or non-ST-segment elevation myocardial infarction (NSTEMI) and patients with stable angina (SA). (Reproduced with permission27).
In a separate observational study of 106 patients with NSTEMI, PCI was deferred as the culprit vessel FFR was >0.75. The one-year event rate was 1.9% mortality, 0.9% target vessel revascularisation and 4.7% for readmission with a cardiac cause, further emphasising the clinical value of FFR in non-culprit vessels of patients with ACS28.
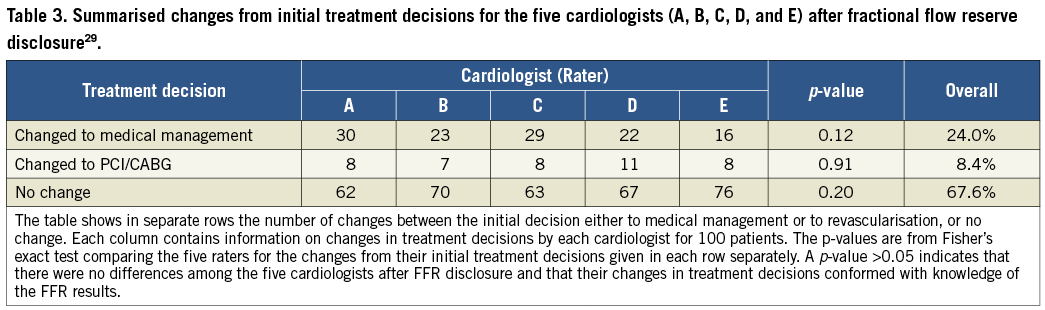
A recent study by our group in patients with UA/NSTEMI looked at the impact of FFR on decision making by a group of experienced interventional cardiologists. After FFR disclosure, the treatment plan was altered in 46% of patients (p=0.0016). Changes in favour of medical therapy occurred in 24% of patients (p=0.0016) (Table 3)29. This figure is in line with 1,075 patients (19% with recent ACS) from a recent French FFR registry, 43% of whom had reclassification of their treatment group following FFR disclosure30.
Ongoing clinical trials of FFR-guided PCI in patients with ACS
Despite the theoretical considerations outlined above, the weight of evidence suggests that non-culprit FFR can provide useful information regarding functional significance in a high proportion of patients with ACS. On this basis, FFR-guided decision making in patients with STEMI and MVD is now being tested in a series of randomised controlled trials.
The COMPARE ACUTE study (NCT01399736) is a randomised controlled trial (RCT) in STEMI patients with MVD in The Netherlands, with an estimated enrolment of 885 patients divided into immediate FFR-guided complete revascularisation versus staged non-culprit PCI (ischaemia-driven) by proven ischaemia or recurrent symptoms. The primary endpoint will be a composite of death, non-fatal MI, CVA or revascularisation at 12 months. This study is estimated to end in 201831.
The COMPLETE study (Complete vs. Culprit-only Revascularisation to Treat Multi-vessel Disease After Primary PCI for STEMI; NCT01740479) is an RCT comparing FFR-guided revascularisation within 72 hrs of primary PCI versus optimal medical therapy for the endpoint of a composite of cardiovascular death or MI at four years. It began recruiting in 2012 and is due to report its findings in 201832.
The Primary PCI in Patients With ST-elevation Myocardial Infarction and Multi-vessel Disease: Treatment of Culprit Lesion Only or Complete Revascularisation (PRIMULTI, NCT01960933) study is an RCT of patients with STEMI and MVD with a comparison of the clinical outcome after complete FFR-guided revascularisation versus treatment of the infarct-related artery only during primary PCI. The primary outcome is all-cause death, MI or revascularisation at 48 months. This study, which is based in Denmark, has finished recruiting and is due to report its findings later this year33.
The FAMOUS – NSTEMI trial (Fractional Flow Reserve versus Angiography in Guiding Management to Optimise Outcomes in Non-ST-Segment Elevation Myocardial Infarction) is a prospective multicentre parallel group 1:1 randomised controlled trial in 350 NSTEMI patients with ≥1 coronary stenosis ≥30% severity (threshold for FFR measurement) (NCT01764334). Patients were randomised during coronary angiography to the FFR-guided group or angiography-guided group. All patients had FFR measurement in all vessels with a coronary lesion of ≥30% diameter stenosis severity. FFR was disclosed to the operator in the FFR-guided group but not disclosed in the angiography-guided group. In the FFR-guided group, an FFR ≤0.80 was an indication for revascularisation by percutaneous coronary intervention (PCI) or coronary artery bypass surgery (CABG), as appropriate. The primary outcome is the between-group difference in the proportion of patients allocated to medical management. This trial is also fully recruited and will present its results this year34.
Guidelines and recommendations
The 2011 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation recommend FFR is ideally performed >5 days after the acute event in order to minimise the impact of any microvascular disturbance18. The current ESC guidelines found no indications for the use of FFR during the acute phase of a STEMI. In fact, the recent Society of Cardiovascular Angiography and Interventions (SCAI) publication entitled “Expert Consensus Statement on the Use of Fractional Flow Reserve, Intravascular Ultrasound, and Optical Coherence Tomography” advises avoidance of the use of FFR altogether in ACS and STEMI, though this is clearly another example of the evidence being ahead of the current guidelines35.
We would offer the following recommendations regarding FFR in ACS and STEMI:
– Non-culprit vessel FFR values should always be interpreted with caution and never performed prior to culprit vessel PCI, in the presence of persistent haemodynamic instability or symptoms.
– Measuring FFR in the culprit vessel of patients with STEMI is not recommended in the acute setting. FFR is reliable in the culprit vessel of patients more than six days after STEMI.
– Measurement errors are a potential problem in patients with extensive STEMI and significant haemodynamic disturbances (elevated LVEDP) as well as extensive microvascular dysfunction.
– High-dose adenosine has been shown to be safe (no excess mortality or MI) in patients with STEMI in a recent Cochrane review35 when used for treatment of no-reflow. Pressure wire studies in haemodynamically stable patients should not be avoided due to unnecessary concerns over adenosine safety given its short half-life and clinical safety as outlined in a recent review by our group36.
– FFR values in the non-culprit vessels of patients with STEMI may serve as a useful guide to treatment particularly if strongly indicative of ischaemia with values <0.75.
– In the acute setting, if an ischaemic FFR value is confirmed then intervention should be considered on an ad hoc or staged basis. If there is concern about a false negative FFR due to high peri-infarct microvascular resistance, then it would be appropriate to consider subsequent non-invasive testing or alternatively a repeat FFR assessment at a later date.
– Measuring FFR in the non-culprit vessels of patients with NSTEMI is beneficial, just as it is in stable patients.
– Measuring FFR in the culprit vessel of patients with NSTEMI appears reliable in the acute setting, but requires further study.
– A negative FFR in the setting of NSTEMI with a likely embolic event should prompt aggressive secondary prevention with a view to plaque stabilisation, though further modalities of assessment including IVUS/OCT/near-infrared spectroscopy may be indicated in order to determine further the underlying pathophysiology and guide the need for stent implantation.
Conclusion
Despite the theoretical concerns, the use of FFR is well validated in patients with NSTEMI in both the culprit and non-culprit vessels. Data regarding the safety and utility of FFR in the non-culprit vessels of patients with STEMI are less well established though there are indications in small clinical studies that it may be a safe and sufficiently accurate modality of assessment. Further studies are required and the clinical trials outlined above will add greatly to what is currently known.
Conflict of interest statement
W. Fearon has received research grant support from St. Jude Medical. K. Oldroyd has received consultancy fees from St. Jude Medical, Boston Scientific, Biosensors and AstraZeneca. The other authors have no conflicts of interest to declare.

