Based on the sound mathematical principles underpinning its derivation and the wealth of clinical outcome data supporting its use, fractional flow reserve (FFR) has become the reference standard for evaluating the functional significance of epicardial coronary artery stenoses in patients with stable ischaemic heart disease. Percutaneous coronary intervention (PCI) for stenoses with an FFR value ≤0.80 improves clinical outcomes and quality of life compared with medical therapy1,2. On the other hand, patients with lesions with an FFR value >0.80 do just as well, if not better, when treated medically1,3. Most of these data, however, are derived from patients with stable ischaemic heart disease.
The validity of FFR relies on the assumption that microvascular resistance is minimised and stable. However, in patients presenting with an acute coronary syndrome (ACS), variable degrees of transient microvascular dysfunction can occur, particularly in the culprit vessel territories of ST-segment elevation myocardial infarction (STEMI), owing to embolisation of ruptured plaque and thrombus, as well as in situ thrombosis, inflammation, and vasoconstriction. Therefore, FFR measurement is not recommended in the culprit vessel in the acute setting of STEMI. However, data suggest that measurement of FFR in non-culprit vessel territories of STEMI, where less, if any, transient microvascular dysfunction is expected to occur, is reliable and can be used to guide revascularisation decisions4.
Two randomised, multicentre studies recently utilised FFR to guide revascularisation decisions for non-culprit lesions in patients with STEMI and multivessel disease. In the DANAMI-3-PRIMULTI trial5, 627 patients presenting with STEMI who had multivessel disease were enrolled and randomised to either infarct-related artery only revascularisation (IRA-only group, n=313) or FFR-guided complete revascularisation (n=314) (with FFR-guided treatment of non-IRA lesions two days after primary PCI). There was a significant reduction in major adverse cardiac events (MACE), a composite of all-cause mortality, non-fatal reinfarction, and ischaemia-driven revascularisation at a median follow-up of 27 months in the patients assigned to FFR-guided complete revascularisation.
The subsequent Compare-Acute trial6 assigned 885 patients presenting with STEMI and multivessel coronary disease to either culprit vessel only PCI or complete revascularisation guided by FFR measurement of the non-culprit lesions at the time of primary PCI. It showed improved overall outcomes in the patients who underwent FFR-guided complete revascularisation. In the DANAMI-3-PRIMULTI trial and the Compare-Acute trial, 31% and 50%, respectively, of the non-infarct-related coronary lesions considered to be angiographically significant were not functionally significant (FFR >0.80). The longer-term outcome of these lesions, as well as the residual disease in the IRA-only PCI group of patients, has not been well studied.
In this issue of EuroIntervention, De Backer et al report a substudy7 of the DANAMI-3-PRIMULTI trial with the aim of understanding better which lesions are most likely to result in a subsequent ischaemia-driven revascularisation and the timing of these events after primary PCI of the IRA only or after FFR-guided complete revascularisation.
As previously reported, ischaemia-driven revascularisation was significantly less frequent in the FFR-guided complete revascularisation group than in the IRA-only group (17/314 patients [5%] vs. 52/313 patients [17%]; p<0.001). In both groups, the primary reason for ischaemia-driven revascularisation was related to non-culprit, non-treated lesions (n=71/82 lesions in the IRA-only group; n=13/26 lesions in the complete revascularisation group). De novo lesions (8/82 and 7/26) or previously treated lesions (3/82 and 6/26 lesions) were less frequent causes of repeat revascularisation, although relatively speaking, in the FFR-guided complete revascularisation group, patients were just as likely to return for revascularisation of a de novo or previously treated lesion as they were a lesion deferred based on FFR. In the IRA-only group, non-culprit untreated lesions with more severe stenosis and in more proximal segments tended to have a higher rate of ischaemia-driven revascularisation. In particular, the majority of patients who presented with angina class IV/unstable angina during follow-up had ≥80% stenosis in the left anterior descending artery or in the right coronary artery.
An important finding of this study is that, in patients with STEMI, the rate of revascularisation of a non-culprit lesion which was deferred based on FFR is very low. Of the 143 deferred lesions in the FFR-guided revascularisation group, repeat revascularisation was performed in 13 non-culprit untreated lesions of 11 patients (five urgent and six non-urgent); only nine of these lesions were actually deferred based on FFR at the time of index admission. Only three patients (1%) were revascularised within one year after the index admission. These results support the safety and utility of FFR-guided complete revascularisation in patients with STEMI, arguing against concerns about global microvascular dysfunction resulting in inaccurate FFR measurement in the non-culprit territories.
There are a couple of other points worth considering when interpreting the results. First, given the open-label design of the study, treating physicians might have been biased towards earlier repeat revascularisation in individuals in the IRA-only PCI group. Arguing against this is the surprising finding that the number of those admitted because of suspected cardiac ischaemia was similar in both groups; one would have expected the FFR-guided complete revascularisation group to have a lower admission rate. Of note, the vast majority of admissions in both groups were due to stable angina; however, the number of patients admitted because of class IV or unstable angina was significantly higher in the IRA-only PCI group. Second, because we do not have follow-up FFR data at the time of repeat revascularisation in either group, we do not know whether the treated lesion was actually responsible for myocardial ischaemia.
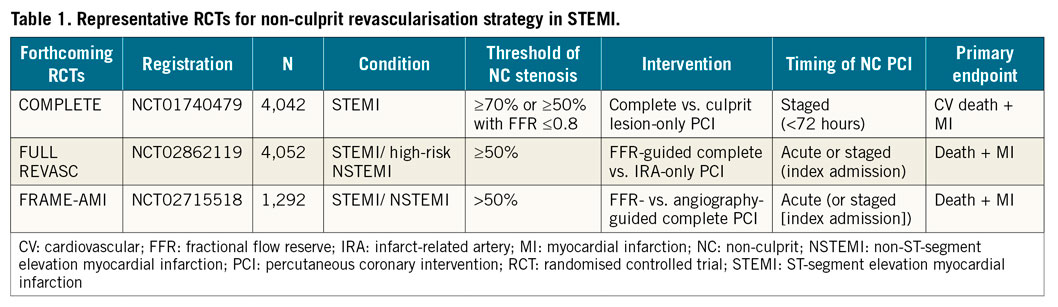
The clinical implications of this study support those of a previous substudy evaluating patients with ACS and showing that functionally complete revascularisation based on FFR does not leave behind untreated lesions which are likely to cause adverse cardiac events8. Moreover, there are no reliable angiographic predictors to indicate which lesion is most likely to require ischaemia-driven revascularisation if left untreated, supporting the role of up-front FFR-guided complete revascularisation. Ongoing larger studies powered for hard clinical endpoints will inform us regarding the differences between angiography-guided versus FFR-guided complete revascularisation (Table 1).
Conflict of interest statement
W. F. Fearon receives institutional research support from Medtronic, Abbott and CathWorks, and has minor stock options with HeartFlow. T. Nishi has no conflicts of interest to declare.

