
Disease in a non-culprit vessel is observed in up to 50% of ST-elevation myocardial infarction (STEMI) patients. Randomised trials, such as PRAMI1, CvLPRIT2, DANAMI-3-PRIMULTI3 and Compare-Acute4, demonstrated improved composite clinical outcomes with complete revascularisation (CR). More recently, the largest study, the COMPLETE trial, showed a reduction in hard clinical endpoints (death and recurrent myocardial infarction [MI]) with CR5. Considered together with a very recently published meta-analysis6, the data strongly support CR in multivessel disease (MVD) STEMI patients and it certainly appears to be worth the effort. It is unlikely that further trials comparing complete versus incomplete revascularisation will be undertaken (although it should be noted that none thus far have included a CTO as the non-infarct-related artery [N-IRA]).
Despite these robust data, a number of questions remain. For example, do the benefits of CR last over the longer term? When should CR be undertaken: pre or post discharge? Is invasive physiological assessment of the bystander lesion essential to determine which lesions should be tackled and, importantly, is a CR strategy cost-effective? The paper by Smits et al7 in the current edition of EuroIntervention addresses two of these questions (longer-term outcomes and cost/efficacy) and allows a chance to discuss the other vexed question that clinicians face – the role of invasive physiological testing in selecting which lesions to treat.
Regarding timing, it is generally accepted that CR should preferably be completed pre discharge as per ESC Guidelines8, although a substudy from the COMPLETE investigators9 suggested that it could be undertaken post discharge (<45 days). A caveat is that these findings may be open to influence bias since CR timing was based on operator choice rather than being randomised.
The paper by Smits et al reports the three-year follow-up of the Compare-Acute study, which randomised 885 patients in a 2:1 fashion with multivessel PCI (N-IRA lesions included if fractional flow reserve [FFR] was <0.80)7. The authors are to be congratulated for low loss to follow-up and the inclusion of cost efficacy of CR in STEMI. This longer-term analysis shows sustained benefit of CR in terms of composite death/MI/repeat revascularisation and stroke.
It is more than interesting that there was no difference in hard endpoint outcomes at this follow-up time, as compared to our recently published CvLPRIT longer-term analysis, and the COMPLETE trial at three years. For example, at a median 5.6 years, CvLPRIT showed a significant reduction in death and MI with CR10, whereas in Compare-Acute and the other FFR-guided strategies this was driven predominantly by ischaemia-driven revascularisation (IDR).
Speculatively, the difference could be based on how lesions were assessed for trial inclusion, with CvLPRIT (and COMPLETE) being almost solely selected by angiographic assessment and the Compare-Acute through the use of physiologic FFR testing. The recent meta-analysis has also shown that differences in hard endpoint outcomes depend on whether angiographic or physiological assessment of non-culprit lesions was used (Figure 1),6. Could it be that FFR is more likely to predict lesion flow and thus revascularisation need/outcome11, whereas angiographic selection is more likely to include potentially more complex disease and so, by default, harder endpoints are reduced in the longer term when non-culprit lesions are treated?
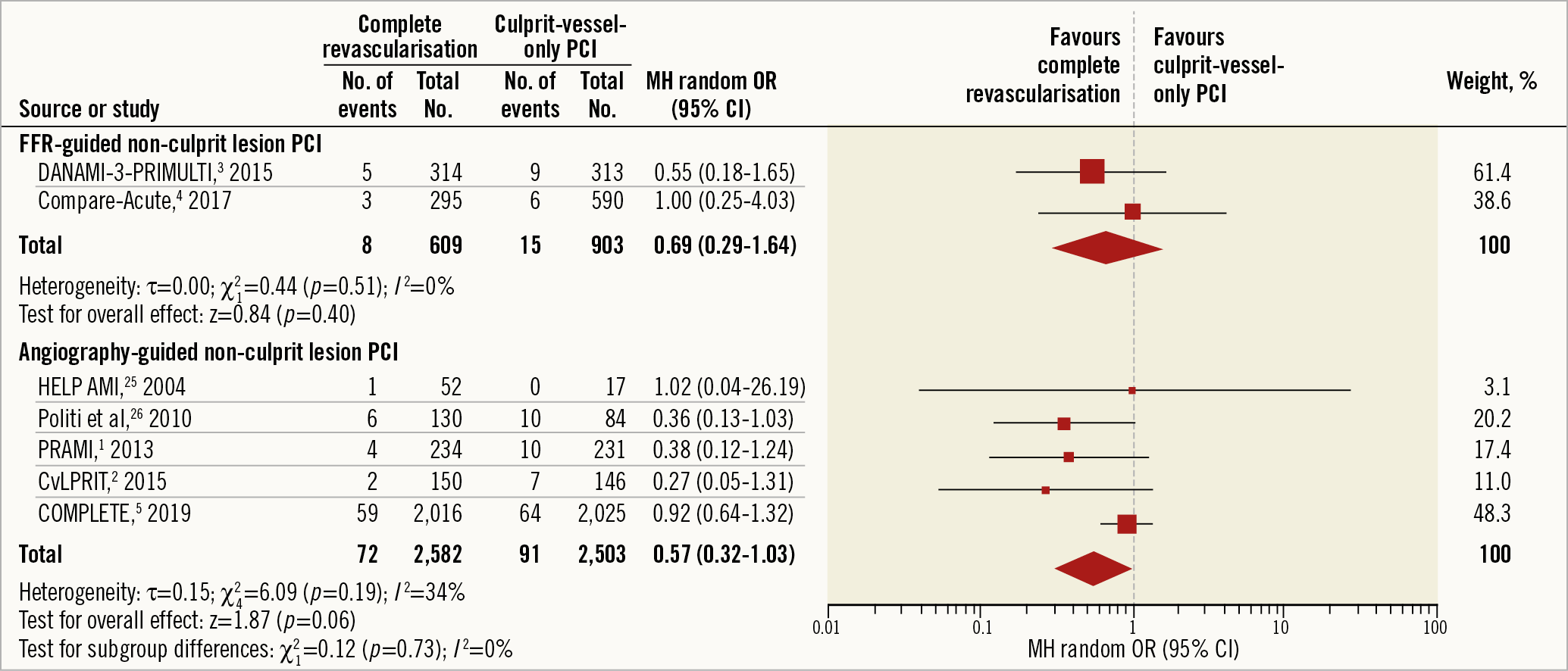
Figure 1. Longer-term cardiovascular death according to method of non-culprit lesion assessment – results from a systematic review and meta-analysis. Reproduced with permission from Bainey et al6.
In CvLPRIT, 85% of patients had a visual assessment of >70% stenosis. In COMPLETE, this was even higher with >95% of lesions having an angiographic severity of >70% (and nearly 60% with at least 80% stenosis) with <1% included with an FFR <0.80 in lesions 50-69%. Physiological assessment was essentially not used in PRAMI, CvLPRIT and COMPLETE but guided trial inclusion in DANAMI-3 and Compare-Acute. In Compare-Acute, the lesion had to be >50% to be included, which may explain why only 50% of lesions had an FFR <0.80. The question is does selection of lesions by FFR predict flow severity (and predominantly follow-up IDR need), whereas angiographic selection (providing they are >70%), by default, selects complexity and vulnerability? For example, if you exclude lesions based on FFR12 by default, then ~25% of lesions in the angiographic range >70%, which may include complex and/or vulnerable ones, would be out.
FFR versus angio-guided – the concept of vulnerable plaque in non-culprit lesions
So why are there outcome differences between these studies, and are they more than academic musings with clinical implications? Why is there discord in outcome endpoints between similar studies that ask the same questions. While the answer may lie in the way in which non-culprit lesions were selected, it may not be that simple and unravelling the concepts is difficult.
Plaque morphology must play a role in predicting hard endpoint outcomes. In particular, thin-cap fibroatheroma (TCFA) is associated with increased plaque rupture since this thin cap separates the necrotic thrombogenic core from the lumen13. The PROSPECT study demonstrated highest major adverse cardiac event (MACE) rates at 3.4 years in coronary lesions that had a combination of minimum lumen area (MLA) <4.0 mm2, high plaque burden (>70%) and TCFA14. Similarly, the VIVA study also used virtual histology intravascular ultrasound (VH-IVUS) to show that MACE in non-culprit lesions was independently associated with VH-TCFA (HR 7.53, p=0.038) and plaque burden >70% (HR 8.13, p=0.011)15. A substudy from the COMPLETE trial showed that 50% of non-culprit lesions were composed of TCFA-vulnerable morphology (Pinilla-Echeverri N. Non-Culprit Lesion Plaque Morphology in Patients With ST-Segment Elevation Myocardial Infarction: Results from the COMPLETE Trial Optical Coherence Tomography (OCT) Substudy. Presented at AHA Scientific Sessions 2019, Philadelphia, PA, USA, 19 November 2019).
Using physiologic testing to select N-IRA lesions may be counterintuitive for predicting long-term hard endpoint outcomes, unless physiologic measures can be shown to correlate with plaque vulnerability.
So, can physiological testing highlight vulnerable plaques in intermediate (or even in severe) lesions? Is further imaging (VH-IVUS, optical coherence tomography [OCT] or near-infrared spectroscopy [NIRS]) still needed in patients to predict vulnerability, even in the more severe lesion or does angiographic severity, by default, predict vulnerability as well?
Although the FAME trial showed that a composite of death and MI was significantly reduced with an FFR-guided strategy compared with an angiography-driven revascularisation16, FFR in the acute setting may be influenced by it being undertaken peri-infarct. While an analysis by Ntalianis et al of 112 non-culprit lesions in 101 patients presenting with acute coronary syndrome (ACS) and MVD showed no change in mean FFR values between index admission and 35 days, in two patients there was a fall in the FFR from non-significant to significant17. Furthermore, in the REDUCE-MVI study of 73 patients, N-IRA FFR values measured during the index procedure showed a mean decrease in the FFR measurement of 0.03 to 30-day follow-up. This was most pronounced in patients with larger infarcts. This suggests that non-culprit lesion (NCL) significance may be underestimated with FFR in the acute setting18, probably due to increased microvascular resistance and blunted adenosine response at the time of infarct. Hence, it is possible in N-IRA lesions assessed by FFR during index admission that the FFR reading could be lower than the follow-up reading, and so some “significant” N-IRA lesions may not included. This may have an impact on hard endpoint outcomes.
On the other hand, relying purely on angiographic assessment of N-IRA lesions may be fraught, with selection of lesions that appear significant at the time of index procedure, but less so on subsequent angiography. Analysis of N-IRA lesions between infarct and post-infarct angiography has shown increase in the MLD of these stenoses as well as re-classification of severity of stenosis by up to 10% diameter severity19. Dönmez et al showed a significant decrease in severity of N-IRA lesions in 20% of patients at follow-up quantitative coronary angiography (QCA)20.
Can FFR predict plaque vulnerability?
There are some data to support a relationship between flow limitation and vulnerability. In a study of CT angio-defined vulnerable lesions, Driessen et al found a strong correlation with hyperaemic FFR (area under the curve [AUC] 0.82 [0.77-0.87]), plaque burden (surely surrogated by FFR) and acute events in an invasive imaging study21. Usui et al correlated OCT TCFA with severity of flow measured by FFR22. On the other hand, the FAME 2 trialists at five years showed (albeit in stable patients) that FFR predicted urgent revascularisation but not hard endpoint outcomes23. There is more important research work to be done on the relationship between FFR and plaque vulnerability but, as it stands, FFR assessment appears unlikely to predict the more important hard endpoint outcomes.
Is CR cost-effective?
With trials5,10 demonstrating hard endpoint outcomes on the basis of angiographic inclusion criteria alone, such questions on the need for/value of FFR are important in terms of time, resource use and cost efficacy. In this context, Smits and colleagues included a cost/benefit analysis, showing a cost reduction for CR at one and three years. However, this analysis is based upon bundled payment (diagnosis-related groups [DRGs]) from a payer perspective, with differing cost benefit demonstrated by the country DRG payment system used. Hence, it lacks a detailed cost-effectiveness breakdown (neither quality of life, quality-adjusted life-years [QALY] nor indeed actual costs used per individual patient admission were included). That said, the study overall was positive in terms of cost/efficacy and so supports the economic evaluation benefit which the CvLPRIT trial also showed in terms of QALY gain (assessed using the EQ-5D questionnaire)24.
However, even if cost-effective, the question remains as to whether FFR is worth doing if it is unlikely to predict patients with hard endpoint benefit: does it aid N-IRA selection in the everyday clinical setting and, if so, for which N-IRAs? Would you consider FFR for all N-IRA (including lesions >70% to help “predict vulnerability”), or only for those with intermediate lesions and, if so, what is “intermediate”? Do you use FFR at all, or solely determine which non-culprit lesions to treat through angiographic severity?
More insightful data are likely to come from the iMODERN trial (NCT03298659) which will assess instantaneous wave-free ratio (iFR)-guided revascularisation of N-IRA with >50% diameter stenosis and iFR ≤0.89 during index procedure/index hospitalisation with all-cause death, recurrent MI and heart failure hospitalisation as one-year outcomes. It was hoped that a formal evaluation of FFR-guided complete revascularisation in STEMI MVD would be possible from the FULL REVASC trial (NCT02862119), but this has halted recruitment in the light of the results from COMPLETE, although it is hoped that the available FFR data may help to answer some of these questions. Furthermore, we await the results of the FLOWER-MI trial (NCT02943954) which will determine whether FFR in addition to angiography improves cardiovascular outcomes. Trial completion is estimated December 2021.
So where does that leave us for everyday practice? If you see a bystander lesion in a STEMI patient that looks angiographically >70% stenosed, then published data suggest that hard endpoint outcomes are improved in the short, medium and longer term by CR. Smits and colleagues’ data suggest that the general use of physiologic measurements appears beneficial and cost-effective but that outcomes are driven by IDR. In current practice, most will only use physiologic measures for lesions between 50 and 70%. Whether physiologic measurements help to define further which patients are more likely to have vulnerable plaques is currently undetermined, but it is possible. Further work linking abnormalities in physiological flow and vulnerability is clearly needed.
There remain unanswered questions within this field. However, in answer to this editorial’s title, CR in the STEMI patient appears well worth it on all fronts.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

