Abstract
Background: In patients with ST-segment elevation myocardial infarction (STEMI) who have multivessel disease, the FLOWER-MI trial found no significant clinical benefit to fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) compared to angiography-guided PCI.
Aims: Our aim was to estimate the cost-effectiveness and cost-utility of FFR-guided PCI, the secondary endpoint of the FLOWER-MI trial.
Methods: Costs, major adverse cardiovascular events (composite of all-cause death, non-fatal myocardial infarction [MI], and unplanned hospitalisation leading to urgent revascularisation), and quality-adjusted life years were calculated in both groups. The incremental cost-effectiveness and cost-utility ratios were estimated. Uncertainty was explored by probabilistic bootstrapping. The analysis was conducted from the perspective of the health care provider with a time horizon of one year.
Results: At one year, the average cost per patient was 7,560€ (±2,218) in the FFR-guided group and 7,089€ (±1,991) in the angiography-guided group (p-value<0.01). The point estimates for the incremental cost-effectiveness and cost-utility ratios found that the angiography-guided strategy was cost saving and improved outcomes, with a probabilistic sensitivity analysis confirming dominance.
Conclusions: The FFR-guided strategy at one year is unlikely to be cost effective compared to the angiography-guided strategy on both clinical and quality of life outcomes.
Introduction
The relevance of fractional flow reserve (FFR)-guided complete revascularisation compared to culprit-only treatment of multiple coronary artery stenoses has recently been evaluated12. With FFR guidance, the haemodynamic relevance of coronary stenoses of intermediate severity (50-70%) can be determined during coronary angiography, allowing appropriate guidance of stent implantation in patients with stable coronary artery disease. In patients with ST-segment elevation myocardial infarctions (STEMI), the Compare-Acute and COMPLETE trials found a reduced rate of events with complete revascularisation guided by FFR measurements, compared with a strategy of culprit-only percutaneous coronary intervention (PCI)12. The potential discrepancy between visual (angiography only) and haemodynamic (FFR guidance) findings may reduce the need for stenting if angiographic stenoses turn out to be without haemodynamic relevance. Although neither trial included an economic evaluation, their results, with a two-fold reduction in major adverse cardiovascular events (MACE), suggested that the additional cost of MACE in the control groups (culprit lesion-only treatment) could offset the initial costs of the FFR-guided complete revascularisation strategy (costs of the guidewire and additional stenting).
In chronic coronary syndromes, the FFR Versus Angiography for Multivessel Evaluation (FAME) study demonstrated improved health outcomes at one year and the ancillary economic evaluation found that FFR was significantly cost saving with an average cost reduction of $2,385 (1,681€)3 per patient4. The potential discrepancy between visual (angiography only) and haemodynamic (FFR guidance) findings may reduce the need for stenting and the added cost of the pressure wire if angiographic stenoses turn out to be without haemodynamic relevance.
The FLOW Evaluation to Guide Revascularization in Multivessel ST-Elevation Myocardial Infarction (FLOWER-MI) trial was designed to assess whether FFR-guided complete revascularisation translated into better clinical outcomes and lower costs compared to angiography-guided complete revascularisation in STEMI patients with multivessel disease (MVD)5. In the angiography-guided group, PCI was performed for all coronary stenoses of ≥50% reduction in vessel diameter by visual estimation and considered amenable to PCI. In the FFR-guided group, FFR was measured in all lesions judged to have a stenosis of ≥50% reduction in vessel diameter by visual estimation and considered amenable to PCI. An FFR value ≤0.80 was considered significant for ischaemia with a recommendation that non-culprit PCI be performed. Although the use of stents was reduced with the FFR-guided strategy, the trial found no evidence of a clinical benefit6. The prespecified economic evaluation was conducted alongside the trial to provide additional data on cost-effectiveness and cost-utility of the FFR-guided strategy.
Methods
Study design and oversight
The FLOWER-MI trial protocol and clinical results have been published56. In short, the investigator initiated a multicentre, randomised trial and compared FFR-guided complete revascularisation in the acute setting of primary PCI to angiography-guided complete revascularisation in STEMI patients.
The study protocol was approved by an ethics committee (CPP Ile de France XI, April 14, 2016) and registered in the ClinicalTrials.gov registry (NCT02943954).
The study was funded by a 2015 grant from the “Programme Hospitalier de Recherche Clinique” (PHRC) issued by the French Ministry of Health. It was sponsored by Assistance Publique-Hôpitaux de Paris, with financial assistance provided by the St. Jude Medical Company, which provided the coronary pressure guidewire (Radi Medical Systems). The authors were solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. The steering committee vouched for the accuracy and completeness of the data and analyses, and for the fidelity of the trial to the protocol, available at NEJM.org6.
Cost-effectiveness and cost-utility analyses
The primary economic analysis was a within-trial cost-effectiveness analysis undertaken from a healthcare system perspective over a one-year period and followed the CHEERS guidelines7. The primary endpoint for the effectiveness was defined as the composite of all-cause death, non-fatal myocardial infarction, and unplanned hospitalisation leading to urgent revascularisation (MACE), at one year between the FFR-guided group and the angiography-guided group, calculated as the total number of events in each group divided by the total population. The cost-effectiveness analysis was complemented by a cost-utility analysis. The utility was expressed as the difference in quality-adjusted life years (QALY) between the two strategies. QALY represents a patient’s survival time weighted by the quality of life, represented by a utility function. Utility values were collected at baseline, day 30, 6 and 12 months using the EQ-5D-5L health-related quality of life questionnaire8. The EQ-5D-5L comprises a descriptive system which is composed of five health dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) with five levels of health state (no problems to extreme problems). The participant’s answers are combined to produce a five-digit number describing the participant’s health status which is converted to a utility value from the country-specific value set. The French EQ-5D-5L value set was used in this economic study and has utility values between −0.525 (worst possible health) and 1 (best possible health)9.
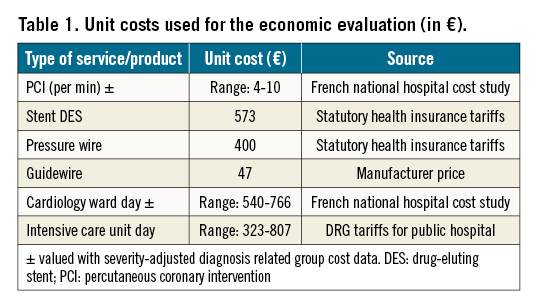
Hospital resource use data were obtained from discharge summaries prospectively collected at 30 days from the index admission. The cost of the index admission was valued with severity-adjusted diagnosis-related group cost data obtained from the national hospital cost study10, adjusted for actual length of stay, by type of unit and actual use of intensive care reported in the patients’ electronic case report forms (eCRF). Unit costs are presented in Table 1. Repeat hospital admissions were identified from the study eCRF for adverse events. Unit cost used for adverse events are presented in Supplementary Table 1.
Total costs were estimated from the date of recruitment until the earliest date of death, withdrawal from the study, or at 12 months. Non-hospital costs were not included in the cost calculations. All costs were valued at 2021 prices.
Both costs and outcomes were undiscounted because of the short time horizon.
The incremental cost-effectiveness ratio (ICER) and incremental cost-utility ratio (ICUR), defined as the difference in cost between the two strategies divided by the difference in effectiveness and utility, were calculated in cost per MACE averted and in cost per QALY gained.
The statistical analyses were performed on the intention-to-treat population. Multivariate imputation by chained equations (MICE) was used to process missing data1112. Imputed datasets were generated using predictive mean matching from a set of imputation models constructed from all potential prognostic factors: sex, age, site, time spent in the trial and by intervention group.
Cost and efficacy data were expressed as mean±standard deviation. Between the two groups, differences in MACE and rehospitalisations were compared with a Poisson model or by negative binomial regression depending on the variance and the mean. The difference in QALYs was compared using Student’s t-test or Mann-Whitney test depending on the distribution. The difference in costs was compared with a permutation test. Other quantitative data were compared using Student’s t-test. Where the assumption of equal variances was not met, a Welch correction was applied. The non-parametric Mann-Whitney test was carried out in the case of non-normal distribution.
The uncertainty of the results was analysed using a non-parametric bootstrap, which provided multiple estimates of the ICER by randomly resampling the patient population 1,000 times. Results were presented as a scatter plot of 1,000 ICERs on the cost-effectiveness plane and transformed into a cost-effectiveness acceptability curve based on the decision-makers’ willingness to pay for an additional QALY.
All the 95% confidence intervals (95% CI) were estimated with this bootstrap technique.
Finally, we performed a subgroup analysis to present the clinical and economic outcomes in patients, according to the type of procedure for non-culprit lesions (complete revascularisation during index procedure or complete revascularisation performed during another, staged procedure).
A p-value less than 0.05 was considered significant.
All health economic analyses were done with R version 4.0.1 (The R Foundation)13.
Results
Patients
Between December 2016 and December 2018, a total of 1,163 patients with STEMI and MVD were enrolled in the trial. Among them, 586 patients were randomly assigned to receive FFR-guided complete revascularisation, and 577 to receive angiography-guided complete revascularisation.
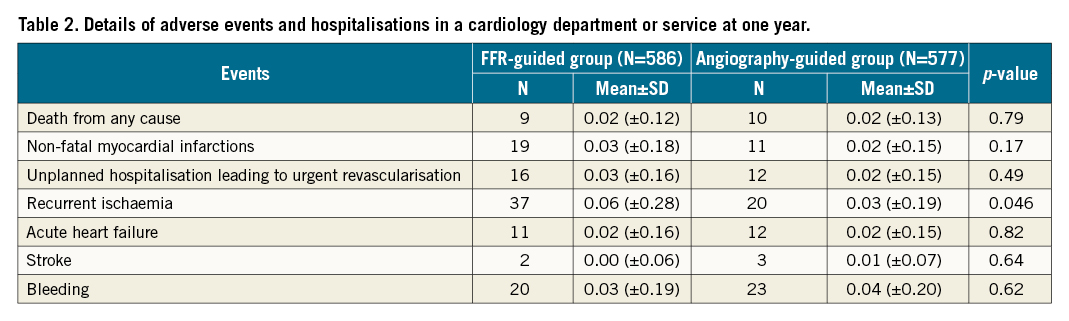
Details of adverse events and hospitalisations in a cardiology department or service at one year are presented in Table 2.
Cost-effectiveness and cost-utility results
Results are presented in Table 3.
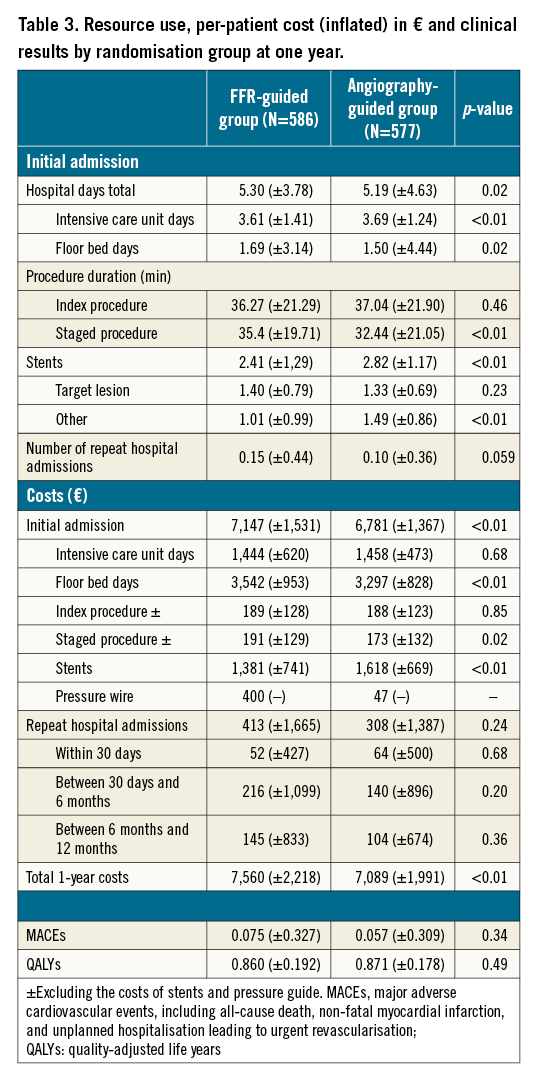
The average length of stay for initial hospitalisation was 5.30 days (±3.78) for the FFR-guided group, and 5.19 days (±4.63) for the angiography-guided group (p-value=0.02). The average total cost per patient at one year was 7,560€ (±2,218) for the FFR-guided group and 7,089€ (±1,991) for the angiography-guided group, i.e., a mean difference of 471€ (95% CI: 232€-712€; p-value<0.01).
Utility scores obtained during follow-up are described in the Supplementary Table 2.
In the FFR-guided group, the mean number of one-year MACE per patient was 0.075 (±0.327) and one-year QALY was 0.860 (±0.192) versus 0.057 (±0.309) and 0.871 (±0.178) in the angiography-guided group (p-value=0.34 and 0.49, respectively).
With a higher cost and lower efficiency and utility scores, FFR-guided complete revascularisation was a dominated strategy, i.e., more expensive and less effective. The set of ICERs estimated by the non-parametric bootstrap are presented by the cloud of points on the cost-effectiveness plane; 85% of these ICERs and 81% of the ICURs were located in the top left-hand quadrant (Central illustration). In addition, the acceptability curve is presented in Figure 1. At a threshold of 100,000€/QALY there was 9% chance that the FFR-guided strategy was cost effective (Figure 1).
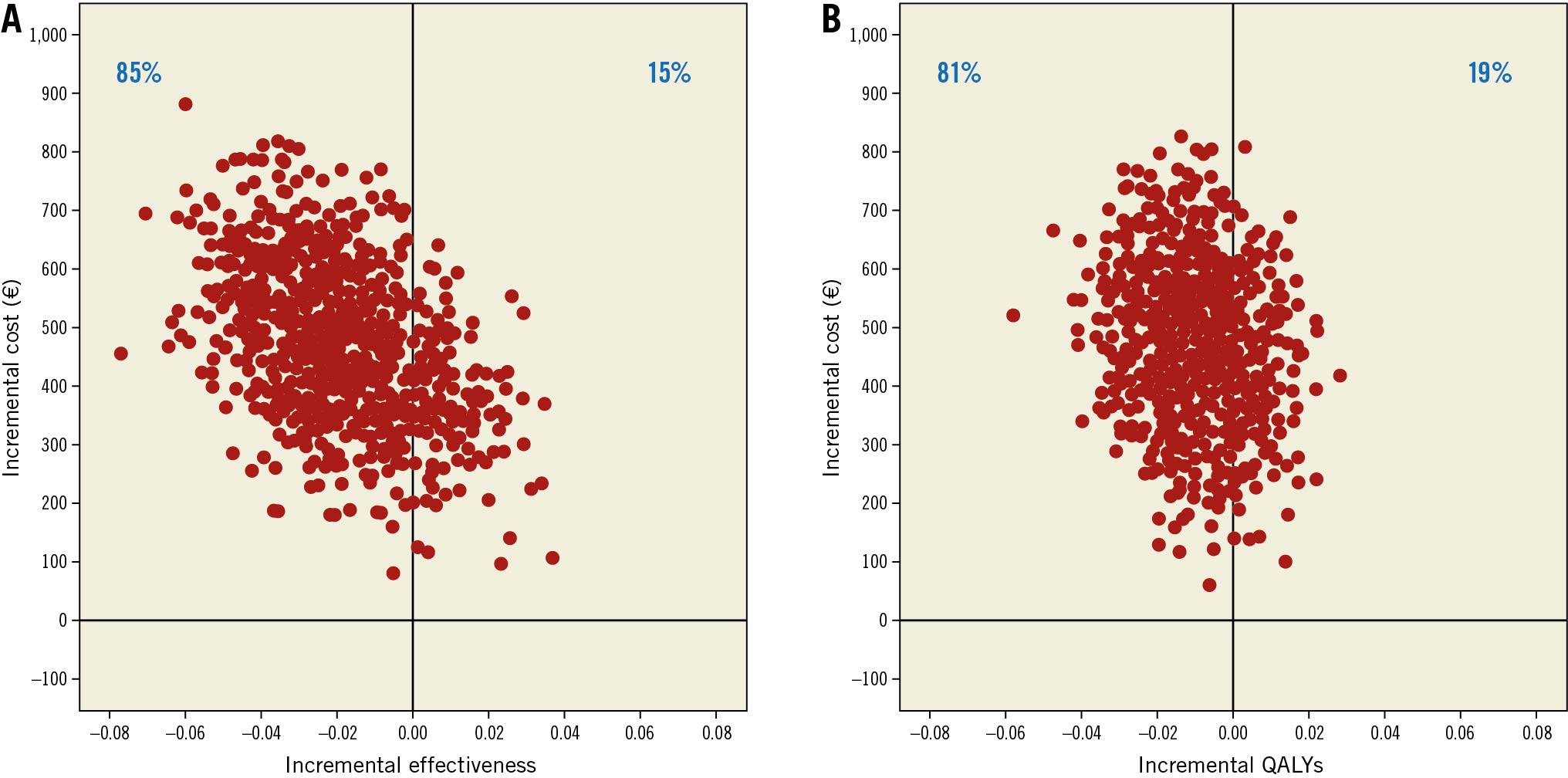
Central illustration. Scatter plots of incremental cost and effectiveness of FFR-guided strategy compared to angiography-guided strategy at one year. A) Scatter plot of incremental cost and effectiveness in Euros per MACE avoided. B) Scatter plot of incremental cost and effectiveness in Euros per quality-adjusted life-year (QALY) gained. MACE: major adverse cardiovascular events, including all-cause death, non-fatal myocardial infarction, and unplanned hospitalisation leading to urgent revascularisation; QALYs: quality-adjusted life years
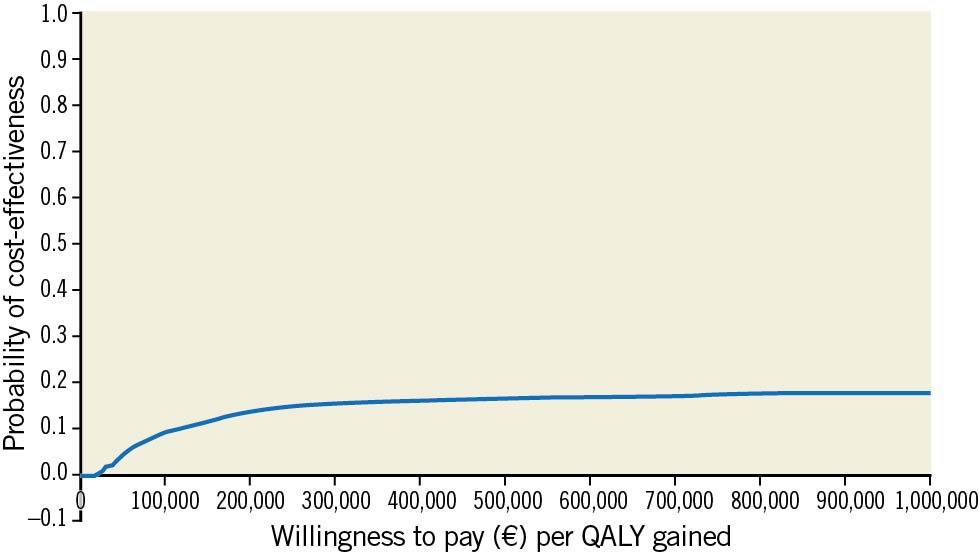
Figure 1. Cost-effectiveness acceptability curve showing the probability that FFR-guided strategy is cost effective compared to angiography-guided strategy at one year. QALYs: quality-adjusted life years
Cost and clinical outcomes according to the type of procedure (immediate or staged) are presented in the Supplementary Table 3. When the procedure was performed at the same time as the primary PCI, the average total cost per patient at one year was 6,497€ (±2,548) for the FFR-guided group, and 6,456€ (±1,825) for the angiography-guided group.
Discussion
We performed the prespecified within-trial incremental cost-effectiveness and cost-utility analyses of the FLOWER-MI trial to inform clinicians on the expected costs and quality of life results of using FFR-guided PCI. The hypothesis was that the additional cost of measuring the FFR would be offset by a decreased use of stents, and by the savings of decreased subsequent events. The FLOWER-MI trial did not show a superiority of the FFR-guided PCI compared to angiography-guided PCI for non-infarct related coronary arteries in patients with STEMI and MVD. The analyses of the joint distribution of costs and outcomes (MACE and quality of life) found a high probability that angiography-guided revascularisation dominated FFR-guided revascularisation, thus yielding a “negative” point estimate of the ICER, with a probabilistic sensitivity analysis showing an 80% probability of FFR-guided PCI being dominated.
Reimbursement and purchasing decisions for devices are often subject to uncertainty at the time of their market entry, without economic evaluation to guide policy makers14. The trial-based economic study of FLOWER-MI provides evidence of efficacy and economic value of two revascularisation strategies in patients with MVD admitted for myocardial infarction.
These results differ from those found in FAME4. Firstly, the FAME and FLOWER-MI studies were carried out on different populations (i.e., in stable patients for FAME and in STEMI patients for FLOWER-MI). Secondly, the severity of non-culprit lesion stenosis based on visual estimation was different between the studies, which could explain the discrepancy in results, particularly regarding the need for stenting. Thirdly, the cost difference could be explained by (i) the substantial decrease in the drug-eluting stents (DES) tariff from 1,100€ at the time of FAME (2010) to 573€ in 2021, (ii) the pressure wire tariff in FLOWER-MI was 400€ vs $650 (458€)3 in FAME, (iii) among 586 patients in the FFR-guided PCI group, 388 (66%) of them had ≥1 PCI in non-culprit lesions and (iv) compared to the angiography-guided group, the mean number of one-year MACE per patient is higher in the FFR-guided group, which increased the costs incurred by the FFR-guided group during follow-up.
Patients in the FLOWER-MI trial had an initial length of stay of five days, one day longer than in the FAME and Compare-Acute trials, and the average number of stents used per patient was higher in both groups of FLOWER-MI than in both other trials14.
The economic results are sensitive to the relative costs of DES and FFR pressure wires, as in the FAME trial, since the use of FFR resulted in a reduction in stent use. In the USA, DES are more expensive than pressure wires15, and up to six times more expensive than in European countries14. In Europe, DES and FFR pressure wires are roughly the same price. In Japan, the prices are different again, with FFR pressure wires being more expensive1617. With a US price three times higher for DES than for pressure wire ($1,656 or 1,155€ vs $650 or 458€)3, FFR additional costs can be offset by a 30% reduction in DES implantation, which is what happened in FAME. In FLOWER-MI, the relative costs of DES and pressure wires in European countries make cost offsets very unlikely.
In practice, the main results of the FLOWER-MI trial have suggested that the FFR-guided strategy for complete revascularisation in STEMI patients with MVD offered no clear clinical benefit over angiography-guided complete revascularisation. The most interesting situation concerns intermediate lesions (i.e., 50 to 70% of stenosis) in which the probability of functional lesions is rare. FFR measurement could be useful for patients especially if the measurement is performed during the index procedure (i.e., after the primary PCI). However, we recently showed that in patients with STEMI undergoing complete revascularisation guided by FFR measurement, those with ≥1 PCI in non-culprit lesions had lower event rates at one year, compared with patients with deferred PCI, suggesting that deferring lesions judged relevant by visual estimation but with FFR >0.80 may not be optimal in this context18.
Limitations
We have identified the following limitations.
First, during the initial procedure, patients in the FFR-guided PCI group received adenosine and intracoronary nitrate. The additional cost of drugs was not included in our calculations, as it would further increase the cost difference in favour of the angiography-guided group. We did not include out-of-hospital costs, but it is unlikely that they would constitute an important limitation since medications and follow-up visits were the same in both groups and represent less than 10% of total costs during the year following STEMI19 (the average cost for medications per year was reported, by social health insurance in 2019, to be 426€ per patient20. Second, we encouraged all investigators to perform complete revascularisation during the index procedure for the treatment of non-culprit lesions. In practice, however, this proved to be unrealistic, and only 4% of the patients (44 out of 1,163) had an immediate intervention for non-culprit lesions. This can be explained by logistical constraints due to randomisation, the organisation of each centre (personnel insufficiently trained for FFR measurement during nights and week-ends in some catheterisation laboratories), and the reluctance of investigators to perform MVD revascularisation during the acute procedure (increased procedural time, prothrombotic and inflammatory milieu at the acute phase of STEMI; increased contrast load with increased risk of contrast-induced nephrotoxicity; radiation exposure). In addition, coronary spasm at the acute stage may lead to possible overestimation of stenosis severity in non-infarct-related arteries, possibly explaining why some patients in the angiography-guided group (3%) did not undergo PCI. Overall, this suggests that FFR measurement at the same time as PCI of the infarct-related artery might be rather unrealistic in routine clinical conditions. Immediate revascularisation of non-culprit lesions would reduce costs but not necessarily favour the FFR-guided group since it can be angiography-guided as well. Only patients with intermediate stenosis could potentially avoid repeat admission. Third, trial-based economic evaluations reflect the practice and unit costs of the investigator’s country, which limits external validity. The known negative correlation between use of resources and unit costs in health care is confirmed by the comparison of our data with the FAME economic evaluation, based upon Medicare unit cost. Bed days, cardiac care unit days and DES are three to four times more expensive in the USA than in France. However, the cost of the study device was not too different, 400€ versus $650 (458€). Fourth, completed at baseline, day 30, 6 and 12 months, the EQ-5D-5L health-related quality of life questionnaire did not necessarily capture the quality of life during adverse events, which may have led to an overestimation of the cost-utility of the FFR-guided strategy. Fifth, the FLOWER-MI study was unblinded which could have affected patients’ perception of their quality of life. The informed consent mentioned that FFR could allow better identification of lesions that needed revascularisation. However, patients also knew that all significant lesions would be treated, either immediately or in a staged procedure. The EQ-5D-5L quality data are consistent with the MACE data, which leads us to believe that the absence of blinding had limited impact on quality of life reporting.
Conclusions
Among patients presenting with STEMI and MVD, the cost-effectiveness and cost-utility were in favour of the angiography-guided PCI, rather than an FFR-guided strategy.
Impact on daily practice
The main results of the FLOWER-MI trial showed that FFR-guided PCI was not superior compared to angio-guided PCI in STEMI patients with MVD. With an 80% probability that FFR-guided PCI is less effective and more expensive, this economic evaluation also highlighted the financial limitations of FFR-guided revascularisation, which may lead the healthcare authorities to reject the use of this innovation for all patients. Potentially, this strategy could be reserved for intermediate lesions (i.e., non-culprit lesions 50-70%). We recently showed that in patients with STEMI undergoing complete revascularisation guided by FFR measurement, those with ≥1 PCI in non-culprit lesions had lower event rates at one year, compared with patients with deferred PCI, suggesting that deferring lesions judged relevant by visual estimation but with FFR >0.80 may not be optimal in this context18. Other data therefore seem necessary to define the use of FFR in acute myocardial infarction. Regarding the trade-offs between the cost of using FFR-guided PCI, the issue of the relative costs of DES and FFR guidewires is not as significant in Europe or Japan as it is in the USA, or countries where DES are priced above guidewires.
Funding
FLOWER-MI is an academic study.
Acknowledgements
The authors are deeply indebted to all patients who accepted to participate in the surveys, and to the physicians who took care of the patients at the participating institutions. The authors would like to thank Anaïs Charles-Nelson, Aurelia Dinut, Dr Juliette Djadi-Prat and Hakima Manseur for their help in managing the FLOWER-MI study.
Conflict of interest statement
E. Puymirat reports grants from the French Ministry of Health and St Jude Medical, during the conduct of the study; lecture and/or consulting fees from: Abbott, Amgen, Astra Zeneca, BMS, Bayer, Biotronik, Boehringer Ingelheim, Daiichi-Sankyo, Lilly, MSD, Novartis, Pfizer, Sanofi and Servier. G. Cayla reports personal fees from Astra Zeneca, Biotronik and Bristol Myers Squibb, Pfizer, Sanofi, and Microport; and grants and personal fees from Medtronic, outside the submitted work. T. Simon reports personal fees from AstraZeneca, Ablative Solutions, Bayer, Novartis, Sanofi and 4Living Biotech; grants from AstraZeneca, Boehringer, Daiichi-Sankyo, Eli Lilly, GSK, Novartis and Sanofi. G. Steg reports personal fees from Amgen, Bristol Myers Squibb, Boehringer Ingelheim, Idorsia, Myokardia, Novartis, Novo Nordisk, Pfizer, Regeneron and Sanofi/Lexicon; personal fees and non-financial support from AstraZeneca, grants and personal fees from Amarin, Bayer, Sanofi and Servier, outside the submitted work. In addition, G. Steg has a patent issued on alirocumab for cardiovascular risk reduction, assigned to Sanofi. G. Montalescot reports grants and personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boston Scientific, Bristol-Myers Squibb, IRIS-Servier, Novartis, Quantum Genomics and Sanofi-Aventis; personal fees from Boehringer-Ingelheim, Cell-Prothera and Europa; grants from COR2ED, CSL Behring, Idorsia, Medtronic, MSD and Pfizer, outside the submitted work. O. Varenne reports grants from Abbott Vascular and Boston Scientific, during the conduct of the study and he reports receiving personal fees from AstraZeneca and personal fees from Servic, outside the submitted work. L. Bonello reports grants from Abbott, AstraZeneca, Biotronik, Boston Scientific, and Bristol Meyer, outside the submitted work. J-L. Georges reports personal fees from AstraZeneca France and Sanofi-Aventis, other from Amgen, outside the submitted work. N. Danchin reports grants from the French Ministry of Health and St Jude Medical, during the conduct of the study; grants, personal fees and non-financial support from Amgen, AstraZeneca, Bayer and BMS; grants and personal fees from Sanofi; and personal fees from Boehringer Ingelheim, Intercept, MSD, Novartis, Servier, Novo Nordisk, UCB and Vifor, outside the submitted work. I. Durand-Zaleski reports grants from the French Ministry of Health, during the conduct of the study; and personal fees from Boston Scientific and Medtronic, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

